Oral health is vital to general health and well-being for all ages, and as with other chronic conditions, oral health problems increase with age. There is a bi-directional link between nutrition and oral health, in that nutrition affects the health of oral tissues and saliva, and the health of the mouth may affect the foods consumed. Evidence suggests that a healthy diet generally has a positive impact on oral health in older adults. Although studies examining the direct link between oral health and protein intake in older adults are limited, some have explored the relationship via malnutrition, which is also prevalent among older adults. Protein–energy malnutrition (PEM) may be associated with poor oral health, dental caries, enamel hypoplasia, and salivary gland atrophy.
- older adults
- protein intake
- periodontitis
- amino acid composition
- oral health
1. Introduction
2. Effect of Various Dietary Protein Sources on Oral Health
Dietary Amino Acid Composition and Its Effect on Oral Health
| Amino Acid | Effect on Oral Health | Material | References |
|---|---|---|---|
| Alanine | Alanine and histidine form citrulline. A higher concentration of citrulline in saliva is correlated with periodontitis. | Human | [58] |
| Arginine | Arginine improves calcium absorption by the formation of soluble complexes with calcium that maintain calcium in an absorbent form, which is important for enamel maturation. Higher concentration saliva in Stage III Grade C generalised periodontitis. |
Human | [58] |
| L-Arginine | L-Arginine monohydrochloride in saliva inhibits bacterial coaggregation in the oral cavity by decreasing the viscosity of extracellular polymeric substances produced by bacteria and altering cellular metabolism resulting in biofilm dispersion and reducing antibiotic tolerance. | Human | [55] |
| Aspartic acid | Adult age estimation is based on aspartic acid racemisation in dentine. | Human | [59] |
| Cysteine | Toxic to oral Streptococci through inhibiting an enzymatic step in the valine-leucine biosynthetic pathway. | Human | [60] |
| Reduces bacterial biofilm adherence and biofilm biomass. | A multi-species plaque-derived biofilm model | [61] | |
| N-Acetyl-L-cysteine (from L-cysteine) | Reduces pain and hypersensitivity of teeth. Protects gingivae from white lesions and oral mucosal inflammation after using bleaching agents. |
Human | [62] |
| As mouthwash, it treats and prevents gingivitis | Human | [63] | |
| Glutamic acid | Higher in Stage III Grade C generalised periodontitis. | Human | [58] |
| Glutamine | Topical administration to patients receiving stomatoxic chemotherapy resulted in 20% decrease in moderate and severe oral mucositis. | Human | [64] |
| Glycine | Glycine supplement reduced dental caries development by 65.7% through the changes in the fatty acid composition of the tooth and a reduction in growth rate (no effect on the retention of either calcium or phosphorus by dietary glycine). | Rodent (rat) | [65] |
| Glycine is an integral part of collagen that is an intrinsic component of the tooth structure. Reduced level of saliva glycine has been associated with collagen degradation. Hence, higher salivary glycine has been associated with reduced risk of dental caries and periodontitis through reduced collagen degradation and decreased collagenase activity, leading to less inflammation in gingiva. | Human | [66][67] | |
| Histidine † | Reduces the risk of dental caries. Lack of histidine and its derivatives in saliva results in chelation, i.e., formation of metal complexes with amino acids, leading to initial lesion and secondary to destruction of the organic matrix by the action of proteolytic bacteria. |
Human | [56] |
| Isoleucine † | Found in carious dentine | Human | [68] |
| Leucine † | Repaired carious enamel. | Human | [69] |
| Leucine-rich amelogenin peptide regulates receptor activator of NF-kappa B ligand (RANKL) expression in cementoblast/periodontal ligament cells. | Rodent (mouse) | [70] | |
| Lysine † | Important for the integrity of dentally attached epithelium to act as a barrier to microbial products. | Lysine decarboxylas extracted on Eikenella corrodens bacterial cell surface | [71] |
| Methionine † | Methionine reduces the adverse effect of fluorides on soft tissue, and this has been found to be optimal for the prevention of the adverse effects of chronic fluoride intoxication together with vitamin E in drinking water. | Rodent (rat) | [72] |
| Phenylalanine † | May inhibit dental caries development. In bacteria, phenylalanine is converted to phenylpropionate or phenylacetate, resulting in alkali environment which is an essential factor in maintaining plaque pH homeostasis. |
Human | [73] |
| Proline | Salivary proline-rich glycoprotein regulates the oral calcium homeostasis by controlling the supersaturated state of saliva with respect to calcium phosphate salts, countering the plaque acidity, formation of dental pellicle, and influencing the composition of plaque. | Human | [74] |
| Moreover, this prevents the adherence of oral microorganisms inhibiting their growth and neutralises acids from biofilms protecting from dental caries. | Human | [75] | |
| Serine and threonine † | Interact with host cytoplasmic phosphoproteins, facilitating internalisation of bacteria. | Primary cultures of human gingival epithelial cells | [76][77] |
| Tryptophan † | Tryptophan metabolites generated from oral supplementation of tryptophan promote regulatory T-cell (Treg) differentiation and suppress proinflammatory T-helper cell (Th)1 and Th17 phenotypes. | Rodent (mice) | [78] |
| Higher saliva tryptophan level was observed in Stage III Grade B generalised periodontitis. | Human | [58] | |
| Tyrosine | Potential biomarker of oral lichen planus (lower levels). Tyrosine is suggested to be involved in the antioxidative defence. |
Human | [79] |
| Valine † | Detected in sound dentine compared to carious dentine. | Human | [68] |
| Homocysteine ‡ | Associated with high narrow palate, mandibular prognathia (protruding lower jaw), crowding and early eruption of teeth and short dental roots. | Human | [80] |
3. Dietary Protein Intake in Older Adults
4. Protein–Energy Malnutrition and Oral Health in Older Adults
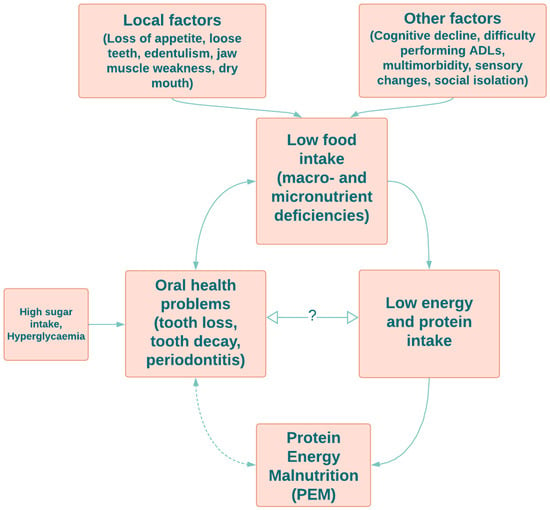
This entry is adapted from the peer-reviewed paper 10.3390/nu14214478
References
- AHMAC. Aboriginal and Torres Strait Islander Health Performance Framework 2017 Report; AHMAC: Canberra, Australia, 2017. Available online: https://www.niaa.gov.au/sites/default/files/publications/2017-health-performance-framework-report_1.pdf (accessed on 22 June 2022).
- Baiju, R. Oral Health and Quality of Life: Current Concepts. J. Clin. Diagn. Res. 2017, 11, ZE21–ZE26.
- Sanders, A.E.; Slade, G.D.; Lim, S.; Reisine, S.T. Impact of oral disease on quality of life in the US and Australian populations. Community Dent. Oral Epidemiol. 2009, 37, 171–181.
- Ruiz-Roca, J.A.; Fuentes, D.M.; García, F.J.G.; Martínez-Beneyto, Y. Oral status of older people in medium to long-stay health and social care setting: A systematic review. BMC Geriatr. 2021, 21, 363.
- Division of Oral Health, Facts about Older Adult Oral Health. 2021. Available online: https://www.cdc.gov/oralhealth/basics/adult-oral-health/adult_older.htm (accessed on 20 June 2022).
- Dye, B.; Thornton-Evans, G.; Li, X.; Iafolla, T. Dental caries and tooth loss in adults in the United States, 2011–2012. NCHS Data Brief 2015, 197.
- Dodington, D.W.; Young, H.E.; Beaudette, J.R.; Fritz, P.C.; Ward, W.E. Improved Healing after Non-Surgical Periodontal Therapy Is Associated with Higher Protein Intake in Patients Who Are Non-Smokers. Nutrients 2021, 13, 3722.
- Eke, P.I.; Thornton-Evans, G.O.; Wei, L.; Borgnakke, W.; Dye, B.A.; Genco, R.J. Periodontitis in US Adults. J. Am. Dent. Assoc. 2018, 149, 576–588.e6.
- González-Moles, M.; Ramos-García, P. State of Evidence on Oral Health Problems in Diabetic Patients: A Critical Review of the Literature. J. Clin. Med. 2021, 10, 5383.
- Kotronia, E.; Brown, H.; Papacosta, A.O.; Lennon, L.T.; Weyant, R.J.; Whincup, P.H.; Wannamethee, S.G.; Ramsay, S.E. Oral health and all-cause, cardiovascular disease, and respiratory mortality in older people in the UK and USA. Sci. Rep. 2021, 11, 16452.
- Deschamps-Lenhardt, S.; Martin-Cabezas, R.; Hannedouche, T.; Huck, O. Association between periodontitis and chronic kidney disease: Systematic review and meta-analysis. Oral Dis. 2019, 25, 385–402.
- Rapp, L.; Maret, D.; Diemer, F.; Ferré, M.L. Dental Caries in Geriatric Dentistry: An Update for Clinicians. Int. J. Oral Dent. Health 2019, 5, 080.
- Chan, A.K.Y.; Tamrakar, M.; Jiang, C.M.; Lo, E.C.M.; Leung, K.C.M.; Chu, C.H. A Systematic Review on Caries Status of Older Adults. Int. J. Environ. Res. Public Health 2021, 18, 10662.
- Chapple, I.L.C.; Bouchard, P.; Cagetti, M.G.; Campus, G.; Carra, M.-C.; Cocco, F.; Nibali, L.; Hujoel, P.; Laine, M.L.; Lingström, P.; et al. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: Consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J. Clin. Periodontol. 2017, 44 (Suppl. S18), S39–S51.
- Choowong, P.; Wali, J.A.; Nguyen, A.T.M.; Jayasinghe, T.N.; Eberhard, J. Macronutrient-induced modulation of periodontitis in rodents—A systematic review. Nutr. Rev. 2021, 80, 1160–1178.
- Hujoel, P.P.; Lingström, P. Nutrition, dental caries and periodontal disease: A narrative review. J. Clin. Periodontol. 2017, 44, S79–S84.
- Hwang, S.-Y.; Park, J.-E. The Relationship Between Periodontal Disease and Nutrient Intake in Korean Adults: The Korea National Health and Nutrition Examination Survey (KNHANES VII) from 2016–2018. Oral Health Prev. Dent. 2022, 20, 313–320.
- Santonocito, S.; Polizzi, A.; Palazzo, G.; Indelicato, F.; Isola, G. Dietary Factors Affecting the Prevalence and Impact of Periodontal Disease. Clin. Cosmet. Investig. Dent. 2021, 13, 283–292.
- Griffin, S.O.; Jones, J.A.; Brunson, D.; Griffin, P.M.; Bailey, W.D. Burden of Oral Disease Among Older Adults and Implications for Public Health Priorities. Am. J. Public Health 2012, 102, 411–418.
- Lingström, P.; Simark Mattsson, C. Chapter 2: Oral Conditions. Monogr. Oral Sci. 2020, 28, 14–21.
- Iorgulescu, G. Saliva between normal and pathological. Important factors in determining systemic and oral health. J. Med. Life 2010, 2, 303–307.
- McArthur, W.P. Oral Immunology. In Encyclopedia of Immunology; Elsevier: Amsterdam, The Netherlands, 1998; pp. 1888–1893.
- Psoter, W.; Reid, B.; Katz, R. Malnutrition and Dental Caries: A Review of the Literature. Caries Res. 2005, 39, 441–447.
- Llena-Puy, C. The rôle of saliva in maintaining oral health and as an aid to diagnosis. Med. Oral Patol. Oral Cir. Bucal 2006, 11, E449–E455.
- Toan, N.; Ahn, S.-G. Aging-Related Metabolic Dysfunction in the Salivary Gland: A Review of the Literature. Int. J. Mol. Sci. 2021, 22, 5835.
- Dodds, M.; Roland, S.; Edgar, M.; Thornhill, M. Saliva A review of its role in maintaining oral health and preventing dental disease. BDJ Team 2015, 2, 15123.
- Kossioni, A.E. The Association of Poor Oral Health Parameters with Malnutrition in Older Adults: A Review Considering the Potential Implications for Cognitive Impairment. Nutrients 2018, 10, 1709.
- Vach, K.; Woelber, J.P. (Eds.) Nutrition and Human Oral Health; MDPI: Basel, Switzerland, 2022.
- Azzolino, D.; Passarelli, P.C.; De Angelis, P.; Piccirillo, G.B.; D’Addona, A.; Cesari, M. Poor Oral Health as a Determinant of Malnutrition and Sarcopenia. Nutrients 2019, 11, 2898.
- Naka, O.; Anastassiadou, V.; Pissiotis, A. Association between functional tooth units and chewing ability in older adults: A systematic review. Gerodontology 2012, 31, 166–177.
- O’Connor, J.-L.P.; Milledge, K.L.; O’Leary, F.; Cumming, R.; Eberhard, J.; Hirani, V. Poor dietary intake of nutrients and food groups are associated with increased risk of periodontal disease among community-dwelling older adults: A systematic literature review. Nutr. Rev. 2020, 78, 175–188.
- Sheiham, A.; Steele, J.; Marcenes, W.; Lowe, C.; Finch, S.; Bates, C.; Prentice, A.; Walls, A. The Relationship among Dental Status, Nutrient Intake, and Nutritional Status in Older People. J. Dent. Res. 2001, 80, 408–413.
- Iwasaki, M.; Hirano, H.; Ohara, Y.; Motokawa, K. The association of oral function with dietary intake and nutritional status among older adults: Latest evidence from epidemiological studies. Jpn. Dent. Sci. Rev. 2021, 57, 128–137.
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-Based Recommendations for Optimal Dietary Protein Intake in Older People: A Position Paper From the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559.
- Deutz, N.E.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936.
- Baum, J.I.; Kim, I.-Y.; Wolfe, R.R. Protein Consumption and the Elderly: What Is the Optimal Level of Intake? Nutrients 2016, 8, 359.
- Bomfim, R.A.; de Souza, L.B.; Corrente, J.E. Tooth loss and its relationship with protein intake by elderly Brazilians—A structural equation modelling approach. Gerodontology 2017, 35, 51–58.
- Kotronia, E.; Brown, H.; Papacosta, A.O.; Lennon, L.T.; Weyant, R.J.; Whincup, P.H.; Wannamethee, S.G.; Ramsay, S.E. Poor oral health and the association with diet quality and intake in older people in two studies in the UK and USA. Br. J. Nutr. 2021, 126, 118–130.
- Woelber, J.P.; Bremer, K.; Vach, K.; König, D.; Hellwig, E.; Ratka-Krüger, P.; Al-Ahmad, A.; Tennert, C. An oral health optimized diet can reduce gingival and periodontal inflammation in humans—A randomized controlled pilot study. BMC Oral Health 2016, 17, 28.
- Morais, J.A.; Chevalier, S.; Gougeon, R. Protein turnover and requirements in the healthy and frail elderly. J. Nutr. Health Aging 2006, 10, 272–283.
- Yeung, S.S.Y.; Lee, J.S.W.; Kwok, T. A Nutritionally Complete Oral Nutritional Supplement Powder Improved Nutritional Outcomes in Free-Living Adults at Risk of Malnutrition: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2022, 19, 11354.
- Thomson, K.H.; Rice, S.; Arisa, O.; Johnson, E.; Tanner, L.; Marshall, C.; Sotire, T.; Richmond, C.; O’Keefe, H.; Mohammed, W.; et al. Effectiveness and cost-effectiveness of oral nutritional supplements in frail older people who are malnourished or at risk of malnutrition: A systematic review and meta-analysis. Lancet Health Longev. 2022, 3, e654–e666.
- Tungare, S.; Paranjpe, A.G. Diet and Nutrition to Prevent Dental Problems; StatPearls: Treasure Island, FL, USA, 2022.
- Krall, E.A.; Wehler, C.; Garcia, R.; Harris, S.S.; Dawson-Hughes, B. Calcium and vitamin D supplements reduce tooth loss in the elderly. Am. J. Med. 2001, 111, 452–456.
- Gondivkar, S.M.; Gadbail, A.R.; Gondivkar, R.S.; Sarode, S.C.; Sarode, G.S.; Patil, S.; Awan, K.H. Nutrition and oral health. Disease-A-Month 2018, 65, 147–154.
- Lee, K.; Kim, J. Dairy Food Consumption is Inversely Associated with the Prevalence of Periodontal Disease in Korean Adults. Nutrients 2019, 11, 1035.
- Lee, Y.; Yoon, Y.; Choi, K.-H. Probiotics-Mediated Bioconversion and Periodontitis. Korean J. Food Sci. Anim. Resour. 2021, 41, 905–922.
- Kato, K.; Toba, Y.; Matsuyama, H.; Yamamura, J.-I.; Matsuoka, Y.; Kawakami, H.; Itabashi, A.; Kumegawa, M.; Aoe, S.; Takada, Y. Milk basic protein enhances the bone strength in overectimised rats. J. Food Biochem. 2000, 24, 467–476.
- Seto, H.; Toba, Y.; Takada, Y.; Kawakami, H.; Ohba, H.; Hama, H.; Horibe, M.; Nagata, T. Milk basic protein increases alveolar bone formation in rat experimental periodontitis. J. Periodontal Res. 2006, 42, 85–89.
- Aimutis, W.R. Bioactive Properties of Milk Proteins with Particular Focus on Anticariogenesis. J. Nutr. 2004, 134, 989S–995S.
- Berlutti, F.; Pilloni, A.; Pietropaoli, M.; Polimeni, A.; Valenti, P. Lactoferrin and oral diseases: Current status and perspective in periodontitis. Ann. Stomatol. 2011, 2, 10–18.
- Reema, S.D.; Lahiri, P.K.; Roy, S.S. Review of casein phosphopeptides-amorphous calcium phosphate. Chin. J. Dent. Res. Off. J. Sci. Sect. Chin. Stomatol. Assoc. 2014, 1, 7–14.
- Sionov, R.V.; Tsavdaridou, D.; Aqawi, M.; Zaks, B.; Steinberg, D.; Shalish, M. Tooth mousse containing casein phosphopeptide-amorphous calcium phosphate prevents biofilm formation of Streptococcus mutans. BMC Oral Health 2021, 21, 136.
- Lopez, M.J.; Mohiuddin, S.S. Biochemistry, Essential Amino Acids; StatPearls: Treasure Island, FL, USA, 2022.
- Kolderman, E.; Bettampadi, D.; Samarian, D.; Dowd, S.E.; Foxman, B.; Jakubovics, N.S.; Rickard, A.H. L-Arginine Destabilizes Oral Multi-Species Biofilm Communities Developed in Human Saliva. PLoS ONE 2015, 10, e0121835.
- Vranić, L.; Granić, P.; Rajić, Z. Basic amino acid in the pathogenesis of caries. Acta Stomatol. Croat. 1991, 25, 71–76.
- Solon-Biet, S.M.; Cogger, V.C.; Pulpitel, T.; Wahl, D.; Clark, X.; Bagley, E.E.; Gregoriou, G.C.; Senior, A.M.; Wang, Q.-P.; Brandon, A.E.; et al. Branched-chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control. Nat. Metab. 2019, 1, 532–545.
- Balci, N.; Kurgan, Ş.; Çekici, A.; Çakır, T.; Serdar, M.A. Free amino acid composition of saliva in patients with healthy periodontium and periodontitis. Clin. Oral Investig. 2021, 25, 4175–4183.
- Sirin, N.; Matzenauer, C.; Reckert, A.; Ritz-Timme, S. Age estimation based on aspartic acid racemization in dentine: What about caries-affected teeth? Int. J. Leg. Med. 2018, 132, 623–628.
- Cowman, R.A.; Baron, S.S.; Fitzgerald, R.J. Cysteine toxicity for oral streptococci and effect of branched-chain amino acids. Infect. Immun. 1983, 39, 1107–1113.
- Rasmussen, K.; Nikrad, J.; Reilly, C.; Li, Y.; Jones, R. N-Acetyl-l-cysteine effects on multi-species oral biofilm formation and bacterial ecology. Lett. Appl. Microbiol. 2015, 62, 30–38.
- Wang, D.; Kaur, K.; Paranjpe, A.; Lee, E.; Wasilewski, M.; Sung, D.; Han, D.; Sung, E.C.; Jewett, A. N-acetyl cysteine prevents pain and hypersensitivity of bleaching agents without affecting their aesthetic appeal; evidence from in vitro to animal studies and to human clinical trials. Transl. Med. Commun. 2019, 4, 19.
- Al-Kamel, A.; Al-Hajj, W.A.; Halboub, E.; Abdulrab, S.; Al-Tahami, K.; Al-Hebshi, N.N. N-acetyl cysteine versus chlorhexidine mouthwashes in prevention and treatment of experimental gingivitis: A randomized, triple-blind, placebo-controlled clinical trial. Clin. Oral Investig. 2019, 23, 3833–3842.
- Peterson, D.E.; Petit, R.G. Phase III study: AES-14 in chemotherapy patients at risk for mucositis . Proc. Am. Soc. Clin. Oncol. 2003, 22, 725.
- Das, S.K.; Harris, R.S. Effect of Dietary Supplementation of Glycine on Caries Development and Lipids in Rat Molars. J. Dent. Res. 1975, 54, 987–992.
- Fonteles, C.S.; Guerra, M.H.; Ribeiro, T.R.; Mendonça, D.N.; de Carvalho, C.B.; Monteiro, A.J.; Toyama, D.O.; Toyama, M.H.; Fonteles, M.C. Association of free amino acids with caries experience and mutans streptococci levels in whole saliva of children with early childhood caries. Arch. Oral Biol. 2009, 54, 80–85.
- Syrjänen, S.; Piironen, P.; Markkanen, H. Free amino-acid content of wax-stimulated human whole saliva as related to periodontal disease. Arch. Oral Biol. 1987, 32, 607–610.
- Lin, Y.H. Changes of dentin matrices during carious process. Tsurumi Shigaku. Tsurumi Univ. Dent. J. 1989, 15, 249–266.
- Mukherjee, K.; Ruan, Q.; Liberman, D.; White, S.N.; Moradian-Oldak, J. Repairing human tooth enamel with leucine-rich amelogenin peptide–chitosan hydrogel. J. Mater. Res. 2016, 31, 556–563.
- Haruyama, N.; Yamaza, T.; Suzuki, S.; Hall, B.; Cho, A.; Gibson, C.W.; Kulkarni, A.B. Leucine rich amelogenin peptide prevents ovariectomy-induced bone loss in mice. PLoS ONE 2021, 16, e0259966.
- Lohinai, Z.; Keremi, B.; Szöko, E.; Tábi, T.; Szabo, C.; Tulassay, Z.; Dicesare, J.C.; Davis, C.A.; Collins, L.M.; Levine, M. Biofilm Lysine Decarboxylase, a New Therapeutic Target for Periodontal Inflammation. J. Periodontol. 2015, 86, 1176–1184.
- Błaszczyk, I.; Birkner, E.; Gutowska, I.; Romuk, E.; Chlubek, D. Influence of Methionine and Vitamin E on Fluoride Concentration in Bones and Teeth of Rats Exposed to Sodium Fluoride in Drinking Water. Biol. Trace Elem. Res. 2011, 146, 335–339.
- Shi, W.; Tian, J.; Xu, H.; Wang, G.; Zhou, Q.; Qin, M. Carbon source utilization patterns in dental plaque and microbial responses to sucrose, lactose, and phenylalanine consumption in severe early childhood caries. J. Oral Microbiol. 2020, 12, 1782696.
- Pateel, D.G.S.; Gunjal, S.; Fong, L.F.; Hanapi, N.S.M. Association of Salivary Statherin, Calcium, and Proline-Rich Proteins on Oral Hygiene: A Cross-Sectional Study. Int. J. Dent. 2021, 2021, 1982083.
- Zakhary, G.; Clark, R.; Bidichandani, S.; Owen, W.; Slayton, R.; Levine, M. Acidic Proline-rich Protein Db and Caries in Young Children. J. Dent. Res. 2007, 86, 1176–1180.
- Oda, D.; Watson, E. Human oral epithelial cell culture I. Improved conditions for reproducible culture in serum-free medium. Vitr. Cell. Dev. Biol. 1990, 26, 589–595.
- Ren, L.; Shen, D.; Liu, C.; Ding, Y. Protein Tyrosine and Serine/Threonine Phosphorylation in Oral Bacterial Dysbiosis and Bacteria-Host Interaction. Front. Cell. Infect. Microbiol. 2022, 11, 814659.
- Lanz, T.V.; Becker, S.; Mohapatra, S.R.; Opitz, C.A.; Wick, W.; Platten, M. Suppression of Th1 differentiation by tryptophan supplementation in vivo. Amino Acids 2017, 49, 1169–1175.
- Darczuk, D.; Krzyściak, W.; Bystrowska, B.; Kęsek, B.; Kościelniak, D.; Chomyszyn-Gajewska, M.; Kaczmarzyk, T. The Relationship between the Concentration of Salivary Tyrosine and Antioxidants in Patients with Oral Lichen Planus. Oxidative Med. Cell. Longev. 2019, 2019, 5801570.
- Björksved, M.; Arnrup, K. Homocystinuria and oral health. A report of 14 cases. Swed. Dent. J. 2012, 36, 101–108.
- Ministry of Health Australia. Nutrient Reference Values for Australia and New Zealand. 2014. Available online: https://www.nrv.gov.au/nutrients/protein (accessed on 10 August 2022).
- Kim, I.-Y.; Schutzler, S.; Schrader, A.; Spencer, H.; Kortebein, P.; Deutz, N.E.P.; Wolfe, R.R.; Ferrando, A.A. Quantity of dietary protein intake, but not pattern of intake, affects net protein balance primarily through differences in protein synthesis in older adults. Am. J. Physiol.-Endocrinol. Metab. 2015, 308, E21–E28.
- Krok-Schoen, J.L.; Price, A.A.; Luo, M.; Kelly, O.J.; Taylor, C.A. Low Dietary Protein Intakes and Associated Dietary Patterns and Functional Limitations in an Aging Population: A NHANES Analysis. J. Nutr. Health Aging 2019, 23, 338–347.
- Mendonça, N.; Granic, A.; Mathers, J.C.; Hill, T.R.; Siervo, M.; Adamson, A.; Jagger, C. Prevalence and determinants of low protein intake in very old adults: Insights from the Newcastle 85+ Study. Eur. J. Nutr. 2018, 57, 2713–2722.
- Volpi, E.; Campbell, W.W.; Dwyer, J.; Johnson, M.A.; Jensen, G.L.; Morley, J.E.; Wolfe, R.R. Is the Optimal Level of Protein Intake for Older Adults Greater Than the Recommended Dietary Allowance? J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 677–681.
- Scholes, G. Protein-energy malnutrition in older Australians: A narrative review of the prevalence, causes and consequences of malnutrition, and strategies for prevention. Health Promot. J. Aust. 2021, 33, 187–193.
- Swinburn, B.A.; Caterson, I.; Seidell, J.C.; James, W.P. Diet, nutrition and the prevention of excess weight gain and obesity. Public Health Nutr. 2004, 7, 123–146.
- WHO. Diet, Nutrition and the Prevention of Chronic Diseases; World Health Organization Technical Report Series; WHO: Geneva, Switzerland, 2003; Volume 916.
- Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; Sisto, A.; Marzetti, E. Anorexia of Aging: Risk Factors, Consequences, and Potential Treatments. Nutrients 2016, 8, 69.
- Wysokiński, A.; Sobów, T.; Kłoszewska, I.; Kostka, T. Mechanisms of the anorexia of aging—A review. AGE 2015, 37, 81.
- Sheetal, A. Malnutrition and its Oral Outcome—A Review. J. Clin. Diagn. Res. 2013, 7, 178–180.
- Kiesswetter, E.; Hengeveld, L.M.; Keijser, B.J.; Volkert, D.; Visser, M. Oral health determinants of incident malnutrition in community-dwelling older adults. J. Dent. 2019, 85, 73–80.
- Psoter, W.J.; Spielman, A.L.; Gebrian, B.; Jean, R.S.; Katz, R.V. Effect of childhood malnutrition on salivary flow and pH. Arch. Oral Biol. 2008, 53, 231–237.
- Cuthbertson, D.; Smith, K.; Babraj, J.; Leese, G.; Waddell, T.; Atherton, P.; Wackerhage, H.; Taylor, P.M.; Rennie, M.J. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2004, 19, 1–22.
- Eggersdorfer, M.; Akobundu, U.; Bailey, R.L.; Shlisky, J.; Beaudreault, A.R.; Bergeron, G.; Blancato, R.B.; Blumberg, J.B.; Bourassa, M.W.; Gomes, F.; et al. Hidden Hunger: Solutions for America’s Aging Populations. Nutrients 2018, 10, 1210.
- Rahman, N.; Walls, A. Chapter 12: Nutrient Deficiencies and Oral Health. Monogr. Oral Sci. 2020, 28, 114–124.
