The availability of clean water is essential for humans wellbeing and the diverse biotic population in the environment. Menkind imposes a significant pressure on food supplies, natural resources, and other commodities. Large-scale anthropogenic activities, such as agriculture and industry, which are practiced to ensure population growth and survival, have caused several harmful environmental effects, including the discharge of pollutants into the aquatic environment. rGO-based TiO2 material is commonly used in light-driven photocatalysis of dyes in an aqueous medium. Because of exceptional properties, rGO-based oxide semiconductors promote electron separation, which results in boosting photo-driven reactions such as the degradation of carcinogenic dyes (e.g., methylene blue) and solar-fuel (hydrogen) production. Preparation of rGO-based TiO2 photocatalysts increases the specific surface area of the nanocomposite, consequently increasing the photocatalytic activity, which is why rGO-based semiconductor photocatalysts have been found to be promising in several applications.
- graphene-based titanium dioxide catalyst
- rGO@TiO2
- nanocomposite
- water treatment
- organic pollutants
1. Introduction
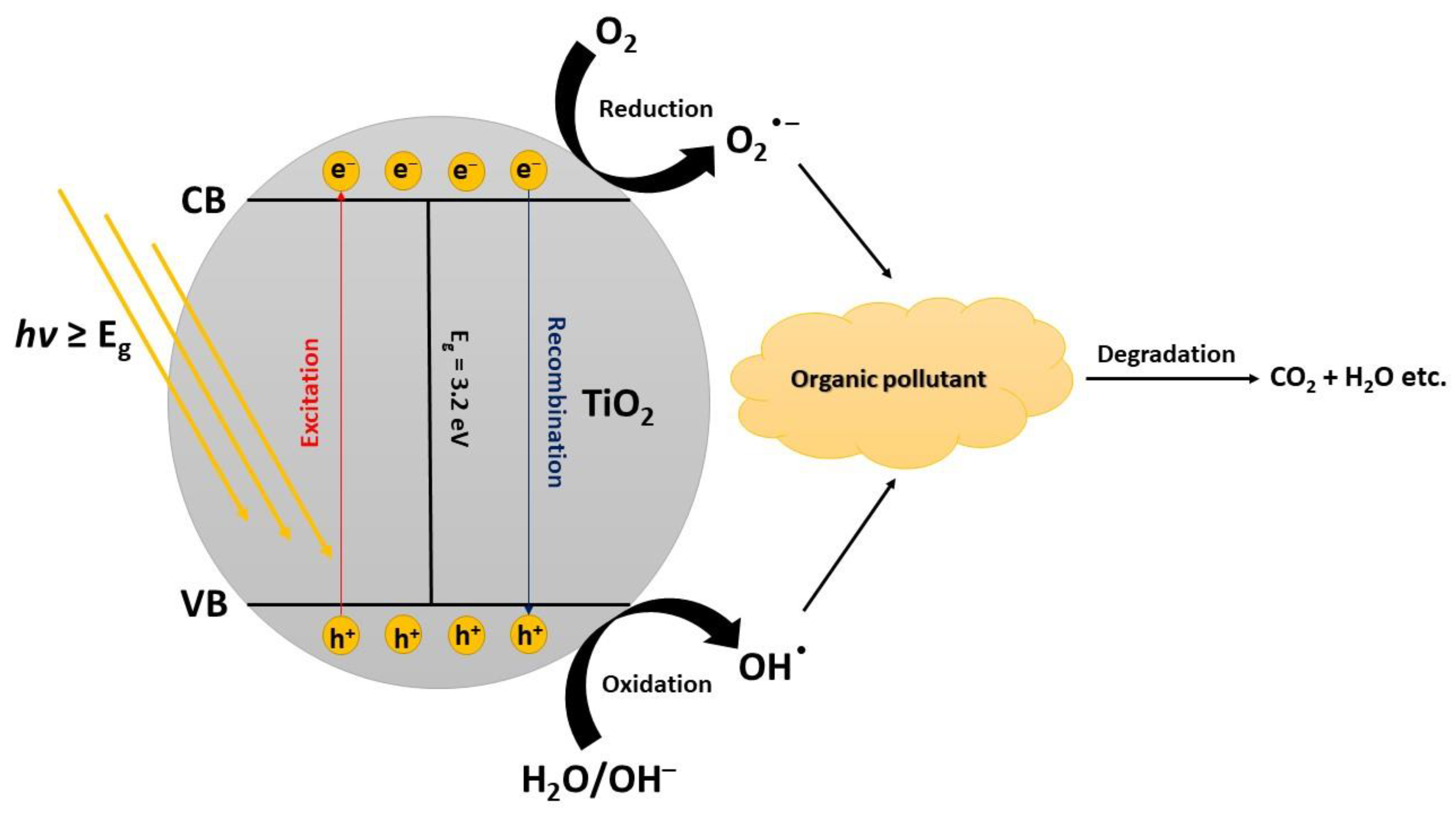
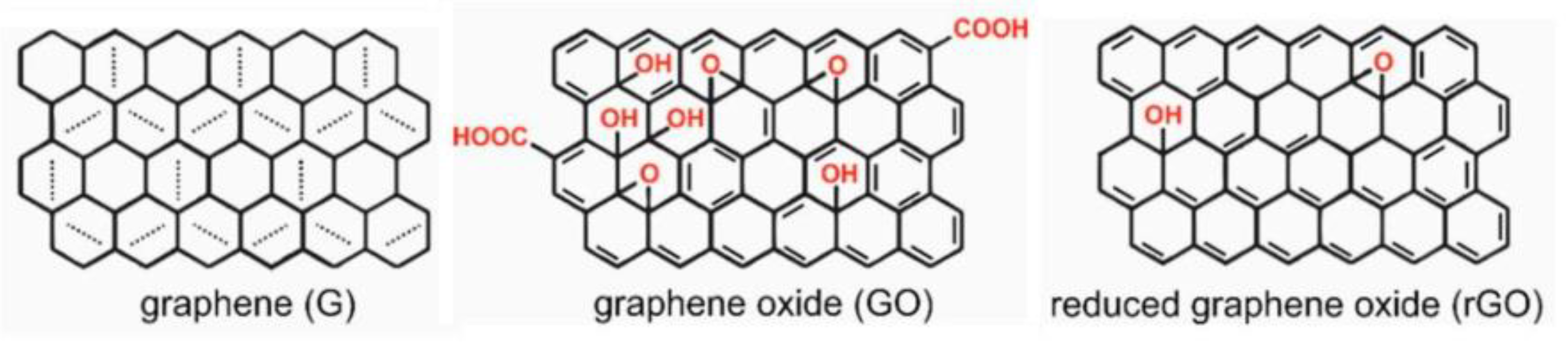
2. Photodegradation of Organic Pollutants
Many studies using rGO@TiO2 nanocomposite were made to improve photocatalytic efficiency. Liu [21] treated Methylene Orange (MO) using rGO@TiO2 nanocomposite for 240 min exposing it to visible light (λ > 400 nm) irradiation and reported ~90% photodegradation of overall organic pollutant. Several authors report the photocatalytic degradation of methylene blue (MB) using rGO@TiO2 nanocomposites, among them Deshmukh et al. [22] who got maximum degradation of MB equal to 91.3% within 30 min of sunlight irradiation. In another study by Mohammadi et al. [23], photocatalytic degradation of MB using rGO@TiO2 composite was even better. 95% and 93% of the overall organic pollutant were removed within 30 min using irradiation from a 200 W Mercury short arc and Osram 500 W Xenon lamp with a cut-off UV filter at 400 nm, respectively.
The investigation of the long-term stability and reusability of prepared rGO@TiO2 photocatalyst is a crucial parameter for its practical application. The stability of prepared rGO@TiO2 photocatalyst is investigated between several consecutive cycles with the same photocatalytic tests. Wanag et al. [5] investigated the stability of prepared rGO@TiO2 photocatalyst under seven cycles. The obtained results show very high activity after five cycles. A substantial decrease in the photoactivity is noted after seventh cycles. Prepared rGO@TiO2 photocatalyst showed high stability during the photodegradation of MB dye.
3. Factors Affecting the Photodegradation of Organic Pollutants
3.1. The Effect of GO to TiO2 Weight Ratio
3.2. Effect of Catalyst Loading
3.3. Effect of Initial Pollutant Concentration
3.4. Effect of Initial pH
3.5. Effect of Water Matrix on the Photocatalytic Degradation of Pollutant
3.6. Effect of Intensity and Wavelength of Light Irradiation
TiO2 has a wide band gap energy (3.0–3.20 eV) which limits its absorption only in the UV region of the solar spectrum. The wavelengths and intensities of UV light irradiation significantly affect the photodegradation of pollutants in an aqueous medium. UV irradiation is thus more frequently practiced than sunlight as it has higher efficiency in the degradation of pollutants. Expanding the photocatalytic degradation of pollutants to visible irradiation is an important aspect to recon with if people want to commercialize the process. Such a system should be functional under natural sunlight as the irradiation source [29]. The intensity of the light also affects the transition rate of electrons from the valence band (VB) to the conduction band (CB). Higher intensity usually leads to significantly higher degradation rates of the photocatalytic process. After saturation when the amount of photons is equal to TiO2 active sites, the rate of photogeneration becomes less dependent on the increase of the light intensity. Therefore, appropriate photon energy distribution contributes to the photodegradation rate [30]. A surplus of photons of given energy cannot contribute to a higher photocatalytic degradation rate because of the limited amounts of active sites on the surface of the catalyst [31].
3.7. Effect of Scavengers
4. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/su141912703
References
- Du, X.; Luo, J.; Qin, Q.; Zhang, J.; Fu, D. Modified TiO2-rGO Binary Photo-Degradation Nanomaterials: Modification, Mechanism, and Perspective. Catal. Surv. Asia 2022, 26, 16–34.
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986.
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349.
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent Advances of Photocatalytic Application in Water Treatment: A Review. Nanomaterials 2021, 11, 1804.
- Wanag, A.; Kusiak-Nejman, E.; Czyżewski, A.; Moszyński, D.; Morawski, A.W. Influence of rGO and preparation method on the physicochemical and photocatalytic properties of TiO2/reduced graphene oxide photocatalysts. Catalysts 2021, 11, 1333.
- Padmanabhan, N.T.; Thomas, N.; Louis, J.; Mathew, D.T.; Ganguly, P.; John, H.; Pillai, S.C. Graphene coupled TiO2 photocatalysts for environmental applications: A review. Chemosphere 2021, 271, 129506.
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-photocatalytic Materials: Possibilities and Challenges. Adv. Mater. 2012, 24, 229–251.
- Tatarchuk, T.; Peter, A.; Al-Najar, B.; Vijaya, J.; Bououdina, M. Photocatalysis: Activity of Nanomaterials. In Nanotechnology in Environmental Science; Wiley: Hoboken, NJ, USA, 2018; pp. 209–292.
- Xie, X.; Kretschmer, K.; Wang, G. Advances in graphene-based semiconductor photocatalysts for solar energy conversion: Fundamentals and materials engineering. Nanoscale 2015, 7, 13278–13292.
- Xiang, Q.; Yu, J.; Jaroniec, M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796.
- Allen, J.M.; Vincent, T.C.; Richard, K.B. Honeycomb carbon: A Review of Graphene What is graphene? Chem. Rev. 2010, 110, 132–145.
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.T. Graphene Derivatives in Photocatalysis. In Graphene-Based Energy Devices; Wiley: Hoboken, NJ, USA, 2015; pp. 249–276.
- Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Jafri, S.H.M.; Li, H. Review on Graphene-, Graphene Oxide-, Reduced Graphene Oxide-Based Flexible Composites: From Fabrication to Applications. Materials 2022, 15, 1012.
- Dideikin, A.T.; Vul, A.Y. Graphene oxide and derivatives: The place in graphene family. Front. Phys. 2019, 6.
- Bianco, A.; Cheng, H.M.; Enoki, T.; Gogotsi, Y.; Hurt, R.H.; Koratkar, N.; Kyotani, T.; Monthioux, M.; Park, C.R.; Tascon, J.M.D.; et al. All in the graphene family-A recommended nomenclature for two-dimensional carbon materials. Carbon 2013, 65, 1–6.
- Eigler, S. Mechanistic insights into the reduction of graphene oxide addressing its surfaces. Phys. Chem. Chem. Phys. 2014, 16, 19832–19835.
- Li, F.; Jiang, X.; Zhao, J.; Zhang, S. Graphene oxide: A promising nanomaterial for energy and environmental applications. Nano Energy 2015, 16, 488–515.
- Guex, L.G.; Sacchi, B.; Peuvot, K.F.; Andersson, R.L.; Pourrahimi, A.M.; Ström, V.; Farris, S.; Olsson, R.T. Experimental review: Chemical reduction of graphene oxide (GO) to reduced graphene oxide (rGO) by aqueous chemistry. Nanoscale 2017, 9, 9562–9571.
- Agarwal, V.; Zetterlund, P.B. Strategies for reduction of graphene oxide–A comprehensive review. Chem. Eng. J. 2021, 405, 127018.
- Oliveira, A.M.L.; Machado, M.; Silva, G.A.; Bitoque, D.B.; Ferreira, J.T.; Ferreira, Q. Graphene Oxide Thin Films with Drug Delivery Function. Nanomaterials 2022, 12, 1149.
- Liu, Y. Hydrothermal synthesis of TiO2-RGO composites and their improved photocatalytic activity in visible light. RSC Adv. 2014, 4, 36040–36045.
- Deshmukh, S.P.; Kale, D.P.; Kar, S.; Shirsath, S.R.; Bhanvase, B.A.; Saharan, V.K.; Sonawane, S.H. Ultrasound assisted preparation of rGO/TiO2 nanocomposite for effective photocatalytic degradation of methylene blue under sunlight. Nano-Struct. Nano-Objects 2020, 21, 100407.
- Mohammadi, M.; Rezaee Roknabadi, M.; Behdani, M.; Kompany, A. Enhancement of visible and UV light photocatalytic activity of rGO-TiO2 nanocomposites: The effect of TiO2/Graphene oxide weight ratio. Ceram. Int. 2019, 45, 12625–12634.
- Maruthamani, D.; Divakar, D.; Kumaravel, M. Enhanced photocatalytic activity of TiO2 by reduced graphene oxide in mineralization of Rhodamine B dye. J. Ind. Eng. Chem. 2015, 30, 33–43.
- Li, T.; Wang, T.; Qu, G.; Liang, D.; Hu, S. Synthesis and photocatalytic performance of reduced graphene oxide–TiO2 nanocomposites for orange II degradation under UV light irradiation. Environ. Sci. Pollut. Res. 2017, 24, 12416–12425.
- Xu, L.; Yang, L.; Johansson, E.M.J.; Wang, Y.; Jin, P. Photocatalytic activity and mechanism of bisphenol a removal over TiO2−x/rGO nanocomposite driven by visible light. Chem. Eng. J. 2018, 350, 1043–1055.
- H.H. Ali, M.; E Goher, M.; D.G. Al-Afify, A. Kinetics and Adsorption Isotherm Studies of Methylene Blue Photodegradation Under UV Irradiation Using Reduced Graphene Oxide-TiO2 Nanocomposite in Different Wastewaters Effluents. Egypt. J. Aquat. Biol. Fish. 2019, 23, 253–263.
- Deepthi, J.; Rajalakshmi, A.S.; Lopez, R.M.; Achari, V.S. TiO2-reduced graphene oxide nanocomposites for the trace removal of diclofenac. SN Appl. Sci. 2020, 2, 480.
- Reza, K.M.; Kurny, A.; Gulshan, F. Parameters affecting the photocatalytic degradation of dyes using TiO2: A review. Appl. Water Sci. 2017, 7, 1569–1578.
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725.
- Cheng, H.Y.; Chang, K.C.; Lin, K.L.; Ma, C.M. Study on isopropanol degradation by UV/TiO2 nanotube. AIP Conf. Proc. 2018, 1946, 020006.
- Kocijan, M.; Ćurković, L.; Bdikin, I.; Otero-Irurueta, G.; Hortigüela, M.J.; Gonçalves, G.; Radošević, T.; Vengust, D.; Podlogar, M. Immobilised rGO/TiO2 Nanocomposite for Multi-Cycle Removal of Methylene Blue Dye from an Aqueous Medium. Appl. Sci. 2022, 12, 385.
