In this research, the extraction of metals from a copper tailing was carried out by means of leaching. The leaching process consisted of dissolving tailing samples using hydrochloric acid solutions. As a result, iron, copper, aluminum, calcium and magnesium, among other elements, were obtained. Different techniques such as Scanning Electron Microscopy-Energy Dispersive Spectroscopy (SEM-EDX), X-ray Diffraction (XRD) and Atomic Absorption Spectroscopy (AAS), where applied to analyze the initial tailing sample, and the leaching products: acid solution and insoluble solid. Variables such as acid concentration, reaction time and reaction temperature were studied. The elements obtained in the acid solution prepared with a tailing sample and hydrochloric acid solution, can be recovered for subsequent applications.
- copper tailing
- leaching
- valorization
Abstract
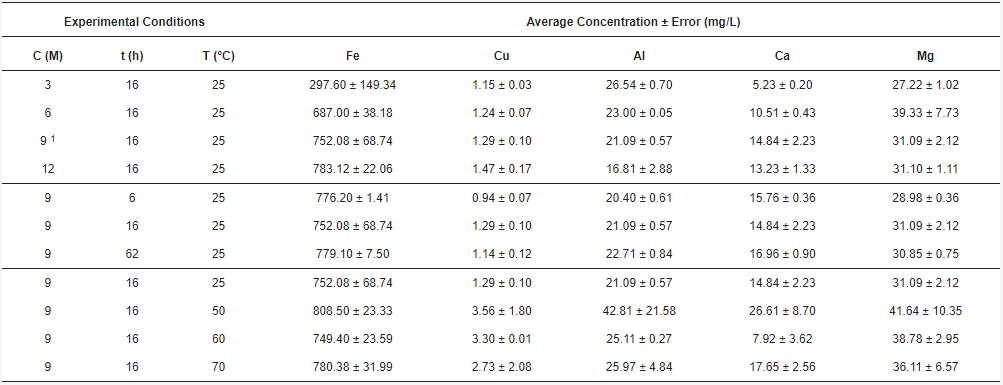
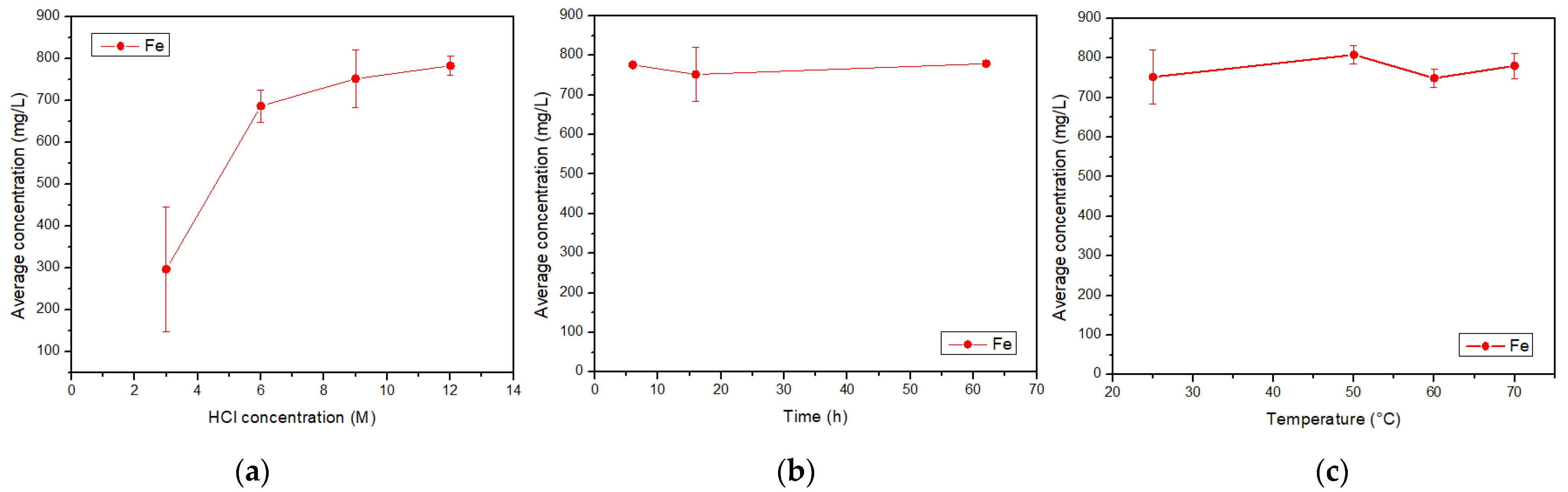
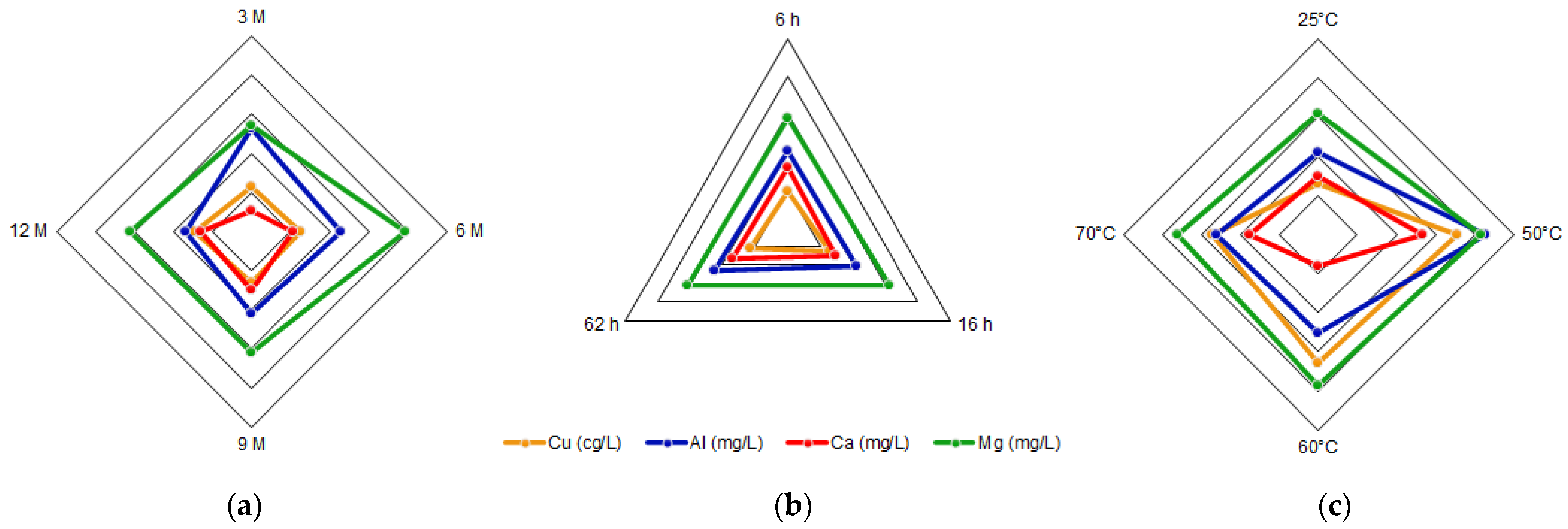
Currently, mining operations have increased the generation of tailings, which contain a variety of elements that can be valorized. In this research, tailing samples were leached with hydrochloric acid of concentrations greater than 3 M, considering the monitoring of iron, copper, aluminum, calcium and magnesium, as relevant elements of the leached solution. Time and temperature were also studied. The original tailing sample was taken by trial pits, and a size distribution analysis was performed. The process generated an insoluble solid, rich in aluminosilicates, and an acid liquid solution with different metal ions. Elemental analyses were performed on liquid samples by Atomic Absorption Spectroscopy (AAS), and solid samples by Scanning Electron Microscopy-Energy Dispersive Spectroscopy (SEM-EDX) and X-ray Diffraction (XRD). Results showed an increasing trend of the iron concentration as a function of the acid concentration. However, copper is not affected by the change in acid concentration, but by time and temperature. Aluminum decreases with acid concentration, keeps constant with time, and yields at 50 °C. In the range of the studied parameters, calcium and magnesium showed a variation without a clear trend. The elements in the acid solution prepared with a tailing from northern Chile can be recovered for subsequent applications.
1. Introduction
2. Materials and Methods
2.1. Characterization Equipment
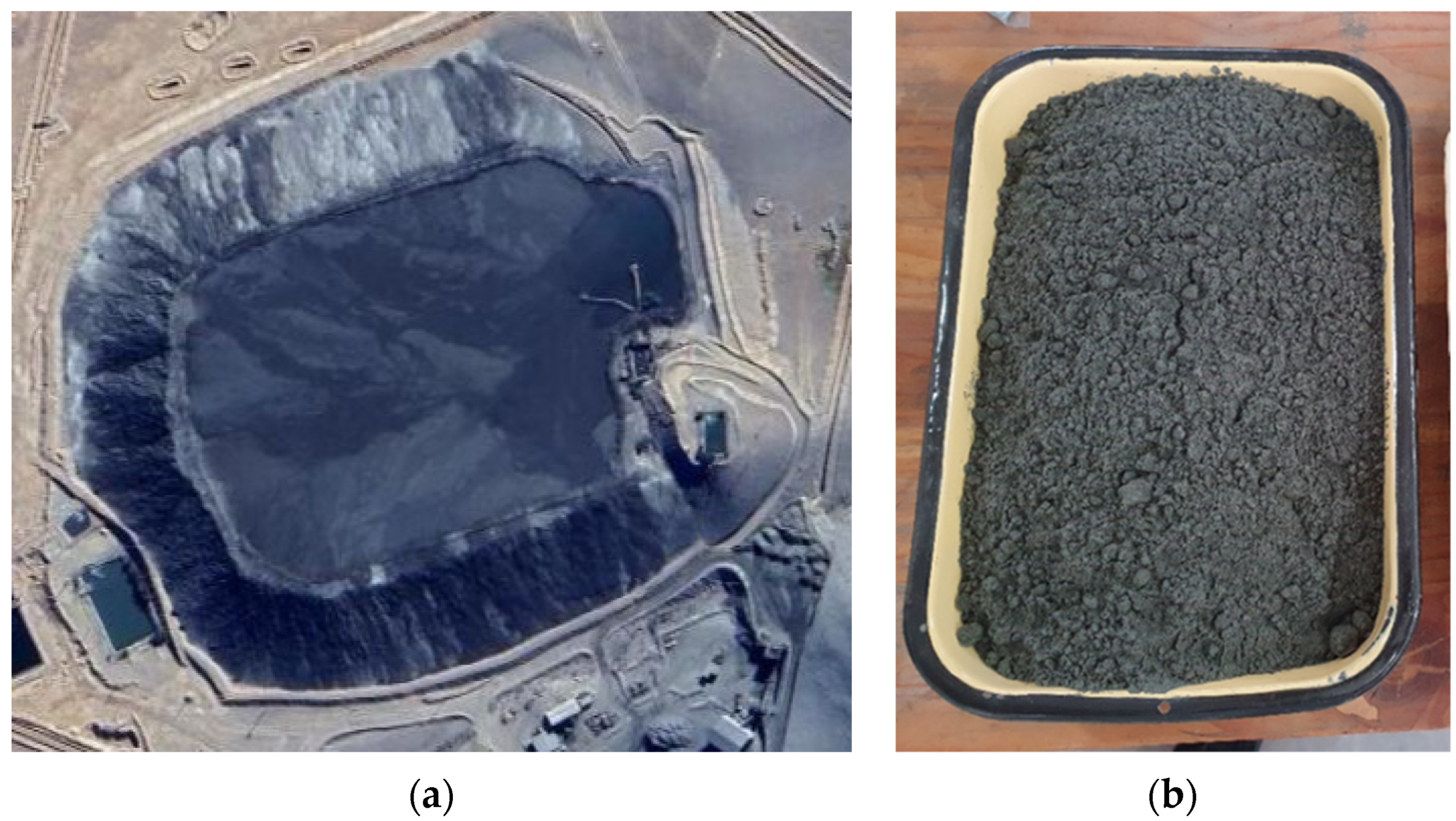
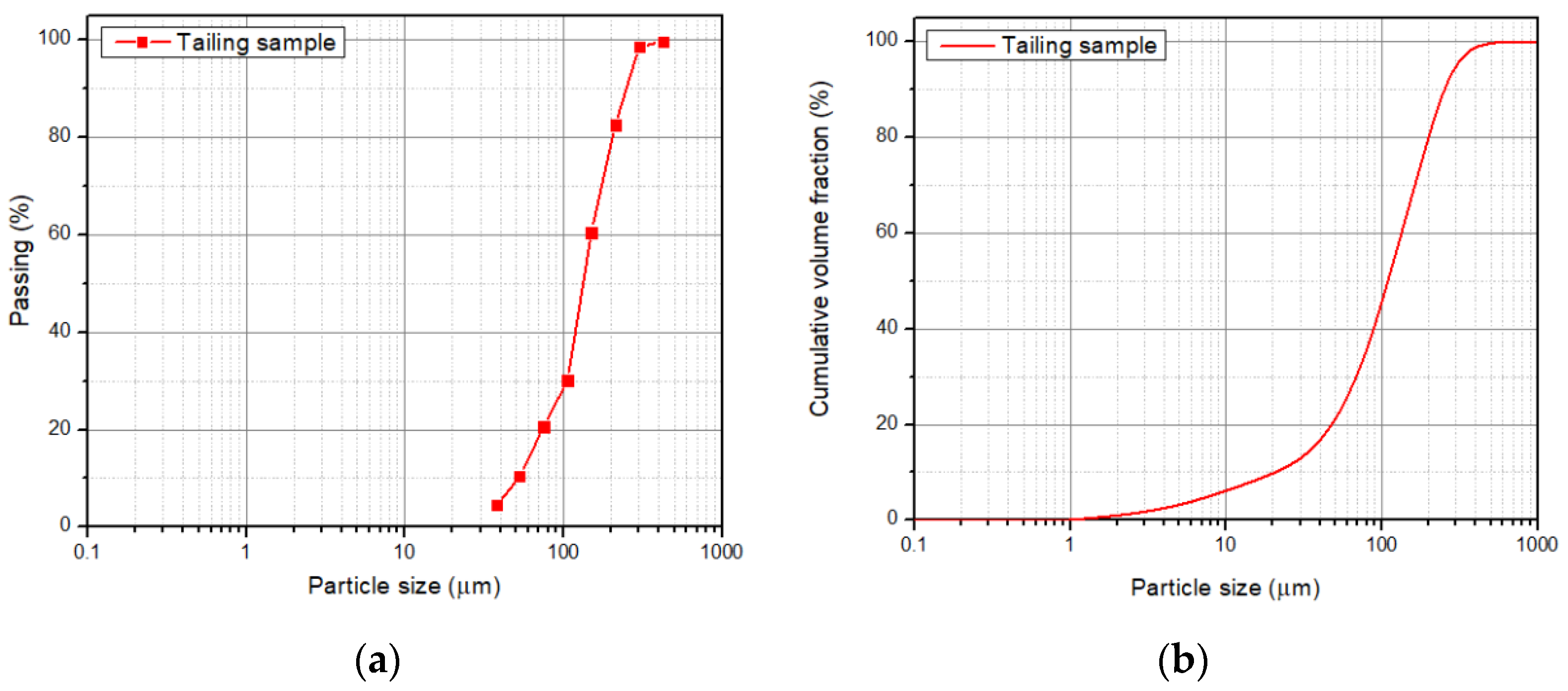
2.2. Collection and Characterization of the Tailing Sample
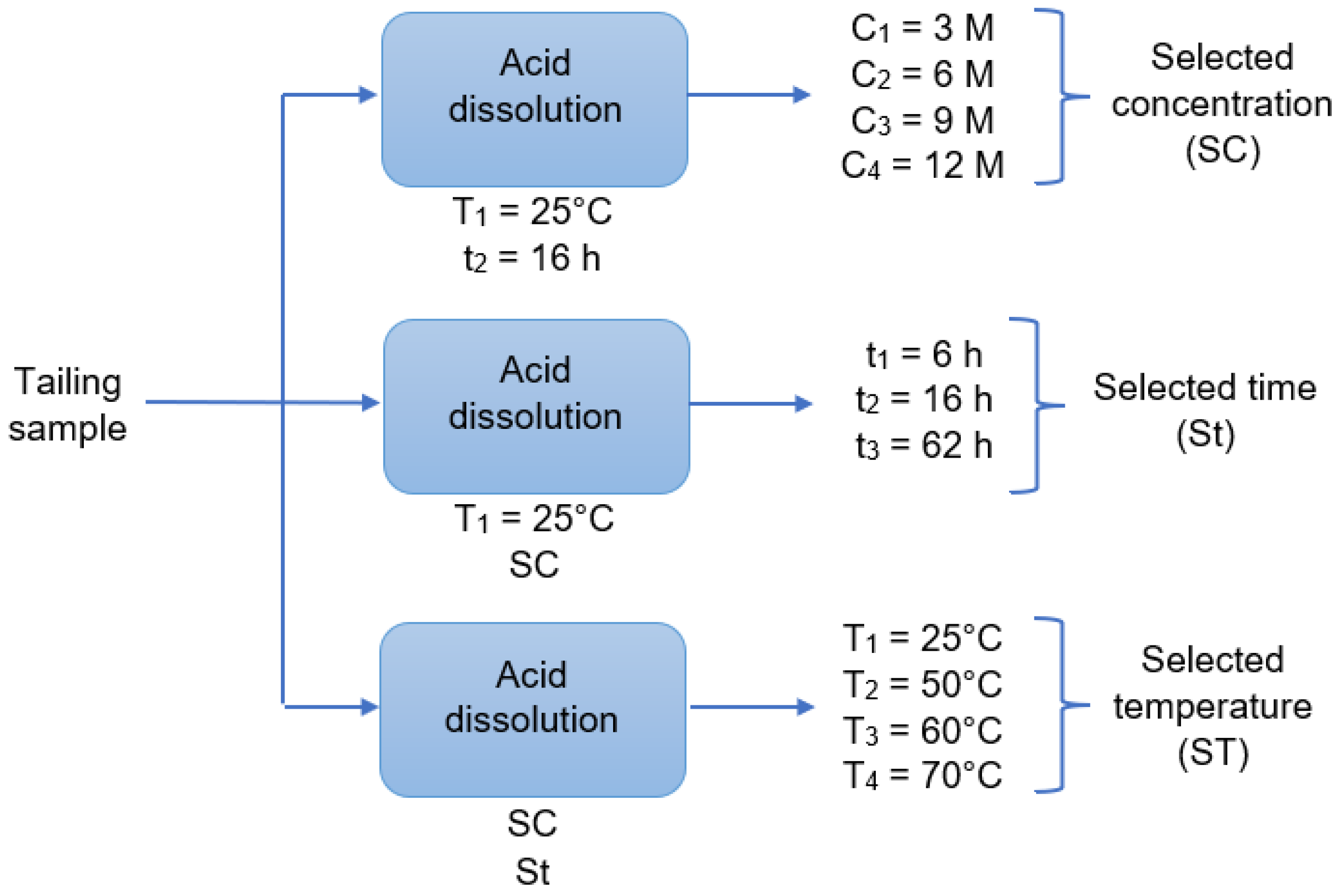
2.3. Leaching Procedure
3. Results
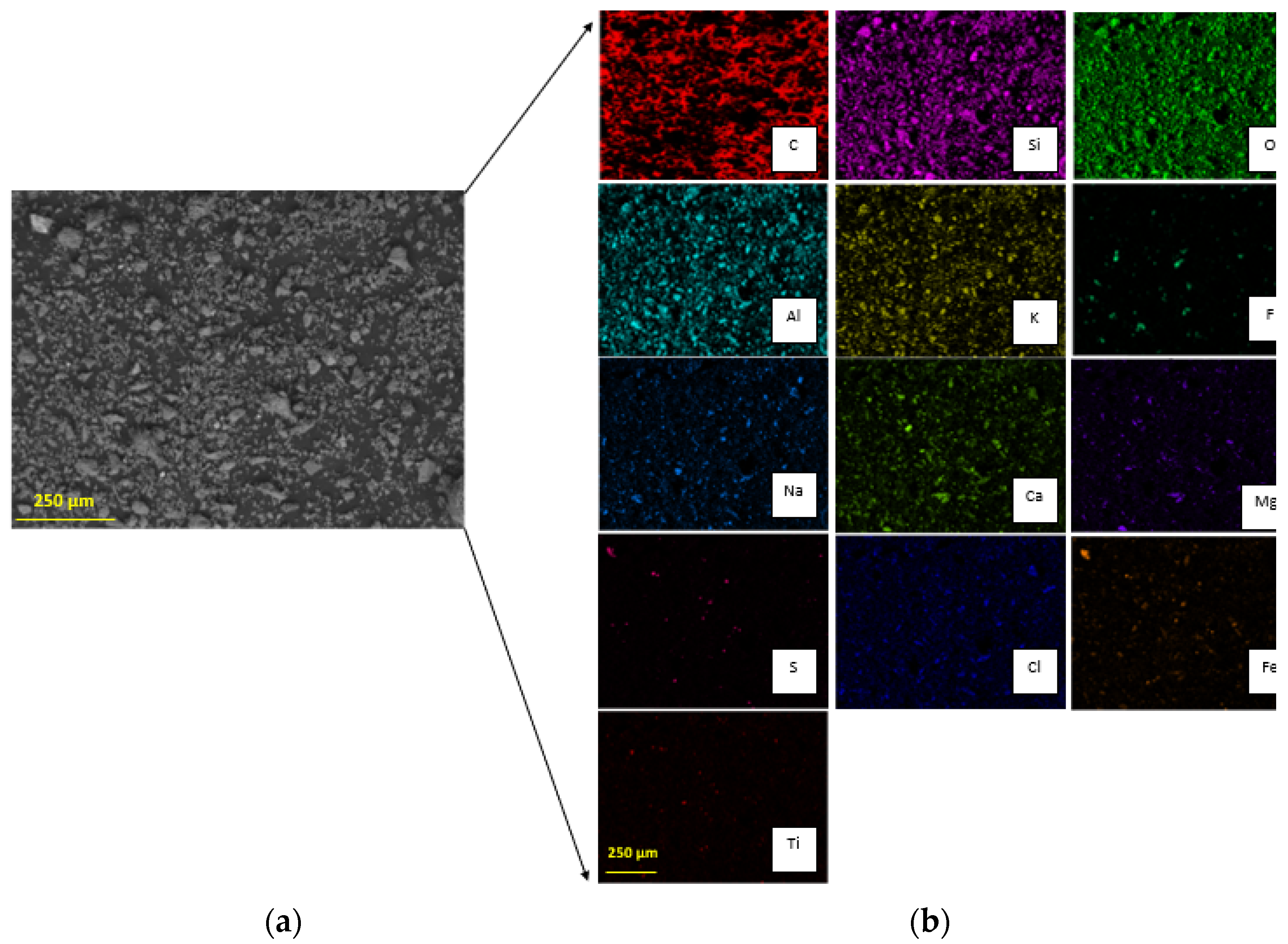
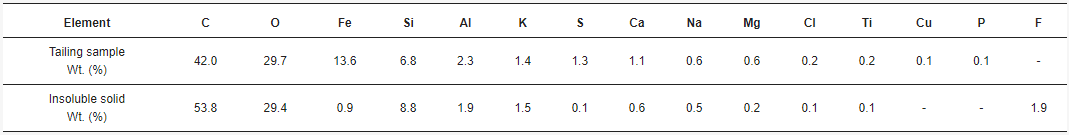
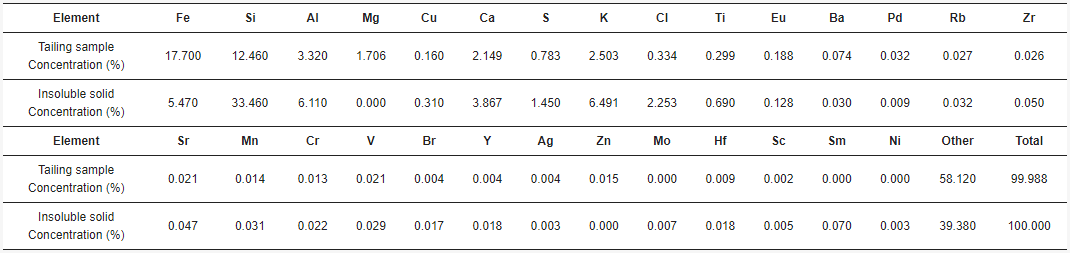
3.1. Tailing Sample Characterization
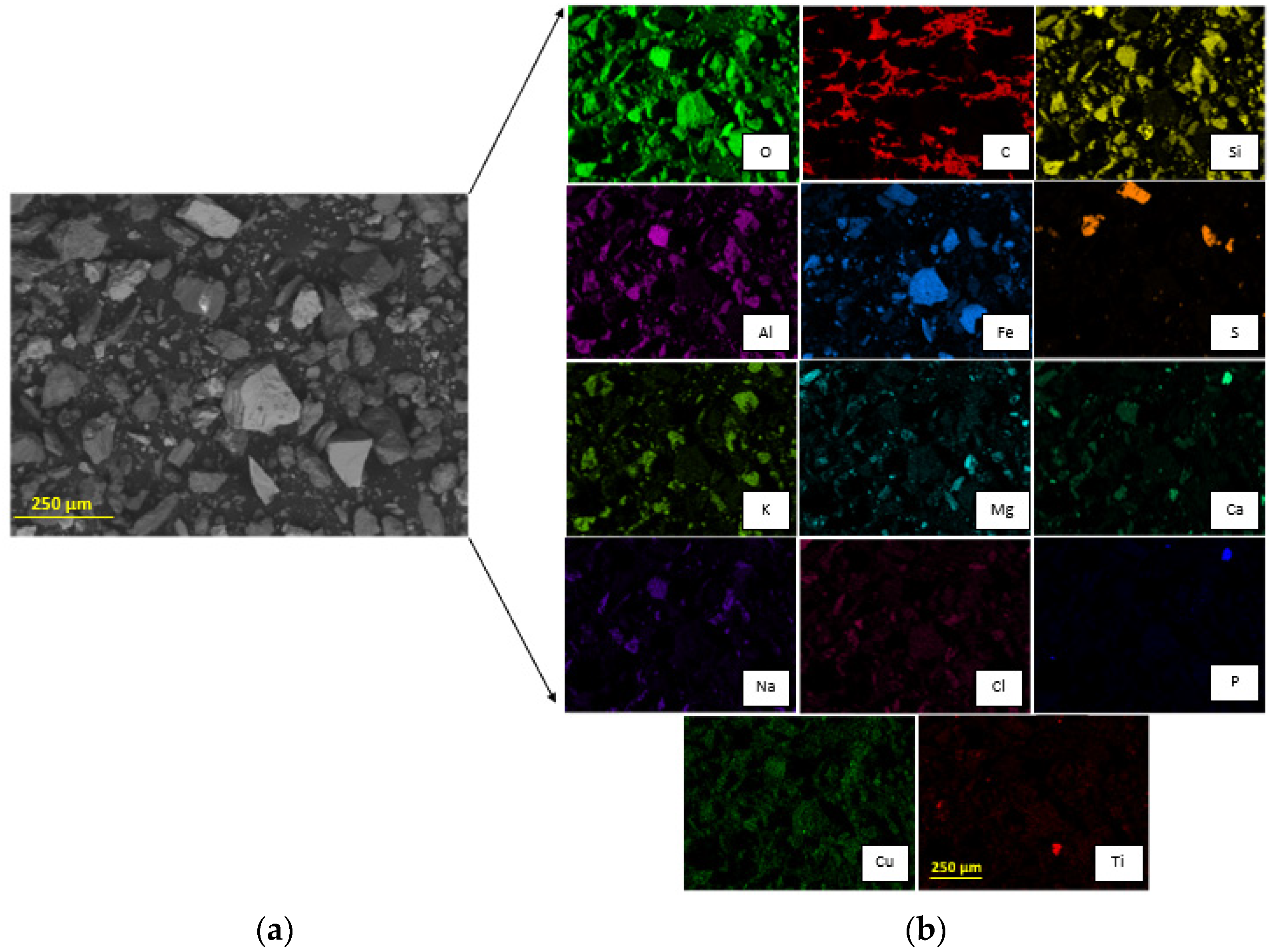
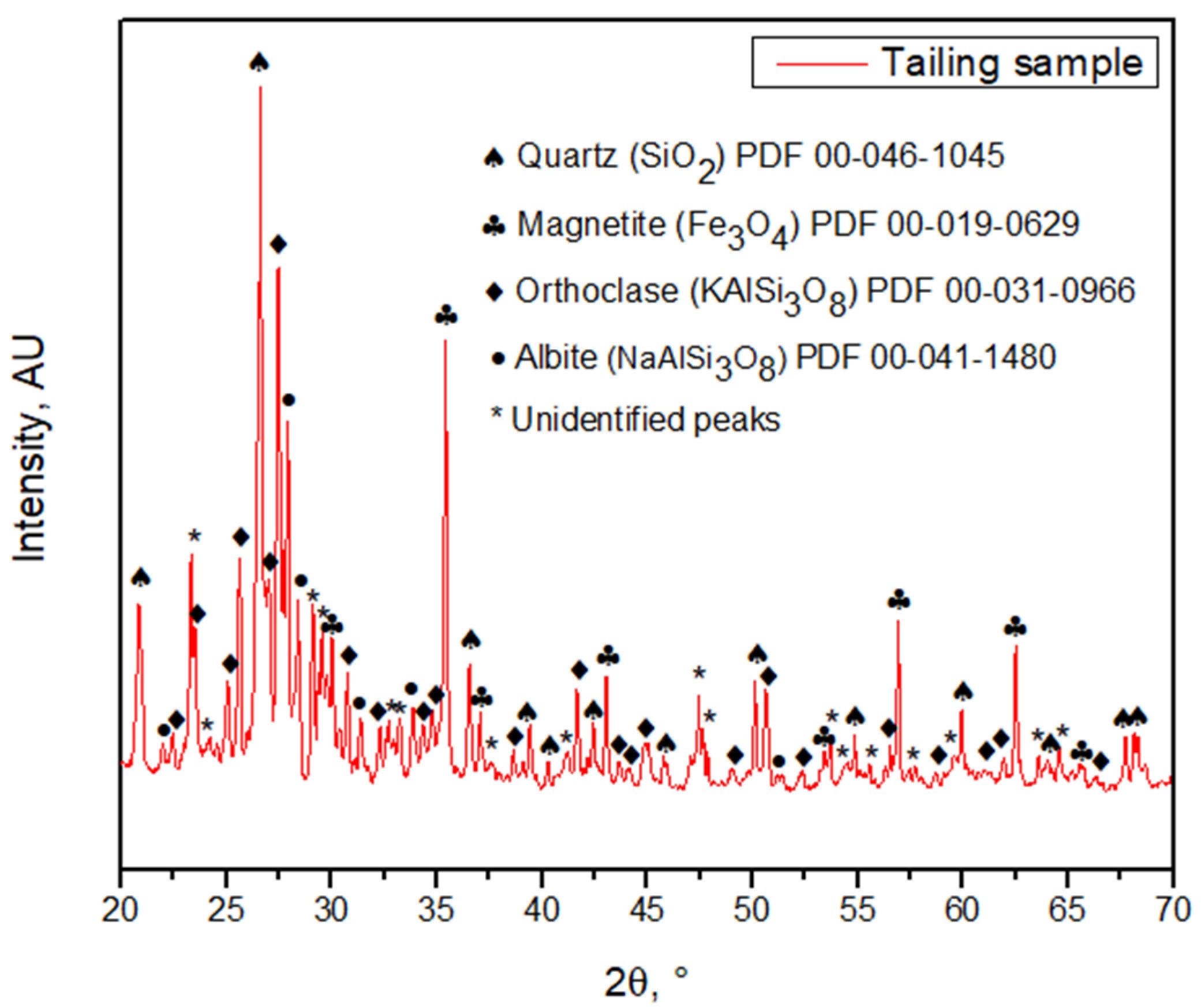
3.2. Acid Solution Characterization
3.3. Insoluble Solid Characterization
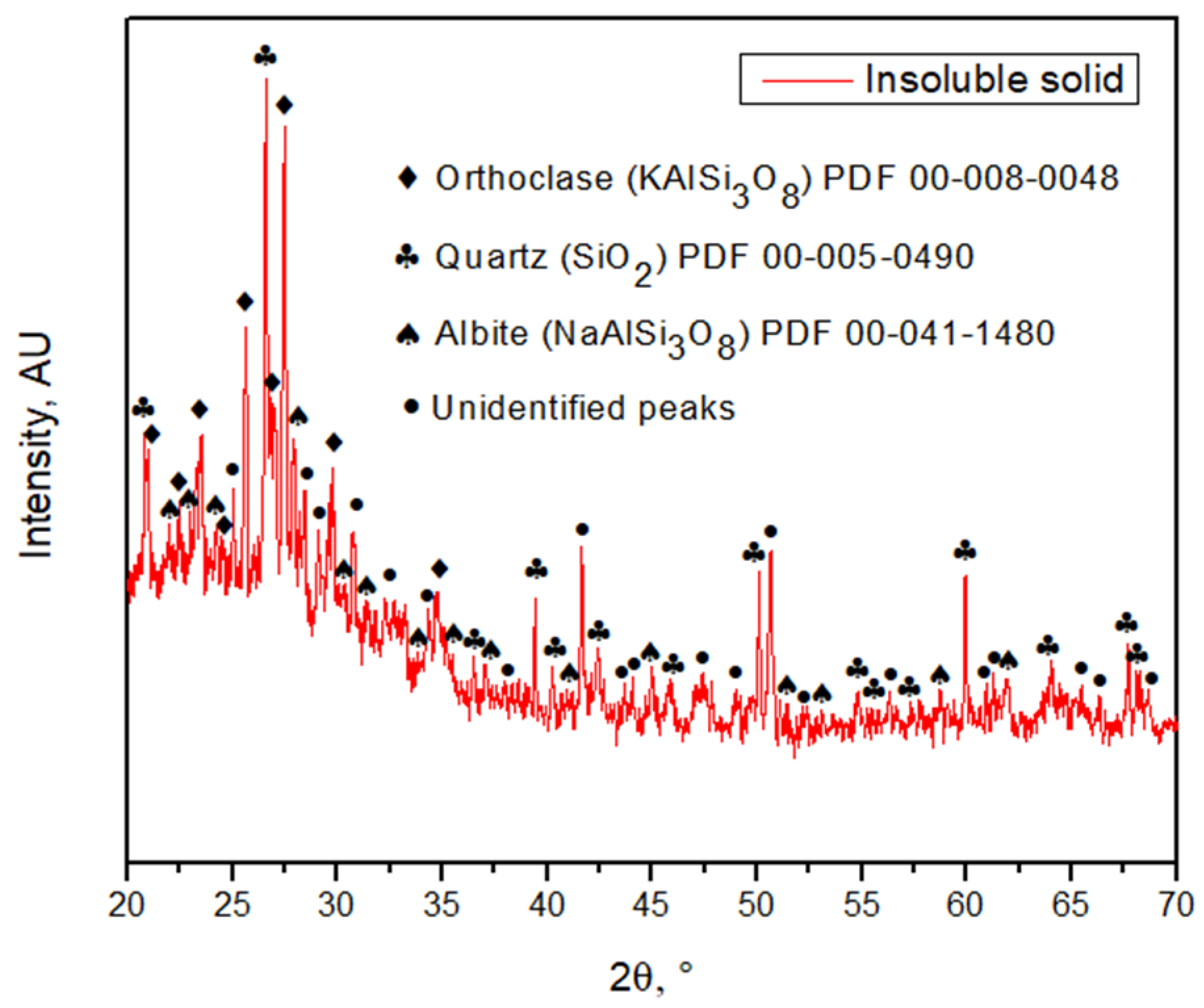
4. Discussion
5. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/met12111924
References
- Aznar-Sánchez, J.A.; García-Gómez, J.J.; Velasco-Muñoz, J.F. Mining Waste and Its Sustainable Management: Advances in Worldwide Research. Minerals 2018, 8, 284.
- Reichl, C.; Schatz, M.; Zsak, G. World Mining Data. Miner. Prod. 2017, 32, 1–261.
- Cortés, S.; Soto, E.E.; Ordóñez, J.I. Recovery of Copper from Leached Tailing Solutions by Biosorption. Minerals 2020, 10, 158.
- Upadhyay, A.; Laing, T.; Kumar, V.; Dora, M. Exploring barriers and drivers to the implementation of circular economy practices in the mining industry. Resour. Policy 2021, 72, 102037.
- Kinnunen, P.; Kaksonen, A.H. Towards circular economy in mining: Opportunities and bottlenecks for tailings valorization. J. Clean. Prod. 2019, 228, 153–160.
- Araya, N.; Kraslawski, A.; Cisternas, L.A. Towards mine tailings valorization: Recovery of critical materials from Chilean mine tailings. J. Clean. Prod. 2020, 263, 121555.
- Valderrama, L.; Santander, M.; Zazzali, B.; Carmona, M. Concentración Magnética Aplicada a Relaves de cobre. HOLOS 2014, 6, 37–44.
- Lacassie, J.P.; Vivallo, W.; Díaz, A.; Ruiz-del-Solar, J. Geoquímica de yacimientos metálicos y de sedimentos, de las regiones de Atacama y Coquimbo, norte de Chile. In Proceedings of the XIV Congreso Geológico Chileno, La Serena, Chile, 4–8 October 2015; pp. 429–432.
- Ristović, I.; Štyriaková, D.; Štyriaková, I.; Šuba, J.; Širadović, E. Bioleaching Process for Copper Extraction from Waste in Alkaline and Acid Medium. Minerals 2022, 12, 100.
- Vargas, F. Copper Tailings as Supplementary Cementitious Material: Activation, Leaching and Environmental Behaviour; Pontificia Universidad Católica de Chile: Santiago de Chile, Chile, 2020.
- Godirilwe, L.L.; Haga, K.; Altansukh, B.; Takasaki, Y.; Ishiyama, D.; Trifunovic, V.; Avramovic, L.; Jonovic, R.; Stevanovic, Z.; Shibayama, A. Copper Recovery and Reduction of Environmental Loading from Mine Tailings by High-Pressure Leaching and SX-EW Process. Metals 2021, 11, 1335.
- Wang, J.; Zhang, Y.; Yu, L.; Cui, K.; Fu, T.; Mao, H. Effective separation and recovery of valuable metals from waste Ni-based batteries: A comprehensive review. Chem. Eng. J. 2022, 439, 135767.
- Chen, M.; Han, Z.; Wang, L. Recovery of valuable metals from copper slag by hydrometallurgy. Adv. Mater. Res. 2011, 402, 35–40.
- Goryachev, A.; Svetlov, A.; Kompanchenko, A.; Makarov, D. Sulfuric Acid Granulation of Copper—Nickel Ore Tailings: Leaching of Copper and Nickel in the Presence of Sulfide Oxidation Activators. Minerals 2022, 12, 129.
- Dimitrijević, M.; Urošević, D.; Milić, S.; Sokić, M.; Marković, R. Dissolution of copper from smelting slag by leaching in chloride media. J. Min. Metall. Sect. B Metall. 2017, 53, 407–412.
- Adrianto, L.R.; Pfister, S. Prospective environmental assessment of reprocessing and valorization alternatives for sulfidic copper tailings. Resour. Conserv. Recycl. 2022, 186, 106567.
- Leiva, E.; Cayazzo, M.; Dávila, L.; Torres, M.; Ledezma, C. Acid Mine Drainage Dynamics from a Paste Tailing Deposit: Effect of Sulfate Content on the Consistency and Chemical Stability after Storage. Metals 2021, 11, 860.
- Google Maps. Tailing Location. Available online: https://www.google.com/maps/@-28.548177,-70.736858,450m/data=!3m1!1e3?hl=en-US (accessed on 19 October 2022).
- Tang, H.; Zhao, L.; Yang, Y.; Han, H.; Wang, L.; Sun, W. Dissolution Kinetics of Chlorine from Iron Ore Sintering Dust. Metals 2021, 11, 1185.
- Šajn, R.; Ristovic, I.; Ceplak, B. Mining and Metallurgical Waste as Potential Secondary Sources of Metals—A Case Study for the West Balkan Region. Minerals 2022, 12, 547.
- Tao, L.; Wang, L.; Yang, K.; Wang, X.; Chen, L.; Ning, P. Leaching of iron from copper tailings by sulfuric acid: Behavior, kinetics and mechanism. R. Soc. Chem. 2021, 11, 5741–5752.
- Mubarak, Y. Leaching of Copper Ores: Effects of Operating Variables. Int. J. Emerg. Trends Eng. Res. 2020, 8, 4226–4235.
- Tayebi-Khorami, M.; Edraki, M.; Corder, G.; Golev, A. Re-Thinking Mining Waste through an Integrative Approach Led by Circular Economy Aspirations. Minerals 2019, 9, 286.
