Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Actin participates in the formation of highly differentiated myofibrils under the regulation of actin-binding proteins (ABPs), which provides a structural basis for the contractile function and morphological change in cardiomyocytes.
- actin-binding proteins
- cardiac hypertrophy
- F-actin
- fetal genes
1. Introduction
The microfilament cytoskeleton is mainly composed of actin and actin-binding proteins (ABPs). Actin is one of the most abundant cytoskeletal proteins in eukaryotes and is involved in cell morphology change, migration, division and other cellular processes [1][2]. Actin takes two forms in cells: actin monomers (also known as globular actin, G-actin) and actin filaments (also known as filamentous actin, F-actin). Actin dynamics are finely regulated by a variety of ABPs (Table 1) [3]. Actin is involved in the formation of sarcomeres in cardiomyocytes [4]. The straight and uniform sarcomeric F-actin is critical for the contractile function of muscle [5]. In addition, actin assembly is thought to be related with autophagy [6][7]. The inhibition of F-actin disassembly can suppress autophagosome formation [8]. Several studies have found that F-actin is significantly accumulated abnormally in hypertrophic cardiomyocytes [9][10][11]. The dysregulation of F-actin accumulation may lead to cardiac hypertrophy through disrupting autophagy and sarcomeric structure. The function of ABPs in the development of cardiac hypertrophy has been gradually elucidated.
Table 1. Actin-binding proteins.
| Types | ABPs | Basic Function | Refs. |
|---|---|---|---|
| G-actin-binding | Profilin, thymosin β4, cofilin | Bound to G-actin | [3][12][13] |
| F-actin-binding | Dystrophin, tropomyosin | Bound to F-actin | [1][3][14] |
| Actin-nucleating | Formin, Arp2/3 complex, proteins with tandem WH2 domains, leiomodin | Nucleation to initiate actin polymerization | [3][15][16][17] |
| Actin-elongating | Formin, tetramers of Ena/VASP | Regulation of actin assembly | [3][16] |
| Actin-bundling | Fimbrin/Plastin, hhLIM, gelsolin | Causes parallel F-actin filaments to closely pack together | [18][19][20][21] |
| Severing | ADF/cofilin, gelsolin, twinfilin, FRL-α, INF-2 | Severs F-actin | [22][23][24][25][26] |
| Capping | Twinfilin, gelsolin, tropomodulin, CapZ, Arp2/3 complex | Caps F-actin to inhibit actin polymerization | [3][27][28][29] |
| Motor | Myosin | Cargo transfer | [30] |
2. ABPs in Cardiac Hypertrophy
2.1. Profilin-1
Profilin is widely expressed in most eukaryotes and has a molecular weight of about 17 kDa [31]. There are various profilin isoforms expressed in different tissues. Profilin-1 is universally expressed, profilin-2 is specifically expressed in the brain and profilin-3 and profilin-4 are specifically expressed in kidney and testis, respectively [32]. Profilin accelerates the nucleotide exchange of G-actin and delivers ATP-G-actin to the growing barbed ends of F-actin through interacting with the poly-proline motifs of formin, vasodilator-stimulated phosphoprotein (VASP) and CDC42-activated Wiskott Aldrich syndrome protein (WASP)/WASP family [12][33][34][35].
Profilin-1 is directly associated with cardiac hypertrophy [36]. Overexpression of profilin-1 in the vascular tissues of FVB/N mice leads to vascular remodeling and hypertension by increasing actin aggregation, which provides mechanical stress for the development of cardiac hypertrophy [37][38]. It has been shown that the protein level of profilin-1 is significantly increased in mammalian hypertrophic hearts (Figure 1). The myocardin-related transcription factor megakaryoblastic leukemia (MKL) induces the expression of the signal transducer and activator of transcription 1 (STAT1) via its SAP-domain (SAF-A/B, acinus and PIAS protein domain) activity, which upregulates PFN expression [39]. Whether this is the explanation for the increased protein level of profilin in cardiac hypertrophy remains to be investigated. In cardiomyocytes, the functional abnormality of profilin-1 can change the abundance or activity of multiple proteins associated with cardiomyopathy. For example, the overexpression of profilin-1 can contribute to decreases in the phosphorylation level of endothelial nitric oxide synthases (eNOS) at Ser1177 in the hearts of spontaneous hypertensive rats [9]. Levels of atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), skeletal muscle α-actin (α-SMA) and phosphorylated ERK1/2 (active form) were significantly increased in neonatal rat ventricular myocytes (NRVMs) following stimulation by phenylephrine or endothelin 1, which can be inhibited by siRNA-directed PFN1 silencing [36]. Increased phosphorylation of ERK1/2 activates the mechanistic (mammalian) target of rapamycin complex 1 (mTORC1) that subsequently inhibits autophagy [40][41][42]. It may be a potential key mechanism of cardiac hypertrophy mediated by the dysregulation of profilin-1 (Figure 1). Additionally, the inhibition of Rho-associated coiled-coil-containing protein kinase pathway (ROCK) can suppress the upregulation of profilin-1 induced by advanced glycation end products (AEGs) in H9c2 cells [43]. By comparison, overexpression of PFN1 results in the reactivation of fetal genes (NPPA and NPPB), an increase in F-actin in myocardium and destruction of myofibrils [36]. These processes can be reversed by inhibiting the expression of profilin-1 [9]. The inhibition of profilin-1 expression in H9c2 cells and Sprague–Dawley rats can attenuate cardiac hypertrophy induced by AEGs [43][44]. In Drosophila, myocyte-specific overexpression of profilin leads to disorders in muscle fibers and sarcomeres, which result in damaged muscle ultrastructure and function [36].
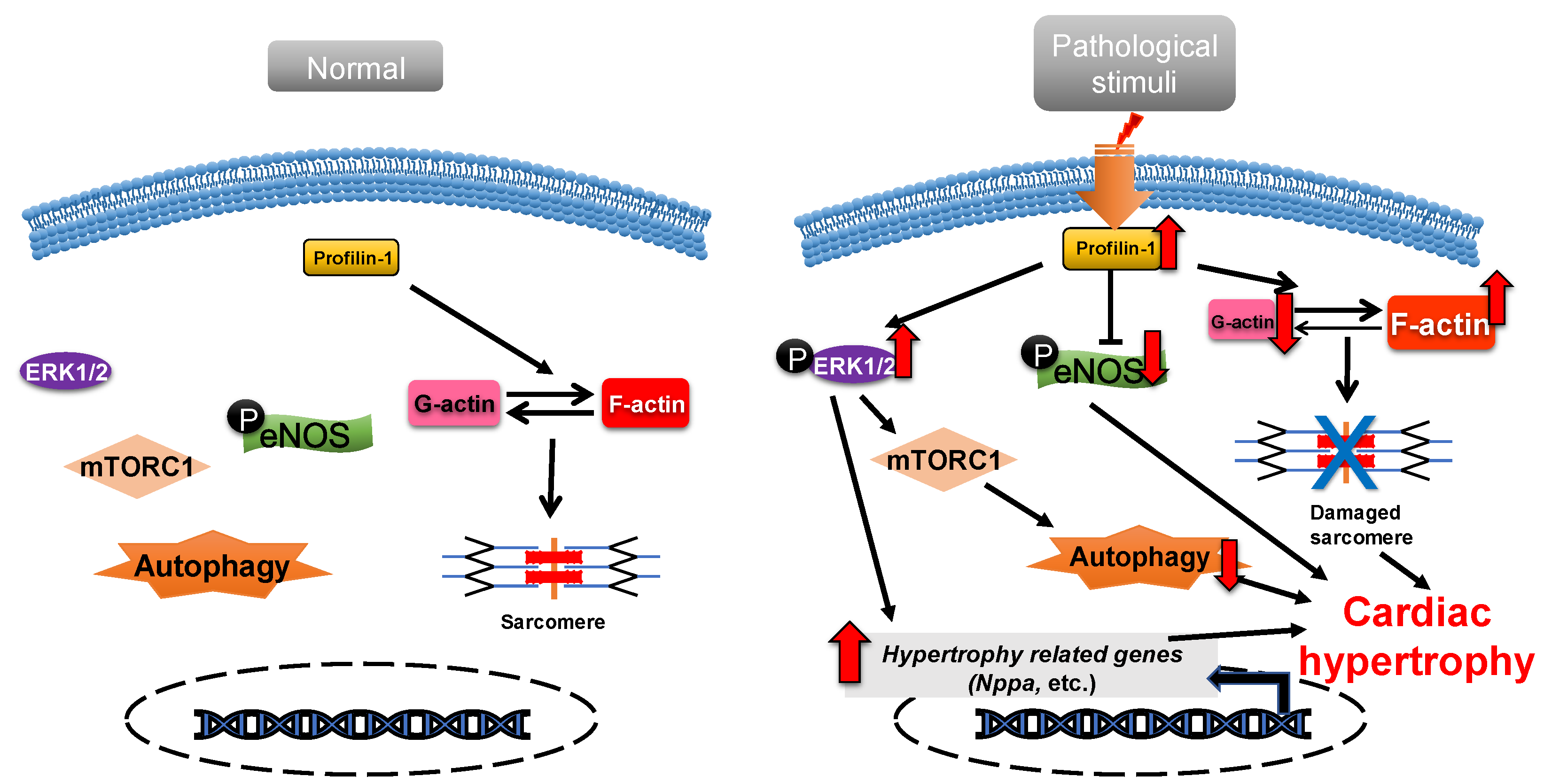
Figure 1. Profilin-1 mediates cardiac hypertrophy. In normal cardiomyocytes, profilin-1 is at a basal level and the fetal genes are not activated. Pathological stimuli increase the protein level of profilin-1, which results in ERK1/2 activation, F-actin accumulation and eNOS inhibition. This results in the reactivation of hypertrophy-related genes, inhibition of autophagy and damage to sarcomere structure and, ultimately, the development of cardiac hypertrophy.
2.2. ADF/Cofilin
Actin-depolymerizing factor (ADF)/cofilin consists of a single ADF homologous domain and has a molecular weight of about 15 kDa. The ADF/cofilin family contains ADF (also known as destrin, mainly expressed in endothelial and epithelial cells) and two cofilin isoforms (cofilin-1 is universal and cofilin-2 is cardio-specific) [45][46]. ADF/cofilin can bind to both G-actin and F-actin and can sever and depolymerize F-actin in regulating actin dynamics, which contributes to the cell contractility power [47]. The activity of cofilin is regulated by phosphorylation primarily from the ROCK/Lin-11, Isl1 and MEC-3 domain kinase (LIMK)/cofilin signaling pathway (Figure 2) [48][49]. Cofilin is inactivated via phosphorylation.
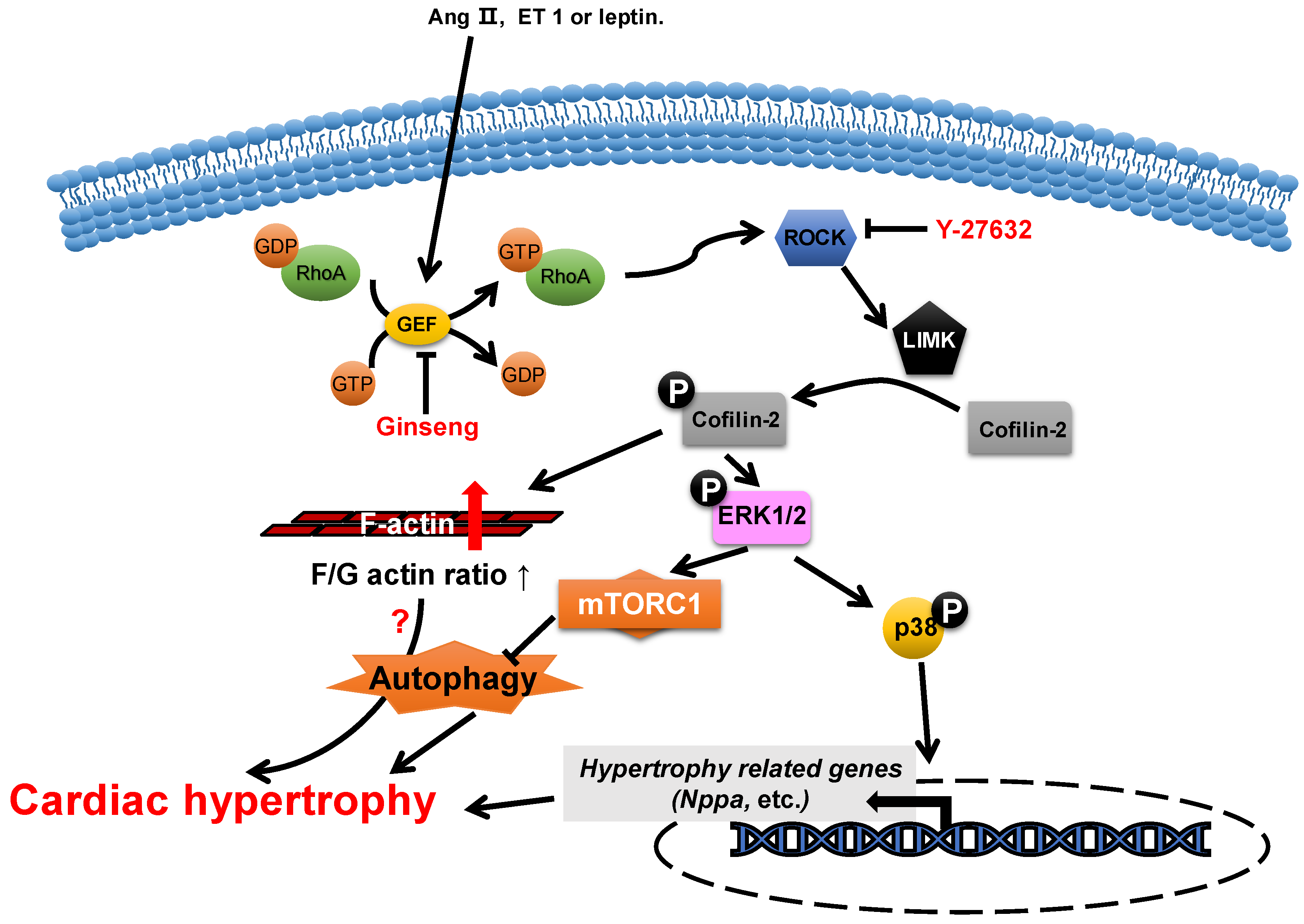
Figure 2. Proposed roles of cofilin-2 in cardiac hypertrophy. Neurohumoral factors (e.g., Ang II, ET 1 and leptin) lead to cofilin-2 phosphorylation through the RhoA/ROCK/LIMK signaling pathway. Phosphorylated cofilin-2 can lead to F-actin accumulation, which may subsequently contribute to cardiac hypertrophy through disrupting autophagy. In addition, it promotes the activation of ERK1/2 and p38, which contributes to the inhibition of autophagy and the reactivation of hypertrophy-related genes, which subsequently cause cardiac hypertrophy.
The abundance change in cofilin-2 does not play a role in the morphogenesis of neonatal rat cardiomyocytes [50], while its activity is closely associated with the development of cardiac hypertrophy. The levels of phosphorylated cofilin-2 are increased in myocardial hypertrophy through the activation of LIM-kinase (LIMK) by ROCK, which is induced by multiple neurohumoral factors, such as angiotensin II [51][52], endothelin 1 [11] and leptin [10][53]. In hypertrophic cardiomyocytes, the increase in levels of phosphorylated cofilin-2 results in an increase in F-actin/G-actin ratios and the levels of phosphorylated ERK1/2 and p38 [11][53][54][55][56]. Y-27632 [11], an inhibitor of ROCK, can reduce the levels of phosphorylated cofilin-2 through the inhibition of ROCK activity, which attenuates endothelin-1-induced neonatal cardiomyocyte hypertrophy, whereas this is achieved in ginseng (Panax quinquefolius) [54] through inhibition of p115Rho guanine nucleotide exchange factor (GEF) activity, which inhibits leptin-induced cardiac hypertrophy. In addition, WD-repeat domain 1 (WDR1), a major cofactor of the ADF/cofilin, has been reported to protect myocardium from myocardial hypertrophic stimuli [5].
2.3. Formin
Formin is a type of multidomain protein consisting of 7 subfamilies and 15 members in human genes. Formins are characterized by the presence of two conserved domains: formin homology 1 (FH1) and FH2. FH1 binds to the profilin–actin complex via poly-proline sequences and brings the G-actin to FH2, which promotes actin nucleation and polymerization [3][57].
2.4. CapZ
CapZ, a type of capping protein, anchors F-actin to the Z disc and regulates actin turnover, which contributes to sarcomere structural changes [58][59]. PIP2, a downstream effector of RAC1, can promote the dissociation of CapZ from F-actin by weakening their binding affinity [60][61][62].
Overexpression of CapZ in transgenic mice can lead to fatal cardiac hypertrophy [59]. It has been shown that hypertrophic agonists, phenylephrine or endothelin can reduce the binding affinity between CapZ and F-actin via PIP2-dependent pathways in NRVMs [63]. This may result in sarcomere remodeling, which induces cardiac hypertrophy. The cyclic mechanical strain activates downstream focal adhesion kinase (FAK) via the mechanotransduction of integrin, which then activates phosphatidylinositol 4-phosphate 5-kinase (PIP5K) through the RhoA/ROCK pathway. PIP5K phosphorylates phosphatidylinositol 4-phosphate (PI4P) in order to produce PIP2, which reduces the affinity of CapZ and F-actin binding, which contributes to the dysregulation of F-actin assembly and cardiac hypertrophy (Figure 3) [62][64][65][66].
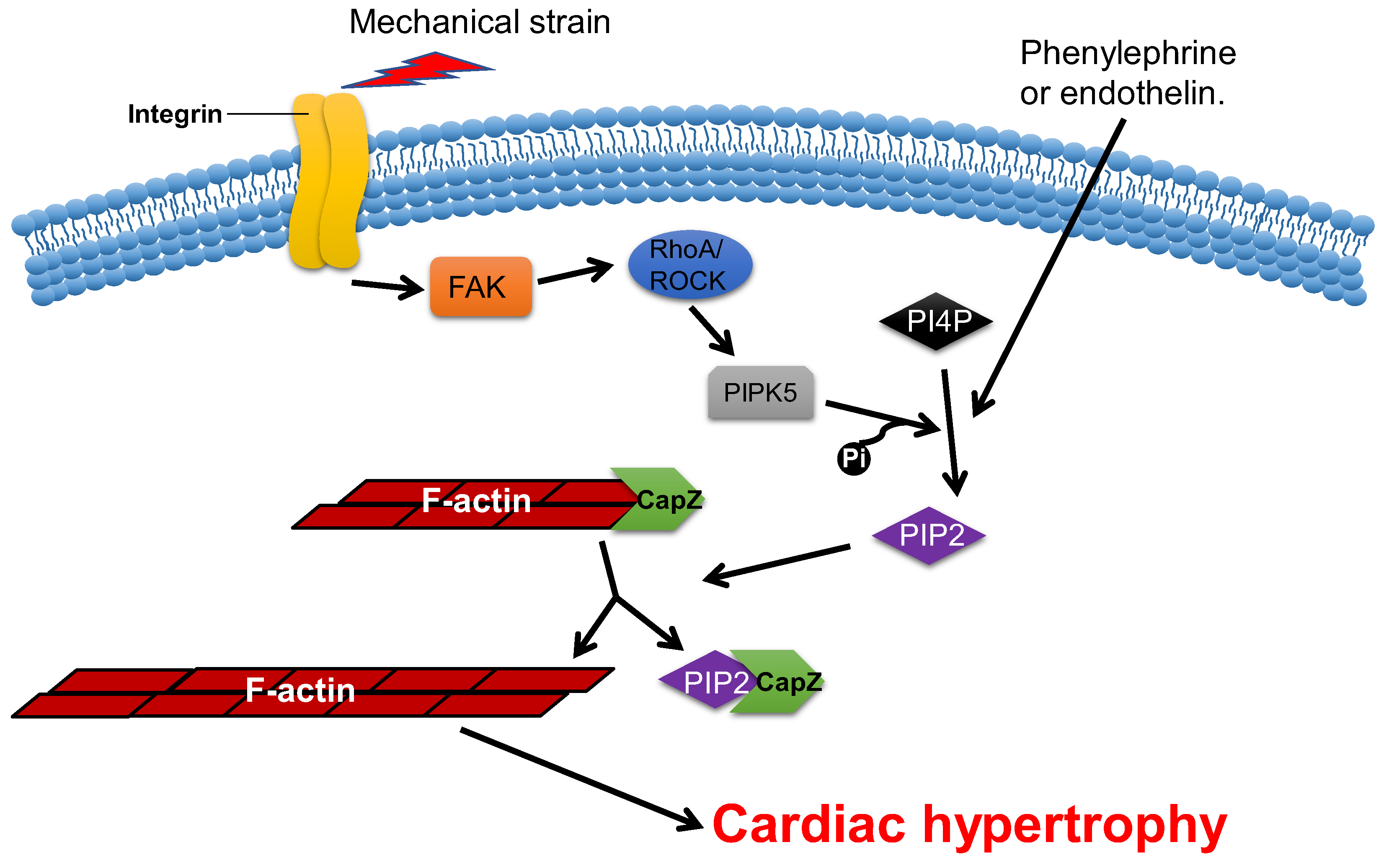
Figure 3. CapZ regulates cardiac hypertrophy. Mechanotransduction leads to the activation of RhoA/Rho-kinase pathway through integrins, which reduce the binding affinity of CapZ and F-actin. It subsequently causes cardiac hypertrophy.
This entry is adapted from the peer-reviewed paper 10.3390/cells11223566
References
- Winder, S.J.; Ayscough, K.R. Actin-binding proteins. J. Cell Sci. 2005, 118, 651–654.
- Chalut, K.J.; Paluch, E.K. The Actin Cortex: A Bridge between Cell Shape and Function. Dev. Cell 2016, 38, 571–573.
- Pollard, T.D. Actin and Actin-Binding Proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a018226.
- Pluess, M.; Ehler, E. Cardiac Cytoarchitecture in Health and Disease. In Cardiac Cytoarchitecture: How to Maintain a Working Heart; Ehler, E., Ed.; Springer: Cham, Switzerland, 2015; pp. 1–14.
- Huang, X.; Li, Z.; Hu, J.; Yang, Z.; Liu, Z.; Zhang, T.; Zhang, C.; Yuan, B. Knockout of Wdr1 results in cardiac hypertrophy and impaired cardiac function in adult mouse heart. Gene 2019, 697, 40–47.
- Kruppa, A.J.; Kendrick-Jones, J.; Buss, F. Myosins, Actin and Autophagy. Traffic 2016, 17, 878–890.
- Aguilera, M.O.; Berón, W.; Colombo, M.I. The actin cytoskeleton participates in the early events of autophagosome formation upon starvation induced autophagy. Autophagy 2012, 8, 1590–1603.
- Zhuo, C.; Ji, Y.; Chen, Z.; Kitazato, K.; Xiang, Y.; Zhong, M.; Wang, Q.; Pei, Y.; Ju, H.; Wang, Y. Proteomics analysis of autophagy-deficient Atg7-/- MEFs reveals a close relationship between F-actin and autophagy. Biochem. Biophys. Res. Commun. 2013, 437, 482–488.
- Zhao, S.H.; Qiu, J.; Wang, Y.; Ji, X.; Liu, X.J.; You, B.A.; Sheng, Y.P.; Li, X.; Gao, H.Q. Profilin-1 promotes the development of hypertension-induced cardiac hypertrophy. J. Hypertens. 2013, 31, 576–586; discussion 586.
- Zeidan, A.; Javadov, S.; Karmazyn, M. Essential role of Rho/ROCK-dependent processes and actin dynamics in mediating leptin-induced hypertrophy in rat neonatal ventricular myocytes. Cardiovasc. Res. 2006, 72, 101–111.
- Hunter, J.C.; Zeidan, A.; Javadov, S.; Kilic, A.; Rajapurohitam, V.; Karmazyn, M. Nitric oxide inhibits endothelin-1-induced neonatal cardiomyocyte hypertrophy via a RhoA-ROCK-dependent pathway. J. Mol. Cell. Cardiol. 2009, 47, 810–818.
- Funk, J.; Merino, F.; Venkova, L.; Heydenreich, L.; Kierfeld, J.; Vargas, P.; Raunser, S.; Piel, M.; Bieling, P. Profilin and formin constitute a pacemaker system for robust actin filament growth. eLife 2019, 8, e50963.
- Safer, D.; Elzinga, M.; Nachmias, V.T. Thymosin beta 4 and Fx, an actin-sequestering peptide, are indistinguishable. J. Biol. Chem. 1991, 266, 4029–4032.
- Rybakova, I.N.; Amann, K.J.; Ervasti, J.M. A new model for the interaction of dystrophin with F-actin. J. Cell Biol. 1996, 135, 661–672.
- Cao, L.; Yonis, A.; Vaghela, M.; Barriga, E.H.; Chugh, P.; Smith, M.B.; Maufront, J.; Lavoie, G.; Méant, A.; Ferber, E.; et al. SPIN90 associates with mDia1 and the Arp2/3 complex to regulate cortical actin organization. Nat. Cell Biol. 2020, 22, 803–814.
- Kuhn, S.; Geyer, M. Formins as effector proteins of Rho GTPases. Small GTPases 2014, 5, e29513.
- Chereau, D.; Boczkowska, M.; Skwarek-Maruszewska, A.; Fujiwara, I.; Hayes, D.B.; Rebowski, G.; Lappalainen, P.; Pollard, T.D.; Dominguez, R. Leiomodin is an actin filament nucleator in muscle cells. Science 2008, 320, 239–243.
- Hosokawa, N.; Kuragano, M.; Yoshino, A.; Shibata, K.; Uyeda, T.Q.P.; Tokuraku, K. Unidirectional cooperative binding of fimbrin actin-binding domain 2 to actin filament. Biochem. Biophys. Res. Commun. 2021, 552, 59–65.
- Sobral, A.F.; Chan, F.Y.; Norman, M.J.; Osorio, D.S.; Dias, A.B.; Ferreira, V.; Barbosa, D.J.; Cheerambathur, D.; Gassmann, R.; Belmonte, J.M.; et al. Plastin and spectrin cooperate to stabilize the actomyosin cortex during cytokinesis. Curr. Biol. 2021, 31, 5415–5428.e10.
- Zheng, B.; Wen, J.K.; Han, M. hhLIM is a novel F-actin binding protein involved in actin cytoskeleton remodeling. FEBS J. 2008, 275, 1568–1578.
- Archer, S.K.; Claudianos, C.; Campbell, H.D. Evolution of the gelsolin family of actin-binding proteins as novel transcriptional coactivators. BioEssays 2005, 27, 388–396.
- Chen, Q.; Courtemanche, N.; Pollard, T.D. Aip1 promotes actin filament severing by cofilin and regulates constriction of the cytokinetic contractile ring. J. Biol. Chem. 2015, 290, 2289–2300.
- Burtnick, L.D.; Urosev, D.; Irobi, E.; Narayan, K.; Robinson, R.C. Structure of the N-terminal half of gelsolin bound to actin: Roles in severing, apoptosis and FAF. EMBO J. 2004, 23, 2713–2722.
- Takács-Kollár, V.; Lőrinczy, D.; Nyitrai, M.; Hild, G. Spectroscopic characterization of the effect of mouse twinfilin-1 on actin filaments at different pH values. J. Photochem. Photobiol. B 2016, 164, 276–282.
- Harris, E.S.; Li, F.; Higgs, H.N. The mouse formin, FRLalpha, slows actin filament barbed end elongation, competes with capping protein, accelerates polymerization from monomers, and severs filaments. J. Biol. Chem. 2004, 279, 20076–20087.
- Gurel, P.S.; Ge, P.; Grintsevich, E.E.; Shu, R.; Blanchoin, L.; Zhou, Z.H.; Reisler, E.; Higgs, H.N. INF2-mediated severing through actin filament encirclement and disruption. Curr. Biol. 2014, 24, 156–164.
- Johnston, A.B.; Hilton, D.M.; McConnell, P.; Johnson, B.; Harris, M.T.; Simone, A.; Amarasinghe, G.K.; Cooper, J.A.; Goode, B.L. A novel mode of capping protein-regulation by twinfilin. eLife 2018, 7, e41313.
- Sun, H.Q.; Yamamoto, M.; Mejillano, M.; Yin, H.L. Gelsolin, a multifunctional actin regulatory protein. J. Biol. Chem. 1999, 274, 33179–33182.
- Bao, Y.; Kake, T.; Hanashima, A.; Nomiya, Y.; Kubokawa, K.; Kimura, S. Actin capping proteins, CapZ (β-actinin) and tropomodulin in amphioxus striated muscle. Gene 2012, 510, 78–86.
- Svitkina, T. The Actin Cytoskeleton and Actin-Based Motility. Cold Spring Harb. Perspect. Biol. 2018, 10, a018267.
- Pinto-Costa, R.; Sousa, M.M. Profilin as a dual regulator of actin and microtubule dynamics. Cytoskeleton 2020, 77, 76–83.
- Jockusch, B.M.; Murk, K.; Rothkegel, M. The profile of profilins. Rev. Physiol. Biochem. Pharmacol. 2007, 159, 131–149.
- Suetsugu, S.; Miki, H.; Takenawa, T. The essential role of profilin in the assembly of actin for microspike formation. EMBO J. 1998, 17, 6516–6526.
- Paul, A.S.; Pollard, T.D. The role of the FH1 domain and profilin in formin-mediated actin-filament elongation and nucleation. Curr. Biol. 2008, 18, 9–19.
- Ferron, F.; Rebowski, G.; Lee, S.H.; Dominguez, R. Structural basis for the recruitment of profilin-actin complexes during filament elongation by Ena/VASP. EMBO J. 2007, 26, 4597–4606.
- Kooij, V.; Viswanathan, M.C.; Lee, D.I.; Rainer, P.P.; Schmidt, W.; Kronert, W.A.; Harding, S.E.; Kass, D.A.; Bernstein, S.I.; van Eyk, J.E.; et al. Profilin modulates sarcomeric organization and mediates cardiomyocyte hypertrophy. Cardiovasc. Res. 2016, 110, 238–248.
- Moustafa-Bayoumi, M.; Alhaj, M.A.; El-Sayed, O.; Wisel, S.; Chotani, M.A.; Abouelnaga, Z.A.; Hassona, M.D.; Rigatto, K.; Morris, M.; Nuovo, G.; et al. Vascular hypertrophy and hypertension caused by transgenic overexpression of profilin 1. J. Biol. Chem. 2007, 282, 37632–37639.
- Elnakish, M.T.; Hassanain, H.H.; Janssen, P.M. Vascular remodeling-associated hypertension leads to left ventricular hypertrophy and contractile dysfunction in profilin-1 transgenic mice. J. Cardiovasc. Pharmacol. 2012, 60, 544–552.
- Joy, M.; Gau, D.; Castellucci, N.; Prywes, R.; Roy, P. The myocardin-related transcription factor MKL co-regulates the cellular levels of two profilin isoforms. J. Biol. Chem. 2017, 292, 11777–11791.
- Oeing, C.U.; Nakamura, T.; Pan, S.; Mishra, S.; Dunkerly-Eyring, B.L.; Kokkonen-Simon, K.M.; Lin, B.L.; Chen, A.; Zhu, G.; Bedja, D.; et al. PKG1alpha Cysteine-42 Redox State Controls mTORC1 Activation in Pathological Cardiac Hypertrophy. Circ. Res. 2020, 127, 522–533.
- Altamirano, F.; Oyarce, C.; Silva, P.; Toyos, M.; Wilson, C.; Lavandero, S.; Uhlen, P.; Estrada, M. Testosterone induces cardiomyocyte hypertrophy through mammalian target of rapamycin complex 1 pathway. J. Endocrinol. 2009, 202, 299–307.
- Ranek, M.J.; Kokkonen-Simon, K.M.; Chen, A.; Dunkerly-Eyring, B.L.; Vera, M.P.; Oeing, C.U.; Patel, C.H.; Nakamura, T.; Zhu, G.; Bedja, D.; et al. PKG1-modified TSC2 regulates mTORC1 activity to counter adverse cardiac stress. Nature 2019, 566, 264–269.
- Yang, D.; Wang, Y.; Jiang, M.; Deng, X.; Pei, Z.; Li, F.; Xia, K.; Zhu, L.; Yang, T.; Chen, M. Downregulation of Profilin-1 Expression Attenuates Cardiomyocytes Hypertrophy and Apoptosis Induced by Advanced Glycation End Products in H9c2 Cells. BioMed Res. Int. 2017, 2017, 9716087.
- Yang, D.; Liu, W.; Ma, L.; Wang, Y.; Ma, J.; Jiang, M.; Deng, X.; Huang, F.; Yang, T.; Chen, M. Profilin-1 contributes to cardiac injury induced by advanced glycation end-products in rats. Mol. Med. Rep. 2017, 16, 6634–6641.
- Bernstein, B.W.; Bamburg, J.R. ADF/cofilin: A functional node in cell biology. Trends Cell Biol. 2010, 20, 187–195.
- Nakashima, K.; Sato, N.; Nakagaki, T.; Abe, H.; Ono, S.; Obinata, T. Two mouse cofilin isoforms, muscle-type (MCF) and non-muscle type (NMCF), interact with F-actin with different efficiencies. J. Biochem. 2005, 138, 519–526.
- Mseka, T.; Cramer, L.P. Actin depolymerization-based force retracts the cell rear in polarizing and migrating cells. Curr. Biol. 2011, 21, 2085–2091.
- Jaffe, A.B.; Hall, A. Rho GTPases: Biochemistry and biology. Annu. Rev. Cell Dev. Biol. 2005, 21, 247–269.
- Hild, G.; Kalmár, L.; Kardos, R.; Nyitrai, M.; Bugyi, B. The other side of the coin: Functional and structural versatility of ADF/cofilins. Eur. J. Cell Biol. 2014, 93, 238–251.
- Rangrez, A.Y.; Hoppe, P.; Kuhn, C.; Zille, E.; Frank, J.; Frey, N.; Frank, D. MicroRNA miR-301a is a novel cardiac regulator of Cofilin-2. PLoS ONE 2017, 12, e0183901.
- Aoki, H.; Izumo, S.; Sadoshima, J. Angiotensin II activates RhoA in cardiac myocytes: A critical role of RhoA in angiotensin II-induced premyofibril formation. Circ. Res. 1998, 82, 666–676.
- Aikawa, R.; Komuro, I.; Nagai, R.; Yazaki, Y. Rho plays an important role in angiotensin II-induced hypertrophic responses in cardiac myocytes. Mol. Cell. Biochem. 2000, 212, 177–182.
- Zeidan, A.; Javadov, S.; Chakrabarti, S.; Karmazyn, M. Leptin-induced cardiomyocyte hypertrophy involves selective caveolae and RhoA/ROCK-dependent p38 MAPK translocation to nuclei. Cardiovasc. Res. 2008, 77, 64–72.
- Moey, M.; Rajapurohitam, V.; Zeidan, A.; Karmazyn, M. Ginseng (Panax quinquefolius) attenuates leptin-induced cardiac hypertrophy through inhibition of p115Rho guanine nucleotide exchange factor-RhoA/Rho-associated, coiled-coil containing protein kinase-dependent mitogen-activated protein kinase pathway activation. J. Pharmacol. Exp. Ther. 2011, 339, 746–756.
- Zeidan, A.; Gan, X.T.; Thomas, A.; Karmazyn, M. Prevention of RhoA activation and cofilin-mediated actin polymerization mediates the antihypertrophic effect of adenosine receptor agonists in angiotensin II- and endothelin-1-treated cardiomyocytes. Mol. Cell. Biochem. 2014, 385, 239–248.
- Lai, D.; Gao, J.; Bi, X.; He, H.; Shi, X.; Weng, S.; Chen, Y.; Yang, Y.; Ye, Y.; Fu, G. The Rho kinase inhibitor, fasudil, ameliorates diabetes-induced cardiac dysfunction by improving calcium clearance and actin remodeling. J. Mol. Med. 2017, 95, 155–165.
- Chesarone, M.A.; DuPage, A.G.; Goode, B.L. Unleashing formins to remodel the actin and microtubule cytoskeletons. Nat. Rev. Mol. Cell Biol. 2010, 11, 62–74.
- Schafer, D.A.; Hug, C.; Cooper, J.A. Inhibition of CapZ during myofibrillogenesis alters assembly of actin filaments. J. Cell Biol. 1995, 128, 61–70.
- Hart, M.C.; Cooper, J.A. Vertebrate isoforms of actin capping protein beta have distinct functions In vivo. J. Cell Biol. 1999, 147, 1287–1298.
- Kim, K.; McCully, M.E.; Bhattacharya, N.; Butler, B.; Sept, D.; Cooper, J.A. Structure/function analysis of the interaction of phosphatidylinositol 4,5-bisphosphate with actin-capping protein: Implications for how capping protein binds the actin filament. J. Biol. Chem. 2007, 282, 5871–5879.
- Montgomery, D.E.; Chandra, M.; Huang, Q.; Jin, J.; Solaro, R.J. Transgenic incorporation of skeletal TnT into cardiac myofilaments blunts PKC-mediated depression of force. Am. J. Physiol. Heart. Circ. Physiol. 2001, 280, H1011–H1018.
- Lin, Y.H.; Warren, C.M.; Li, J.; McKinsey, T.A.; Russell, B. Myofibril growth during cardiac hypertrophy is regulated through dual phosphorylation and acetylation of the actin capping protein CapZ. Cell. Signal. 2016, 28, 1015–1024.
- Hartman, T.J.; Martin, J.L.; Solaro, R.J.; Samarel, A.M.; Russell, B. CapZ dynamics are altered by endothelin-1 and phenylephrine via PIP2- and PKC-dependent mechanisms. Am. J. Physiol. Cell Physiol. 2009, 296, C1034–C1039.
- Li, J.; Russell, B. Phosphatidylinositol 4,5-bisphosphate regulates CapZβ1 and actin dynamics in response to mechanical strain. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1614–H1623.
- Lin, Y.H.; Li, J.; Swanson, E.R.; Russell, B. CapZ and actin capping dynamics increase in myocytes after a bout of exercise and abates in hours after stimulation ends. J. Appl. Physiol. 2013, 114, 1603–1609.
- Li, J.; Mkrtschjan, M.A.; Lin, Y.H.; Russell, B. Variation in stiffness regulates cardiac myocyte hypertrophy via signaling pathways. Can. J. Physiol. Pharmacol. 2016, 94, 1178–1186.
This entry is offline, you can click here to edit this entry!
