1. Palladium
Reported for the first time by Cacchi and co-workers, the palladium-catalyzed hydroarylation between olefines and arylhalides or pseudohalides is formally known as a reductive Heck reaction [
51,
52,
53,
54]. Although its disclosure dates back to the 1980s, its asymmetric version has only been developed recently. Between 2010 and 2011 Sigman described the hydroarylation of dienes and styrenes trough the reductive formation of π-allyl palladium intermediates under oxidative conditions [
55,
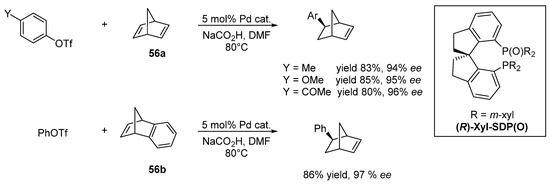
56]. Despite the use of chiral ligands, only poor enantioselectivity was achieved. In 2013 Liu and Zhou reported the desymmetrization of substituted cyclopentenes through an asymmetric Heck reaction and the first highly enantioselective hydroarylation of bicyclic olefins (
56a and
56b) with a Pd catalyst [
57]. The ligand of choice for the asymmetric hydroarylation is the 1,1′-spirobiindane-7,7′-bisphosphine oxide,(R)-Xyl-SDP(O), belonging to the phosphine oxides family tested first for the Heck reaction, while the hydride donor was the sodium formate (
Figure 1). For the first time in the asymmetric hydroarylation catalyzed by Pd, the reaction was achieved with high yields and ee up to 90% in all cases.
Figure 1. Asymmetric hydroarylation catalyzed by Pd/(R)-Xyl-SDP(O).
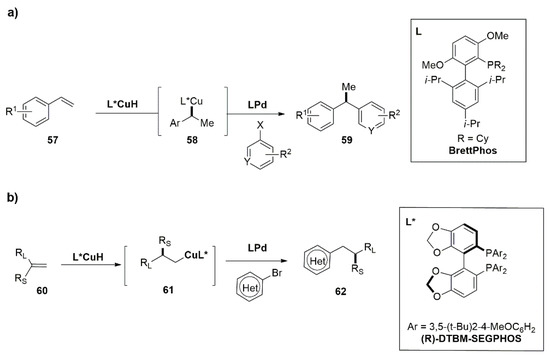
The combination of Cu and Pd as cooperative catalysis in the asymmetric hydroarylation or hydrocyanation is described by the works of Buchwald’s group [
58,
59,
60]. The asymmetric hydroarylation of
57 and
60 leads to the synthesis of 1,1-diarylalkanes (
59) and of arenes with β-stereogenic center (
62).
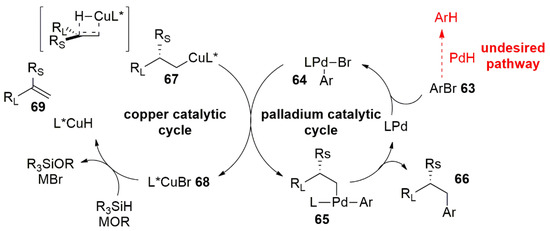
Both processes are performed on terminal double bonds and start from the CuH catalyst, generated in situ from a suitable Cu(I) or Cu(II) salt and the chiral ligand. Figure 2 shows investigated substrates and products as well as the structures of chiral ligands. When the hydrocupration is performed on styrene derivatives (57), a benzylic Cu(I) intermediate (58) is formed (Figure 2a). In the second case, when 1,1-disubstituted olefins (60) are employed, the reaction proceeds with anti-Markovnikov regioselectivity, giving the Cu(I) alkyl intermediate (61) (Figure 2b). In Figure 3, the complete catalytic cycle is reported: simultaneously with the copper activity, the Pd(0) complex is oxidatively added to the aryl bromide (63), forming the aryl palladium intermediate (64), which converges in a stereospecific transmetalation with the organocopper species (67). The enantioenriched products (66) are finally furnished via reductive elimination of chiral Pd(II) alkyl complexes (65), regenerating the Pd(0)-Ligand compound. The Cu(I) salt generated in the first catalytic cycle returns the CuH-ligand catalyst by treatment with the silane (R3SiH) and the base (MOR). It is noteworthy that the metal cycles are required to be matched with attention to avoid undesirable side reactions such as aryl bromide reduction. In the first work of Buchwald’s group, the ligands used for the copper and palladium catalytic processes were different and must be chosen properly to furnish a good reactivity of one metal without deactivating the other. After testing several ligands for both the processes, the BrettPhos for the Pd and the DTBM-SEGPHOS for the Cu turned out to be the best ligands in terms of yields and enantioselectivity. The optimized procedure has proven to work well with several aryl bromides and with a variety of vinyl arenes furnishing the products of the resulting hydroarylation reaction with good yields and ee.
Figure 2. Asymmetric hydroarylation by Cu/Pd cooperative catalysis for the synthesis of 1,1-diarylalkanes (59) (a) and for the synthesis of arenes with β-stereogenic center (62) (b).
Figure 3. Proposed catalytic cycle of reaction in Figure 2b.
The issue of the employment of two different ligands was overcome in a recent work where the ligand of choice, the DTBM-SEGPHOS, is the same for both the metals. The asymmetric hydroarylation through the dual Pd-CuH catalysis described in this work, is performed on numerous 1,1-disubstituted alkenes and aryl bromides providing access in a single-step process to arenes with β-stereogenic centers, motifs founded in many drugs and natural products [
61,
62,
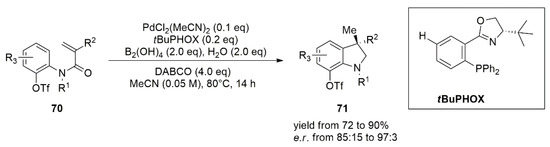
63]. After Buchwald’s and Zhou’s works, Zhu and co-workers developed an asymmetric intramolecular reductive Heck reaction of
N-aryl acrylamides [
64]. The reaction protocol developed by Zhu exploits a diboron–water specie as a hydride donor for the reduction of C(sp
3)-Pd intermediate. Synthesis of enantioenriched 3,3-disubstituted oxindoles (
71) with a quaternary stereocenter was achieved with high yields and ee using a catalytic amount of PdCl
2(MeCN)
2-ligand as catalyst, DABCO (1,4-diazabicyclo[2.2.2]octane) as base, and a stoichiometric amount of B
2(OH)
4/H
2O as the hydride donor. Choice of the chiral ligand determines not only the enantioselectivity of the reaction, but also the reaction pathways: when PPh
3 is used the reaction leads to the carboborylation product, while using (S)-tBuPHOX as ligand, the reaction pathway switches, affording hydroarylation products (
Figure 4).
Figure 4. Asymmetric intramolecular reductive Heck reaction of N-aryl acrylamides (70).
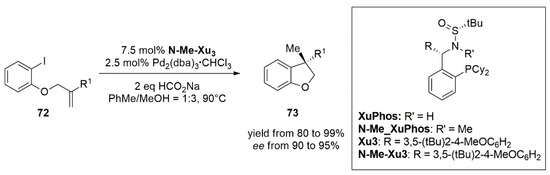
The following year, Zhang’s group reported the first example of highly enantioselective intramolecular hydroarylation of allyl aryl ethers through a reductive Heck reaction catalyzed by palladium [
65]. Similar benzofuran scaffolds were obtained by direct hydroarylation reaction employing rhodium as transition metal catalyst. Here, the reaction was performed exploiting a new chiral sulfonamide monophosphine ligand,
N-Me-XuPhos, prepared in a one-pot synthesis by the deprotonation of the dicyclohexyl phosphine borane with nBuLi and treatment with 1,2-dibromobenzene. Subsequent quenching by NH
4Cl aq. or MeOTf, and deprotection performed with Et
2NH afforded XuPhos and
N-Me-XuPhos with moderate yields and excellent diastereoselectivities. The hydroarylation of allyl aryl ethers (
72) using
N-Me-XuPhos ligands, afforded a variety of optically active 3,3-Disubstituted 2,3-Dihydrobenzofuran (
73) (
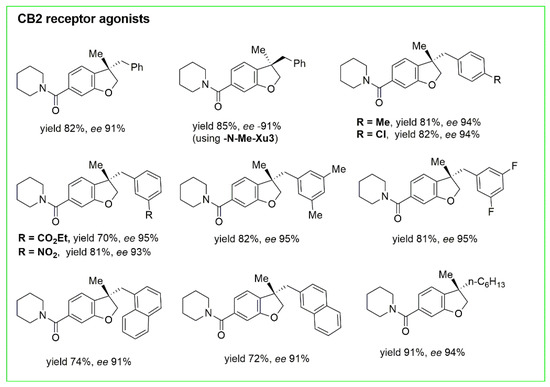
Figure 5) and a series of CB2 receptor agonists with high chemo- and enantioselectivity (70–91% yields with 91–95% ee) (
Figure 6).
Figure 5. Asymmetric intramolecular reductive Heck reaction of allyl aryl ethers (72).
Figure 6. CB2 receptor agonists obtained with synthesis in Figure 5.
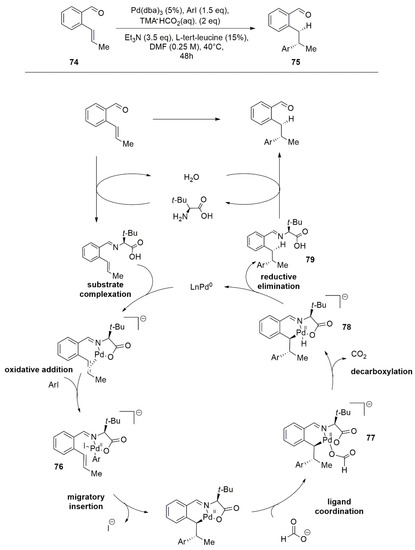
Inspired by asymmetric organocatalysis, Engle and coworkers have developed an enantioselective reductive Heck hydroarylation of alkenes that takes advantage of a chiral transient directing group (TDG) [
66]. The chiral TDG class selected are chiral amines, in particular amino acids (L-tert-leucine in
Figure 7), which lead to a stereocontrolled migratory insertion with alkenyl benzaldehydes (
74) under mild conditions. The authors have hypothesized that the reaction occurs through dual catalytic cycles (
Figure 7) where, after the coordination with the imine, the oxidative addition of the aryl iodide leads to the active palladium intermediate (
76) followed by migratory insertion of aryl moiety and the additional formate coordination. Following this, the intermediate (
77) decarboxylates afford a Pd-H species (
78). Finally, the reductive elimination returns the L-Pd(0) species and subsequent dissociation conducts to the product (
79).
Figure 7. Palladium reductive Heck hydroarylation of alkenes (74) assisted by L-tert-leucine.
2. Nickel
Hydroarylation reactions involving catalytic organoligand/Ni systems and pre-activated arene are more represented compared to the direct counterpart (
Section 2.4). Between 2019 and 2021 Zhu’s group published several works in high-impact journals where the catalytic systems involved Nickel and preactivated arenes in hydroarylation reactions [
67,
68,
69,
70,
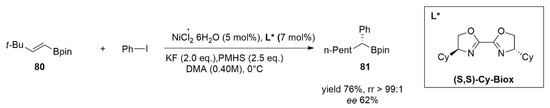
71]. At first, a racemic version of the hydroarylation of boron-containing alkenes (
80) was reported. In this reaction, NiH species generated in situ catalyze both the chain walking process and subsequent cross-coupling, leading to α-functionalized alkyl boronate species with excellent chemo- and regioselectivity. Moreover, the asymmetric version of the reaction performed on these substrates by using (S,S)-Cy-Biox as the chiral ligand (L* in the scheme of
Figure 8) delivered the product (
81) in good yield but with low enantioselectivity.
Figure 8. Hydroarylation of boron containing alkene (80) and benzyl iodide by using Ni/(S,S)-Cy-Biox.
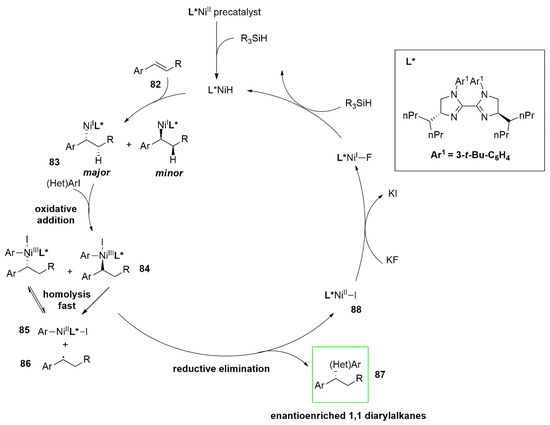
A highly enantio- and regioselective NiH-catalyzed reductive hydroarylation of vinylarenes (82) with aryl iodides was reported. The developed protocol involves the employment of a bis imidazoline (BIm)–nickel complex as catalyst. The proposed mechanism (Figure 9) shows that after formation of an alkyl–nickel intermediate (83) by the reaction between the catalyst and the alkene, the oxidative addition of aryl iodide occurs. Homolysis of the Ni-C bond could form a Ni(II)/benzylic radical pair in fast equilibrium with the Ar-Ni(III)-alkyl intermediate (84). Next, the enantio-determining step of the process is due to the enantioselective recombination of the benzylic radical (86) with the Ar-Ni(II)-I complex (85). An irreversible reductive elimination of the Ar-Ni(III)–alkyl complex (84) provides an enantioenriched 1,1-diarylalkane product (87) and nickel(I) iodide (88). Regeneration of the catalyst L-Ni(I)H occurs through ligand exchange with KF and subsequent reaction with a stoichiometric hydrosilane reagent. After a screening of the ligands and reaction optimization, numerous alkenes and aryl iodide components bearing a variety of functional groups proved to be suitable for the reaction allowing the synthesis of enantioenriched 1,1 diarylalkanes.
Figure 9. NiH-catalyzed asymmetric reductive hydroarylation of vinylarenes (82) with aryl iodides.
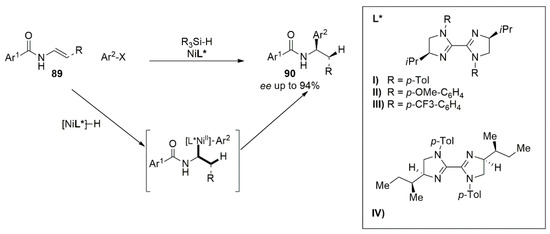
The same system based on chiral bis imidazoline–Nickel complex gave excellent results, also for the enantioselective hydroarylation of
N-acyl enamines. Furthermore, the employment of chiral BIm-Ni systems in the asymmetric reductive hydroarylation of vinyl amides (
89) for the synthesis of enantioenriched α-arylbenzamides (
90) was reported by Nevado [
72]. Different aromatic substituents on the ligand’s nitrogen atoms and isopropyl side chain were tested, leading to the product in moderate yields but with good enantioselectivity. The employment of the ligand
L*IV and the optimization of the reaction conditions allowed for the obtaining of a wide variety of α-arylbenzamides in good yields and with high enantioselectivity (
Figure 10).
Figure 10. Bis-imidazoline-Nickel complex employed to synthesize enantioenriched α-arylbenzamides (90).
Mechanistic studies suggested that the presence of radical scavengers do not significantly affect the reaction outcome. Oxidative addition of the chiral alkyl-aryl-Ni
III complex to the vinyl amide leads to the first intermediate, which allows the insertion of the aryl moiety in a highly enantioselective manner. Notably, DFT calculations showed that the amide moiety plays a pivotal role in the stabilization of the Ni center and thus to stereodefined Csp3-Csp2 bond formation. The use of aryl- or vinylboronic species in the asymmetric hydroarylation/hydroalkenylation of styrenes and 1,3-dienes was reported by the research groups of Mei, Murcum and Zhou [
73,
74,
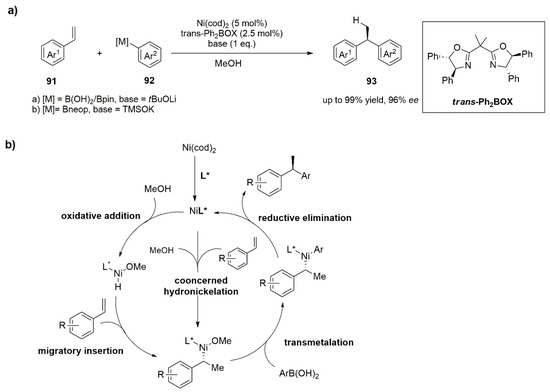
75]. Inspired by Zhou and co-workers’ studies, Stanley’s group recently reported the catalytic enantioselective hydroarylations of vinylarenes (
91) with aryl boronic acids in the presence of a catalyst generated in situ by 2.5 mol% of the nickel precatalyst Ni(cod)
2 and 0.5 mol% of the chiral bisoxazoline ligand trans-Ph
2BOX aimed at the synthesis of chiral 1,1-diarylethanes (
93) (
Figure 11a) [
76]. As reported in
Figure 11b, the reactions performed with arylboronic acids having electron-withdrawing substitutents allowed the preparation of the corresponding 1,1-diarylethanes with less than 80% ee, while boronic acids having electron-donating groups gave the desired products with greater than 90% ee. These results are consistent that the transmetallation with the aryl-boron nucleophile is a determinant for the enantioselectivity of the reaction. In addition to the presence of electrondeficient arylboronic acids, the absence of a base slows down the rate of trasmetalation, and isomerization of the nickel benzyl species may cause loss of enantioselectivity.
Figure 11. (a) Synthesis of chiral 1,1-diarylethanes (93) trough Ni/ trans-Ph2BOX. Catalyst are generated in situ. (b) Mechanism of concerted hydronickelation.
3. Rhodium
Asymmetric arylation reaction between aryl– or alkenyl–boronic acids and olefins has been widely studied by Hayashi and co-workers since the 90s [
77,
78,
79,
80]. Recently, Hayashi’s group has published a work about the Rh-catalyzed asymmetric hydroarylation of 3-pyrrolines [
27]. After optimization of reaction conditions in terms of solvent, temperature, use or not of the additive KOH and the opportune choice of the ligand, the desired hydroarylated product was achieved with high chemoselectity and excellent enantiomeric excess, eliminating the formation of byproducts. Coordination of the hydroxorhodium complex with the ligand leads to the [Rh(OH)(coe)
2]
2/(R)-segphos catalyst system affording the reaction under neutral conditions. The asymmetric hydroarylation of 3-pyrrodines with arylboroxines provides a variety of 3-arylpyrrolidines which are important biologically active compounds.
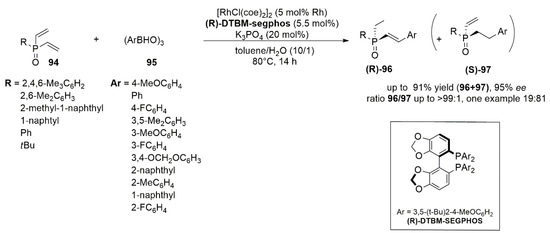
Aryloboroxines ((ArBHO)
3) (
95) have been also used as key reagents in the asymmetric hydroarylation of divinylphosphine oxides (
94) (RP(O)(CH=CH
2)
2) [
81]. The reaction provides the corresponding monoarylated products (
96 and
97) giving the deasymmetrization and the enantioselective formation of the product with a P-stereogenic center. Also in this case, a Segphos ligand, the (R)-DTBM-segphos was found to be the best ligand regarding chemo- and enantioselectivity (
Figure 12). Carbon–carbon bond formation occurs between one of the two vinyl groups and the aryl moiety, while the other double bond is reduced to ethyl group. Divinylphosphine oxides and aryl boroxines bearing different substituents were tested under the optimized reaction conditions. When the reaction is performed between the aryl boroxine and the divinylphosphine oxide with bulky substituents (R- in the list of
Figure 12), high yields and ee in the formation of the product
(R)-96 were observed, while the use of less bulky substituents leads to a lowering of yield and/or enantioselectivity. Concerning the substituted arylboroxines, para- or meta- substituents lead to product
96 in high yields with good enantioselectivity. Ortho-substitution leads to an increase in ee but also to a decrease in
96/97 selectivity, up to an inversion in favor of the product
97 (ratio
96/
97 = 19/81) when the 2-fluorophenylboronic acid is used.
Figure 12. Asymmetric hydroarylation of divinylphosphine oxides (94) and aryl boroxines (95). Catalyst complex: Rh/(R)-DTBM-segphos.
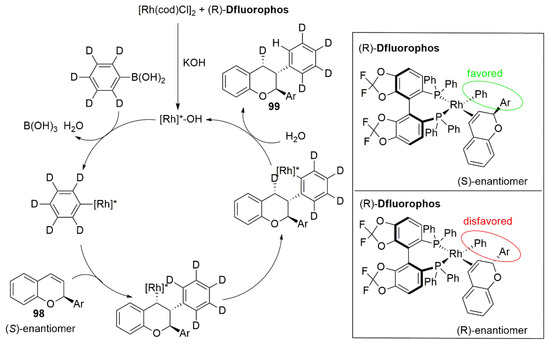
In the same year, Wang and co-workers reported an example of Rh-catalyzed asymmetric hydroarylation for kinetic resolution and dynamic kinetic resolution of chromenes [
82]. This strategy exploits the preferential coordination of the Rh-Ligand complex with one of the two enantiomers of the racemic mixture while the formation of the other intermediate is much less favorable because of steric repulsion (
Figure 13). Therefore, one enantiomer (
98) undergoes the transformation in 2,3-diaryl chromene (
99) through the asymmetric hydroarylation reaction leaving the other optically pure unreacted flavene.
Figure 13. Rhodium-catalyzed hydroarylation of 2-aryl chromenes: Deuterium-labeling experiment and proposed mechanism.
After optimization of reaction conditions and screening of several biphosphines ligands, which showed that (R)-Difluorphos gave the best results, the authors examined the scope with respect of variously substituted flavenes and aryl boronic acids. Good yields and excellent ee were obtained in most cases, proving that the substituents on both the benzopyran and C2-phenyl ring have limited effect on the enantioselectivity and most of the aryl boronic acids provides the desired product with good results. Further application of this strategy was developed for dynamic resolution of chromene acetals providing the desired products, which could be easily transformed into chiral isoflavones or hydroxycoumarin.
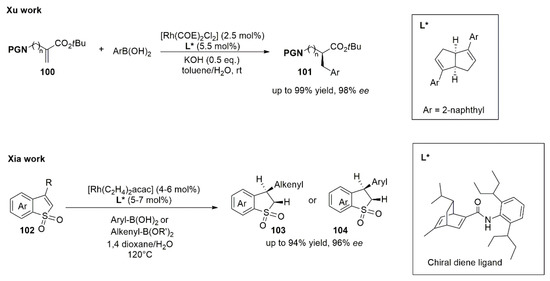
Analogously to the Zhu work about the employment of diboron–water species as a hydride source for the Pd-catalyzed asymmetric synthesis of disubstituted oxindoles, Xu and co-workers have recently developed a Rh(I)-diene catalytic system able to exploit water as proton source for the asymmetric hydroarylation of α-aminoalkyl acrylates (
100) with boronic acids [
83]. In the same year, Xia’s group developed an efficient protocol using a well-designed chiral Rh-diene catalyst with arylboronic acids and alkenylboronic acids in the enantioselective hydroarylation/hydroalkenylation of benzo[b]thiophene 1,1-dioxides (
102) [
84]. This protocol is free of base and additive, and it uses water as co-solvent. Structures of employed chiral ligands were in
Figure 14 together with scheme of reactions.
Figure 14. Diboron–water species as hydride source for the Rh-catalyzed asymmetric synthesis of α-aminoalkyl acrylates (101) (by Xu) and benzo[b]thiophene 1,1-dioxides (103 or 104) (by Xia).
This entry is adapted from the peer-reviewed paper 10.3390/catal12101289