Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Health Care Sciences & Services
Chemotherapy as an adjuvant therapy that has largely failed to significantly improve outcomes for aggressive brain tumors; some reasons include a weak blood brain barrier penetration and tumor heterogeneity. Recently, there has been interest in designing effective ways to deliver chemotherapy to the tumor.
- focused chemotherapy
- brain tumors
- drug delivery
1. Introduction
There has been no significant improvement in the survival of patients with primary brain tumors although there has been extensive development of drug delivery methods to the central nervous system (CNS). Therapies such as surgical resection, radiotherapy, and chemotherapy have improved the survival in certain tumors, such as medulloblastoma, while glioblastomas still have a poor prognosis. The different responses to pharmacotherapy may be due to differences in the blood brain barrier penetration and tumor microenvironment. To overcome these barriers, several drug delivery methods, such as nanoparticles, convection enhanced delivery, focused ultrasound, and intranasal delivery have been designed [1]. These drug delivery systems are designed to introduce therapeutic substances to the CNS, while controlling the rate, time, and region of its release.
2. Evolution of Drug Delivery to the Brain
In 1914, it was reported that salvarsan and neosalvarsan both used for syphilis did not enter the brain, via blood, after IV administration [2]. By 1950, lipid soluble drugs, such as tricyclic antidepressants were designed, which traversed the blood brain barrier (BBB) well [3]. The earliest understanding and definition of the BBB was established by McIntosh and Fildes in 1916 [4]. In 1972, the lipid solubility effects on the BBB transport were further explained by Oldendorf [5]. In 1963, the first drug delivery method through the lateral ventricle was developed by Ommaya who designed a reservoir for the administration of intrathecal antibiotics [6]. In 1979, osmotic disruption of the BBB was introduced for drug delivery [7]. In 1982, transnasal drug delivery was identified as a method to bypass the blood brain barrier [8]. By 1994, different transcranial delivery methods started to emerge, such as intracerebral implants and convection enhanced delivery [9,10]. In 1986, BBB mediated transcytosis was used, and antibodies targeting BBB associated receptors to enhance the specific targeting of therapies were developed [11,12]. In 1990, a liposomal mediated transfer was first described as an advance into the history of CNS drug delivery [13]. In 2001, microbubble and ultrasound focused disruption of the BBB was first used as a drug delivery enhancing method [14]. In 2011, the targeted delivery of siRNA to the brain was established using exosome vesicles [15]. Figure 1 is an outline of the history of CNS drug delivery.
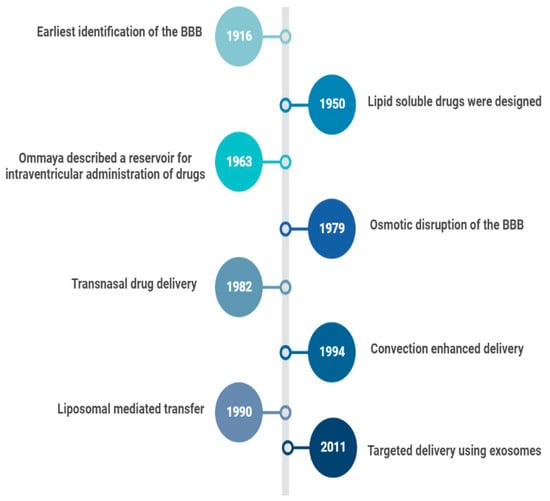
Figure 1. History of CNS Drug delivery evolution.
3. Methods of Drug Delivery
3.1. Non-Viral Nanoparticles
Nanoparticles have long been used as tools for drug delivery. They can penetrate the leaky tumor capillaries due to their small size, explained by their enhanced permeability and retention effect [16]. Transporter ligands and receptors can direct the uptake of the nanoparticle through the BBB to a specified target [16]. The ligand is not of a therapeutic value, but it facilitates the proper targeting and delivery of the drug [16]. A well-known example is the low-density lipoproteins undergoing transcytosis through brain endothelial cells [17]. In one phase 2 clinical study, investigating enhanced penetration in gliomas, a cell penetrating peptide was conjugated with ANG1005, angiopep-2 paclitaxel conjugate [18]. Angiopep-2 is a ligand fashioned to bind to a low-density lipoprotein receptor-related protein-1 (LRP-1), which enhances LRP-1-mediated transcytosis [18]. In experimental trials, it improved the outcome in glioblastoma mouse models [19].
3.2. Exosomes
Different cells secrete small extracellular vesicles called exosomes. They are stable and can remain in the circulation for a long time. Those isolated from brain endothelial cells regulate the process of transport across the BBB [20]. Exosomes have been utilized to carry small molecules and nucleic acids. In addition to carrying useful materials, exosomes have adhesive proteins on their surface [21]. To combat tumor development, an exosome containing siRNA specific vascular endothelial growth factor (VEGF) was able to release its content into brain tumors of zebrafish. VEGF expression was significantly impacted by the siRNA [22]. Additional studies suggest that brain endothelial derived exosomes may bypass the BBB and noninvasively deliver chemotherapeutic drugs [23].
3.3. Active Transport through Blood-Brain Barrier
Amino acids can cross the BBB using different carriers. Linking drugs to these amino acids can help in the delivery of these drugs. Methotrexate (MTX)-lysine conjugate was designed to improve the permeability of the BBB to methotrexate [24]. Methotrexate was transported using the same mechanism of lysine amino acid transport. Although peptide carriers are promising, the synthesis process and optimization is exceedingly complex. More work must be performed to improve the ease of use before sustained usage can be accomplished. Additionally, prodrugs (esters) are potential BBB drug delivery methods. Furthermore, dimers can be utilized as they can carrier the drug of choice and another versatile compound. Lastly, due to their diversity and optimization, nanoparticles (organic or inorganic) possess a tremendous promise in being coupled with drug delivery [25].
3.4. Microbubble-Enhanced
Microbubble-enhanced delivery is a noninvasive technique, which increases the permeability of the BBB for different treatments. It was found to reduce the expression of different junctional proteins that are responsible for the integrity of the BBB without damaging brain tissue [26]. This technique was reported to increase the local brain concentration of doxorubicin and to enhance drug passage across both the BBB and the blood brain tumor barrier (BBTB) [27]. In early 2000, Hynynen and co-workers used MRI-guided US in combination with a microbubble to open the BBB [28]. Adding the microbubble technique mitigated the brain injury caused by the ultrasound waves. Moreover, Herceptin, the monoclonal antibody used to treat breast cancer brain metastasis was reported to efficiently reach the brain when US and microbubbles were used simultaneously [28].
3.5. Convection Enhanced Delivery (CED)
In the early 1990s, Edward Oldfield and colleagues designed CED [29]. Notably, CED alters the permeability of the BBB and allows for subsequent targeted drug delivery. The drug delivery is localized but invasive [30]. Utilizing CED involves one or more intracranial catheters connected to an external infusion pump, enabling therapeutic substances to be delivered to the target tissues via a pressure gradient. With local infusion, the brain parenchyma receives a higher therapeutic concentration with less systemic side effects [31].
There are currently multiple ongoing clinical trials utilizing CED, most of which are for glioblastoma (GBM) and diffuse intrinsic pontine glioma (DIPG) [32]. In GBM, recurrence mainly occurs in the peritumoral area, therefore, CED utilization is vital for tumor recurrences as it perfuses different areas of the tumor [33]. CED allows for the direct delivery of the drug to the tumor bed resulting in high local concentrations with minimal systemic absorption [34] (Figure 2).
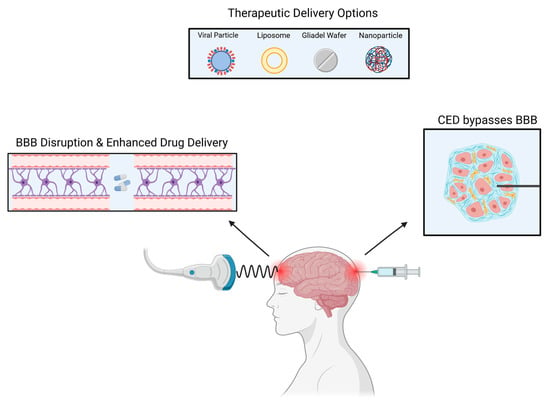
Figure 2. Several methods can be used to deliver drugs to intracranial tumors.
A wide range of therapeutic elements can be delivered through CED such as chemotherapeutic agents, imaging tracers, proteins, viruses, liposomes, and nanoparticles [35,36,37]. Lastly, in addition to other limiting factors, the ideal CED drug has not yet been found with the best therapeutic index. Due to CED’s heterogeneous pressure gradient, the concentration of drugs in the treated area is not uniform, resulting in an inhomogeneous distribution. There is also an ‘intrinsic’ backflow of solutes and air bubbles adjacent to the catheter due to catheter-induced tissue damage and reflux [38]. A new avenue of research involves MRI coupled CED. The utilization of an MRI is crucial to maintain visual confirmation and to avoid complications. Additionally, to prevent reflex, a device referred to as reflux-resistant infusion cannula ensures reflux induced tissue damage is mitigated [39].
3.6. Laser Interstitial Thermal Therapy (LITT)
Laser interstitial thermal therapy (LITT) utilizes photons for the ablation of tumors to induce subsequent necrosis. LITT can disrupt the impermeability of the BBB—allowing for the enhanced delivery and efficacy of drug therapies [40]. Reportedly, LITT can increase permeability by up to 30 days post-treatment. This increased permeability allows for molecules as large as immunoglobulins to cross the BBB [41]. Notably, LITT has successfully been used in conjunction with doxorubicin to treat glioblastomas [42]. Although LITT has demonstrated a positive impact against forms of metastasis, additional research needs to specifically delineate LITTs impact on additional tumor subtypes [40,43].
3.7. Nanoparticles
It is possible to deliver drugs efficiently using nanoparticles, which can be composed of lipids, polymers, or metallic particles. Nanoparticles can cross the BBB in various ways; through enhanced permeability and retention (EPR), endocytosis, and receptor-mediated transcytosis (Figure 3). Nanoparticles encapsulate drugs to increase the plasma half-life and to enable their entry into the brain parenchyma, as displayed in Figure 1 [44]. Several types of cancers were successfully treated with nanoparticles [45]. In EPR, the nanoparticle utilizes the leaky blood vessel of solid tumors, which can access the tumor locally [46]. Extravasation of the nanoparticle releases the encapsulated drugs into the body slowly. In most organs, nanoparticles cannot cross the normal vasculature, which reduces both peripheral and systemic toxicity [47,48]. Nanoparticles can traverse BBB leakages, making them a potential means of delivering drugs to brain tumors. Nevertheless, during clinical studies, nanoparticles could not reach tumors at therapeutic levels [49,50].
Figure 3. Enhanced permeability and receptor-mediated transcytosis allow nanoparticles to carry drugs with longer plasma half-lives into the brain parenchyma.
3.8. Intranasal Delivery
An alternative method of overcoming the BBB is intranasal delivery. The nasal cavity allows easy access without interference from the BBB to the brain parenchyma. Drugs are transported from the neuroepithelium of the nasal cavity to the central nervous system, paracellularly, transcellular, and neuronally. However, the use of intranasal medicines is not suitable for all drugs. The bioavailability of lipophilic drugs with a low molecular weight is generally more significant than hydrophilic drugs charged with a charge. For example, improving drug bioavailability with liposomes, cyclodextrins, and nanoparticles is possible. Furthermore, nose delivery of drugs avoids first-pass metabolism, thereby preserving their effectiveness [51].
Clinical trials using intranasal delivery of drugs have only yielded limited results. Malignant gliomas have been treated with perillyl alcohol intranasally. Perillyl alcohol administered four times daily resulted in 45% of cases surviving six months without progression [52]. Non-specificity of drugs can cause toxicity when delivered intranasally. Targeting tumor cells can minimize this toxicity [53]. It is also possible to reduce the toxicity in the surrounding brain tissue by using an intranasal drug delivery with microbubble mediated FUS. When these methods are combined, drug uptake in tumor regions is increased and targeted [54].
3.9. Intra-Arterial Delivery
Direct injection of drugs into an artery close to a tumor is referred to as intra-arterial drug delivery [55]. Drugs are released into blood vessels after cannulation in the targeted area (Figure 4). Additionally, hyperosmolar drugs, such as mannitol can be used to open the BBB on a local level [56]. However, the survival rate was not significantly improved in several clinical trials and cases. A group of patients with ependymoma were treated with cetuximab, carmustine, and bevacizumab intra-arterially, with the treatment being successful [57]. GBM patients were treated with intra-arterial drug delivery in several clinical studies. A combination of nimustine, bevacizumab, and carboplatin with other conventional chemotherapies resulted in survival rates ranging from 20 weeks to 10 months [58,59].
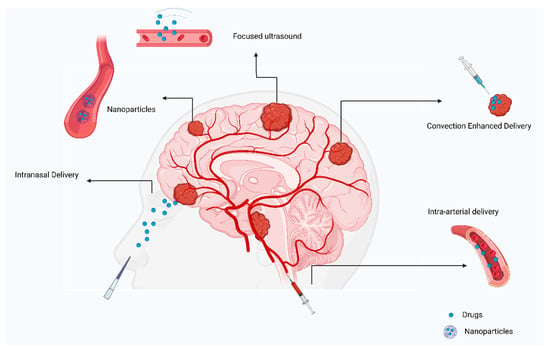
Figure 4. Review of current approaches to treating primary brain tumors.
This entry is adapted from the peer-reviewed paper 10.3390/curroncol29110696
This entry is offline, you can click here to edit this entry!
