Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
X-linked hypophosphatemia (XLH) is the most common genetic form of rickets and osteomalacia and is characterized by growth retardation, deformities of the lower limbs, and bone and muscular pain. Spontaneous dental abscesses caused by endodontic infections due to dentin dysplasia are well-known dental manifestations. When dentin affected by microcracks or attrition of the enamel is exposed to oral fluids, oral bacteria are able to invade the hypomineralized dentin and pulp space, leading to pulp necrosis, followed by the formation of a periapical gingival abscess.
- XLH
- dentin dysplasia
- oral management
1. Introduction
A spontaneous formation of a periapical gingival abscess or fistula around a visibly healthy tooth with no evidence of dental caries or trauma is a typical dental manifestation of X-linked hypophosphatemia (XLH) [1][2][3]. Owing to microcracks or attrition of the enamel, dentin is exposed to the bacteria abundant in the oral cavity, which invade the hypomineralized dentin and pulp space leading to pulp necrosis and periapical gingival abscess formation [1][2][3].
2. Oral Management of X-Linked Hypophosphatemia
There is no fundamental treatment for dentin dysplasia in XLH. Early detection and management of pulp infection improve the prognosis of the tooth [2][3]. Short-term periodical dental check-ups are required for XLH patients [4][5][6][7]. The consensus statement of XLH recommends twice-yearly dentist visits [8]. The principle of dental management in XLH patients is to preserve pulp vitality [2]. Professional tooth cleaning, application of topical fluoride, and fissure sealing are recommended [2][4][5][9][10]. Pit and fissure sealing of the enamel is effective in preventing the invasion of oral bacteria [2][3][4][5][9]. Exposed dentin due to attrition or cracking of the enamel should be repaired as soon as possible [11]. The bonding strength of adhesive composite restorations is assumed to be reduced due to mineralization defects in the dentin. Prolonged etching times or a total etch system increases the risk of pulp irritation; therefore, a self-etch system is recommended [5][9].
The crucial purpose of oral management of XLH is to protect vital pulp from being infected by oral bacteria. The vitality of the tooth should be carefully monitored [2]. Early coronal restoration of teeth at high risk is strongly recommended, especially in patients who are diagnosed early with many abscesses [12]. Composite resin crowns in the anterior region, stainless steel crowns for primary teeth and immature permanent teeth, and permanent crowns for permanent teeth in the posterior region are recommended [12][13][14]. Full ceramic crowns should be avoided because of the extensive tooth reduction required when compared with metal crowns [2]. Large pulp chambers and prominent pulp horns should be considered during the preparation of the tooth to prevent exposure or irritation of the pulp [2][9].
When apical periodontitis is detected, a periapical radiograph is taken, and the dentist must decide whether to perform endodontic treatment or extract the tooth. Systemic antibiotics are used in cases of acute abscess [3]. Primary teeth play an important role as space maintainers for permanent successors, and dentists should preserve them for as long as possible until replacement [15]. Early extraction before replacement leads to loss of space for the eruption of permanent teeth [15]. Space maintenance is necessary after the extraction of primary teeth before replacement [15]. Obturation of the root canal system in XLH patients should aim to fill any voids to achieve maximal density, due to the increased risk of reinfection of the root canal because of dentin dysplasia [2]. The use of thermoplasticized techniques using a virtually insoluble sealer is recommended for permanent teeth [2]. Working length should be determined accurately, taking into account the short roots commonly found in XLH patients [2]. In contrast, for primary teeth, the use of calcium hydroxide and iodoform (Ca(OH)2/iodoform), which are absorbed during root resorption, is recommended [16]. Additionally, for permanent teeth undergoing root formation, Ca(OH)2 or mineral trioxide aggregate (MTA) is recommended before obturation to promote apexification [2][17][18].
Once the pulp starts to become necrotic, the blood supply stops, and the devitalized tooth tends to break down [19]. Teeth with broken roots are indicated for extraction [20]. Prosthetic crowns are necessary for teeth that have undergone root canal treatment (Figure 1). The thin dentin perforates easily and does not support restorative posts for prosthetic crowns in permanent teeth [2][9]. The application of posts in the roots should be avoided to prevent root fracture [2][9].
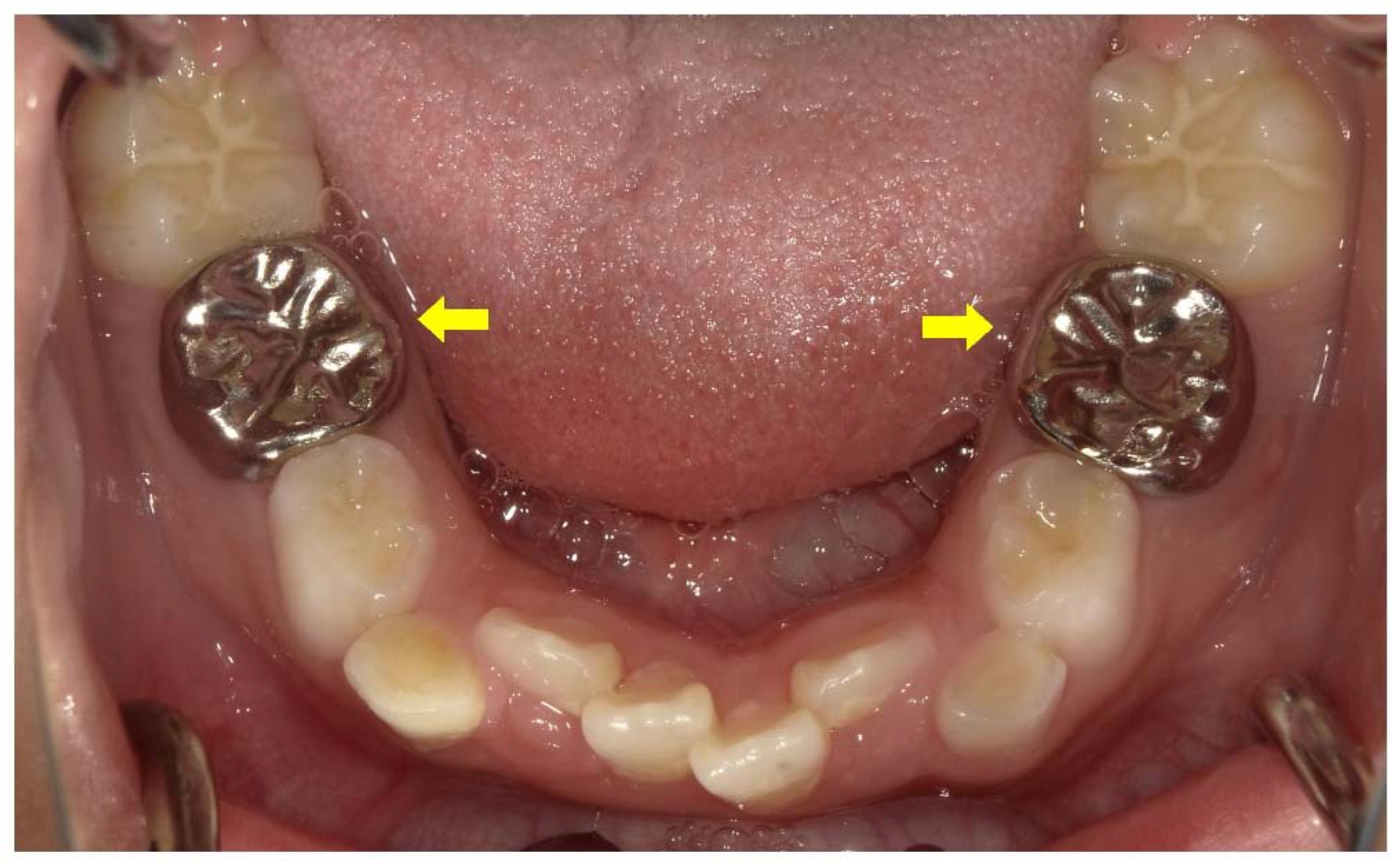
Figure 1. Treatment of a male X-linked hypophosphatemia (XLH) patient aged 8 years 3 months involved full coverage of the primary mandibular second molars with stainless steel crowns (arrows) after root canal treatment associated with a cystic swelling.
There is no established orthodontic treatment for XLH patients [21]. Traumatic orthodontic forces sometimes cause pulp necrosis [6]. It is important to prevent traumatic forces during orthodontic treatment of XLH patients. Orthodontic treatment involves the movement of teeth and extensive remodeling of the alveolar bone [3]. This treatment sometimes results in the loss of permanent teeth in XLH patients with uncontrolled rickets of the jaw [22]. Optimizing conventional medical treatment of XLH is considered mandatory before the initiation of orthodontic treatment [3][8]. A longer period of retention and observation is necessary in cases with abnormal bone remodeling to confirm the stability of the resulting occlusion [23]. The high frequency of permanent tooth loss secondary to endodontic infections or periodontitis often leads to the need for dental implants [8]. Several reports have described cases of XLH patients who have had dental implants [6][24]. Standard surgical protocols in adults with XLH who are not receiving conventional therapy resulted in a decreased success rate compared with healthy control individuals [8]. Some studies reported that the interruption of conventional therapy in XLH may have a negative influence on bone healing around implants [3]. Dental implant surgery should be performed after 3–6 months of medical treatment, which should be continued for 6 months following the implant surgery [8]. The healing time should be extended up to 6 months [8].
3. Dental Effects of Conventional Therapy or Burosumab in X-Linked Hypophosphatemia
The conventional medical therapy of XLH has consisted of oral phosphate and active vitamin D supplementation [25][26]. However, this therapy has certain limitations related to efficacy and safety [25]. A humanized monoclonal antibody for FGF23 (burosumab) was recently approved as a promising treatment for XLH [25][26][27].
Early intervention with conventional therapy is reported to have a beneficial effect on dental status [28][29][30][31][32][33][34]. The missing and filled teeth index of patients treated since early childhood is similar to that of the healthy, age-matched controls [30]. This therapy improves dentin mineralization and formation, reducing the size of the dental pulp canal and chamber in both the primary and permanent dentitions [3][30]. Dentin mineralization of permanent teeth especially, which mineralize after birth, can be restored by the treatment [33]. The primary dentition usually shows more severe symptoms than the permanent dentition [32]. This can also be explained by the low levels of calcium and phosphorus during odontogenesis of the primary dentition [32]. However, this therapy cannot completely eliminate dentin dysplasia [35]. Remaining defects may result from the early exposure of odontoblasts and the surrounding osteoblasts to hypophosphatemia before the commencement of conventional therapy, and from intrinsic cell disturbances linked to the genetic alteration [30]. Additionally, unlike bone, dentin is not remodeled and is not involved in the regulation of calcium and phosphate metabolism [36]. The effects of burosumab on the dentition of XLH patients has not yet been reported. A post hoc analysis of a 64-week, open-label, randomized controlled study of 61 children with XLH aged 1–12 years revealed that dental abscesses occurred in 3 of 12 (25%) younger (<5 years) children in the conventional therapy group, while 0 of 20 (0%) younger children from the burosumab group developed dental abscesses [37]. However, in older children (5–12 years) with XLH, dental abscesses presented more frequently with burosumab (8/15, 53%) than with conventional therapy (0/20, 0%). Dental caries, which were reported more frequently in the burosumab group (9/29, 31%) than the conventional therapy group (2/32, 6%), occurred slightly more often in older than younger children who received conventional therapy (2/20, 10% vs. 0/12, 0%), and slightly more often in younger than older children who received burosumab (5/14, 36% vs. 4/15, 27%). On the basis of the results of this study, the protective effects of burosumab seem to be weaker, or at least not more intense, against the development of dental abscesses compared with conventional therapy; however, a longer duration study is needed.
4. Importance of Medical and Dental Collaboration in X-Linked Hypophosphatemia
Without appropriate dental management, spontaneous periapical gingival abscess formation in XLH patients finally leads to early loss of teeth and a reduced quality of life [38][39]. Early oral management soon after diagnosis and follow-up throughout life by dentists are recommended for XLH patients [4][5][6][7]. There is a need for a system in which medical doctors explain the importance of oral care to parents of children with XLH and ensure they find appropriate dental care [8]. The alveolar bone status is particularly important when XLH patients receive orthodontic treatment or dental implants [8]. Dentists should consult with the patient’s medical doctor about the status of rickets control with medical treatment [3][8].
Primary incisors emerge into the oral cavity at around 6 months of age, and the primary dentition is complete by the age of 2 years [40]. Most XLH patients are diagnosed at approximately 1–2 years of age when their delayed walking or bowed legs are observed by pediatricians [41][42]. Spontaneous periapical abscesses sometimes lead to an XLH diagnosis [9][11][43]. Pediatric dentists must never overlook dental abscesses in teeth that appear to be intact. A system should be established by which dentists can immediately refer patients to pediatricians when this first dental sign of XLH is observed.
This entry is adapted from the peer-reviewed paper 10.3390/endocrines3040056
References
- Goodman, J.R.; Gelbier, M.J.; Bennett, J.H.; Winter, G.B. Dental problems associated with hypophosphataemic vitamin D resistant rickets. Int. J. Paediatr. Dent. 1998, 8, 19–28.
- Sabandal, M.M.; Robotta, P.; Bürklein, S.; Schäfer, E. Review of the dental implications of X-linked hypophosphataemic rickets (XLHR). Clin. Oral Investig. 2015, 19, 759–768.
- Duplan, M.B.; Norcy, E.L.; Courson, F.; Chaussain, C. Dental and periodontal features and management in XLH children and adults. Int. J. Bone Frag. 2021, 1, 74–79.
- Baroncelli, G.I.; Angiolini, M.; Ninni, E.; Galli, V.; Saggese, R.; Giuca, M.R. Prevalence and pathogenesis of dental and periodontal lesions in children with X-linked hypophosphatemic rickets. Eur. J. Paediatr. Dent. 2006, 7, 61–66.
- Douyere, D.; Joseph, C.; Gaucher, C.; Chaussain, C.; Courson, F. Familial hypophosphatemic vitamin D-resistant rickets—Prevention of spontaneous dental abscesses on primary teeth: A case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, 525–530.
- Lee, B.N.; Jung, H.Y.; Chang, H.S.; Hwang, Y.C.; Hwang, I.N.; Oh, W.M. Dental management of patients with X-linked hypophosphatemia. Restor. Dent. Endod. 2017, 42, 146–151.
- Akif, D.; Tuba, A.A.; Esra, E.; Tulga, Ö.F. Dental Management of Hypophosphatemic Vitamin D Resistant Rickets. J. Pediatr. Res. 2018, 5, 221–224.
- Haffner, D.; Emma, F.; Eastwood, D.M.; Duplan, M.B.; Bacchetta, J.; Schnabel, D.; Wicart, P.; Bockenhauer, D.; Santos, F.; Levtchenko, E.; et al. Clinical practice recommendations for the diagnosis and management of X-linked hypophosphataemia. Nat. Rev. Nephrol. 2019, 15, 435–455.
- Batra, P.; Tejani, Z.; Mars, M. X-linked hypophosphatemia: Dental and histologic findings. J. Can. Dent. Assoc. 2006, 72, 69–72.
- Marin, A.; Morales, P.; Jiménez, M.; Borja, E.; Ivanovic-Zuvic, D.; Collins, M.T.; Florenzano, P. Characterization of Oral Health Status in Chilean Patients with X-Linked Hypophosphatemia. Calcif. Tissue Int. 2021, 109, 132–138.
- Wato, K.; Okawa, R.; Matayoshi, S.; Ogaya, Y.; Nomura, R.; Nakano, K. X-linked hypophosphatemia diagnosed after identification of dental symptoms. Ped. Dent. J. 2020, 30, 115–119.
- Seow, W.K.; Romaniuk, K.; Sclavos, S. Micromorphologic features of dentin in vitamin D-resistant rickets: Correlation with clinical grading of severity. Pediatr. Dent. 1989, 11, 203–208.
- Breen, G.H. Prophylactic dental treatment for a patient with vitamin D-resistant rickets: Report of case. ASDC J. Dent. Child. 1986, 53, 38–43.
- Seow, W.K.; Latham, S.C. The spectrum of dental manifestations in vitamin D-resistant rickets: Implications for management. Pediatr. Dent. 1986, 8, 245–250.
- Laing, E.; Ashley, P.; Farhad, B.N.; Dalgit, S.G. Space maintenance. Int. J. Pediatr. Dent. 2009, 19, 155–162.
- Mortazavi, M.; Mesbahi, M. Comparison of zinc oxide and eugenol, and Vitapex for root canal treatment of necrotic primary teeth. Int. J. Pediatr. Dent. 2004, 14, 417–424.
- Lee, J.S. Ca(OH)2 apexification of pulp necroses of the permanent incisors in a case of X-linked hypophosphataemic rickets—The 60-month check-up: A case report. Ped. Dent. J. 2021, 31, 112–116.
- Bradley, H.; Dutta, A.; Philpott, R. Presentation and non-surgical endodontic treatment of two patients with X-linked hypophosphatemia: A case report. Int. Endod. J. 2021, 54, 1403–1414.
- Rosen, E.; Beitlitum, I.; Tsesis, I. The preservation of teeth with root-originated fractures. Evid. Based Endod. 2018, 3, 2.
- Yoshino, K.; Ito, K.; Kuroda, M.; Sugihara, N. Prevalence of vertical root fracture as the reason for tooth extraction in dental clinics. Clin. Oral Investig. 2015, 19, 1405–1409.
- Makrygiannakis, M.A.; Dastoori, M.; Athanasiou, A.E. Orthodontic treatment of a nine-year-old patient with hypophosphatemic rickets diagnosed since the age of two: A case report. Int. Orthod. 2020, 18, 648–656.
- Gibson, C.; Mubeen, S.; Evans, R. X-linked hypophosphatemic rickets: Orthodontic considerations and management. A case report. J. Orthod. 2022, 49, 205–212.
- Kawakami, M.; Takano-Yamamoto, T. Orthodontic treatment of a patient with hypophosphatemic vitamin D-resistant rickets. ASDC J. Dent. Child. 1997, 64, 395–399.
- Resnick, D. Implant placement and guided tissue regeneration in a patient with congenital vitamin D-resistant rickets. J. Oral Implantol. 1998, 24, 214–218.
- Kinoshita, Y.; Fukumoto, S. X-Linked Hypophosphatemia and FGF23-Related Hypophosphatemic Diseases: Prospect for New Treatment. Endocr. Rev. 2018, 39, 274–291.
- Tajima, T.; Hasegawa, Y. Treatment of X-Linked Hypophosphatemia in Children. Endocrines 2022, 3, 522–529.
- Fukumoto, S. FGF23-related hypophosphatemic rickets/osteomalacia: Diagnosis and new treatment. J. Mol. Endocrinol. 2021, 66, R57–R65.
- Larmas, M.; Hietala, E.L.; Similä, S.; Pajari, U. Oral manifestations of familial hypophosphatemic rickets after phosphate supplement therapy: A review of the literature and report of case. ASDC J. Dent. Child. 1991, 58, 328–334.
- Seow, W.K. The effect of medical therapy on dentin formation in vitamin D-resistant rickets. Pediatr. Dent. 1991, 13, 97–102.
- Chaussain-Miller, C.; Sinding, C.; Wolikow, M.; Lasfargues, J.J.; Godeau, G.; Garabédian, M. Dental abnormalities in patients with familial hypophosphatemic vitamin D-resistant rickets: Prevention by early treatment with 1-hydroxyvitamin D. J. Pediatr. 2003, 142, 324–331.
- Chaussain-Miller, C.; Sinding, C.; Septier, D.; Wolikow, M.; Goldberg, M.; Garabedian, M. Dentin structure in familial hypophosphatemic rickets: Benefits of vitamin D and phosphate treatment. Oral Dis. 2007, 13, 482–489.
- Beltes, C.; Zachou, E. Endodontic management in a patient with vitamin D-resistant Rickets. J. Endod. 2012, 38, 255–258.
- Linglart, A.; Biosse-Duplan, M.; Briot, K.; Chaussain, C.; Esterle, L.; Guillaume-Czitrom, S.; Kamenicky, P.; Nevoux, J.; Prié, D.; Rothenbuhler, A.; et al. Therapeutic management of hypophosphatemic rickets from infancy to adulthood. Endocr. Connect. 2014, 3, R13–R30.
- Econs, M.J. Conventional Therapy in Adults With XLH Improves Dental Manifestations, But Not Enthesopathy. J. Clin. Endocrinol. Metab. 2015, 100, 3622–3624.
- Okawa, R.; Hamada, M.; Takagi, M.; Matayoshi, S.; Nakano, K. A Case of X-Linked Hypophosphatemic Rickets with Dentin Dysplasia in Mandibular Third Molars. Children 2022, 9, 1304.
- Vital, S.O.; Gaucher, C.; Bardet, C.; Rowe, P.S.; George, A.; Linglart, A.; Chaussain, C. Tooth dentin defects reflect genetic disorders affecting bone mineralization. Bone 2012, 50, 989–997.
- Ward, L.M.; Glorieux, F.H.; Whyte, M.P.; Munns, C.F.; Portale, A.A.; Högler, W.; Simmons, J.H.; Gottesman, G.S.; Padidela, R.; Namba, N.; et al. Effect of Burosumab Compared With Conventional Therapy on Younger vs Older Children With X-linked Hypophosphatemia. J. Clin. Endocrinol. Metab. 2022, 107, e3241–e3253.
- Nguyen, C.; Celestin, E.; Chambolle, D.; Linglart, A.; Biosse Duplan, M.; Chaussain, C.; Friedlander, L. Oral health-related quality of life in patients with X-linked hypophosphatemia: A qualitative exploration. Endocr. Connect. 2022, 11, e210564.
- Baroncelli, G.I.; Zampollo, E.; Manca, M.; Toschi, B.; Bertelloni, S.; Michelucci, A.; Isola, A.; Bulleri, A.; Peroni, D.; Giuca, M.R. Pulp chamber features, prevalence of abscesses, disease severity, and PHEX mutation in X-linked hypophosphatemic rickets. J. Bone Miner. Metab. 2021, 39, 212–223.
- Schour, I.; Massler, M. The development of the human dentition. J. Am. Dent. Assoc. 1941, 28, 1153–1160.
- Dahir, K.; Roberts, M.S.; Krolczyk, S.; Simmons, J.H. X-Linked Hypophosphatemia: A New Era in Management. J. Endocr. Soc. 2020, 4, bvaa151.
- Petje, G.; Meizer, R.; Radler, C.; Aigner, N.; Grill, F. Deformity correction in children with hereditary hypophosphatemic rickets. Clin. Orthop. Relat. Res. 2008, 466, 3078–3085.
- Archard, H.O.; Witkop, C.J. Hereditary hypophosphatemia (vitamin D-resistant rickets) presenting primary dental manifestations. Oral Surg. 1966, 22, 184–193.
This entry is offline, you can click here to edit this entry!
