Post-transition metals are a set of metallic elements in the periodic table located between the transition metals to their left, and the metalloids to their right. Depending on where these adjacent groups are judged to begin and end, there are at least five competing proposals for which elements to include: the three most common contain six, ten and thirteen elements, respectively (see image). All proposals include gallium, indium, tin, thallium, lead, and bismuth. Physically, post-transition metals are soft (or brittle), have poor mechanical strength, and have melting points lower than those of the transition metals. Being close to the metal-nonmetal border, their crystalline structures tend to show covalent or directional bonding effects, having generally greater complexity or fewer nearest neighbours than other metallic elements. Chemically, they are characterised—to varying degrees—by covalent bonding tendencies, acid-base amphoterism and the formation of anionic species such as aluminates, stannates, and bismuthates (in the case of aluminium, tin, and bismuth, respectively). They can also form Zintl phases (half-metallic compounds formed between highly electropositive metals and moderately electronegative metals or metalloids). The name is universally used, but not officially sanctioned by any organization such as the IUPAC. The origin of the term is unclear: one early use was in 1940 in a chemistry text. Alternate names for this group are B-subgroup metals, other metals, and p-block metals; and at least thirteen other labels.
- aluminates
- metalloids
- metallic elements
1. Applicable Elements
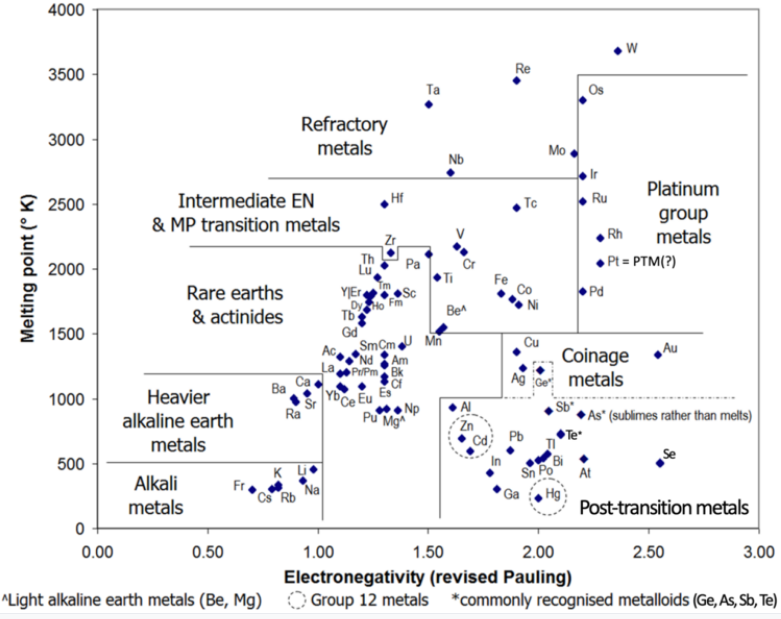
Usually included in this category are the group 13–15 metals in periods 4–6: gallium, indium and thallium; tin and lead; and bismuth. Other elements sometimes included are the group 11 metals copper, silver and gold (which are usually considered to be transition metals); the group 12 metals zinc, cadmium and mercury (which are otherwise considered to be transition metals); and aluminium, germanium, arsenic, selenium, antimony, tellurium, and polonium (of which germanium, arsenic, antimony, and tellurium are usually considered to be metalloids). Astatine, which is usually classified as a nonmetal or a metalloid, has been predicted to have a metallic crystalline structure. If so, it would be a post-transition metal. Elements 112–118 (copernicium, nihonium, flerovium, moscovium, livermorium, tennessine, and oganesson) may be post-transition metals; insufficient quantities of them have been synthesized to allow sufficient investigation of their actual physical and chemical properties.
Which elements start to be counted as post-transition metals depends, in periodic table terms, on where the transition metals are taken to end.[4] In the 1950s, most inorganic chemistry textbooks defined transition elements as finishing at group 10 (nickel, palladium and platinum), therefore excluding group 11 (copper, silver and gold), and group 12 (zinc, cadmium and mercury). A survey of chemistry books in 2003 showed that the transition metals ended at either group 11 or group 12 with roughly equal frequency.[5] Where the post-transition metals end depends on where the metalloids or nonmetals start. Boron, silicon, germanium, arsenic, antimony and tellurium are commonly recognised as metalloids; other authors treat some or all of these elements as nonmetals.
2. Rationale
The diminished metallic nature of the post-transition metals is largely attributable to the increase in nuclear charge going across the periodic table, from left to right.[6] The increase in nuclear charge is partially offset by an increasing number of electrons but as these are spatially distributed each extra electron does not fully screen each successive increase in nuclear charge, and the latter therefore dominates.[7] With some irregularities, atomic radii contract, ionisation energies increase,[6] fewer electrons become available for metallic bonding,[8] and "ions [become] smaller and more polarizing and more prone to covalency."[9] This phenomenon is more evident in period 4–6 post-transition metals, due to inefficient screening of their nuclear charges by their d10 and (in the case of the period 6 metals) f14 electron configurations;[10] the screening power of electrons decreases in the sequence s > p > d > f. The reductions in atomic size due to the interjection of the d- and f-blocks are referred to as, respectively, the 'scandide' or 'd-block contraction',[11] and the 'lanthanide contraction'.[12] Relativistic effects also "increase the binding energy", and hence ionisation energy, of the electrons in "the 6s shell in gold and mercury, and the 6p shell in subsequent elements of period 6."[13]
3. Nomenclature
The origin of the term post-transition metal is unclear. An early usage is recorded by Deming, in 1940, in his well-known[14] book Fundamental Chemistry.[15] He treated the transition metals as finishing at group 10 (nickel, palladium and platinum). He referred to the ensuing elements in periods 4 to 6 of the periodic table (copper to germanium; silver to antimony; gold to polonium)—in view of their underlying d10 electronic configurations—as post-transition metals.
4. Descriptive Chemistry
- This section outlines relevant physical and chemical properties of the elements typically or sometimes classified as post-transition metals. For complete profiles, including history, production, specific uses, and biological roles and precautions, see the main article for each element. Abbreviations: MH—Mohs hardness; BCN—bulk coordination number.[16]
4.1. Group 11
The group 11 metals are typically categorised as transition metals given they can form ions with incomplete d-shells. Physically, they have the relatively low melting points and high electronegativity values associated with post-transition metals. "The filled d subshell and free s electron of Cu, Ag, and Au contribute to their high electrical and thermal conductivity. Transition metals to the left of group 11 experience interactions between s electrons and the partially filled d subshell that lower electron mobility."[17] Chemically, the group 11 metals in their +1 valence states show similarities to other post-transition metals;[18] they are occasionally classified as such.[19]



Copper is a soft metal (MH 2.5–3.0)[20] with low mechanical strength.[21] It has a close-packed face-centred cubic structure (BCN 12).[22] Copper behaves like a transition metal in its preferred oxidation state of +2. Stable compounds in which copper is in its less preferred oxidation state of +1 (Cu2O, CuCl, CuBr, CuI and CuCN, for example) have significant covalent character.[23] The oxide (CuO) is amphoteric, with predominating basic properties; it can be fused with alkali oxides (M2O; M = Na, K) to give anionic oxycuprates (M2CuO2).[24] Copper forms Zintl phases such as Li7CuSi2[25] and M3Cu3Sb4 (M = Y, La, Ce, Pr, Nd, Sm, Gd, Tb, Dy, Ho, or Er).[26]
Silver is a soft metal (MH 2.5–3)[27] with low mechanical strength.[28] It has a close-packed face-centred cubic structure (BCN 12).[29] The chemistry of silver is dominated by its +1 valence state in which it shows generally similar physical and chemical properties to compounds of thallium, a main group metal, in the same oxidation state.[30] It tends to bond covalently in most of its compounds.[31] The oxide (Ag2O) is amphoteric, with basic properties predominating.[32] Silver forms a series of oxoargentates (M3AgO2, M = Na, K, Rb).[33] It is a constituent of Zintl phases such as Li2AgM (M = Al, Ga, In, Tl, Si, Ge, Sn or Pb)[34] and Yb3Ag2.[35]
Gold is a soft metal (MH 2.5–3)[36] that is easily deformed.[37] It has a close-packed face-centred cubic structure (BCN 12).[29] The chemistry of gold is dominated by its +3 valence state; all such compounds of gold feature covalent bonding,[38] as do its stable +1 compounds.[39] Gold oxide (Au2O3) is amphoteric, with acidic properties predominating; it forms anionic hydroxoaurates M[Au(OH)4] where M = Na, K, 1⁄2Ba, Tl; and aurates such as NaAuO2.[40] Gold is a constituent of Zintl phases such as M2AuBi (M = Li or Na);[41] Li2AuM (M = In, Tl, Ge, Pb, Sn)[42] and Ca5Au4.[35]
Roentgenium is expected to be similar to its lighter homologue gold in many ways. It is expected to have a close-packed body-centered cubic structure. It should be a very dense metal, with its density of 28.7 g/cm3 surpassing all known stable elements. Roentgenium chemistry is expected to be dominated by the +3 valence state, similarly to gold, in which it should similarly behave as a transition metal. Roentgenium oxide (Rg2O3) should be amphoteric; stable compounds in the −1, +1, and +5 valence states should also exist, exactly analogous to gold. Roentgenium is similarly expected to be a very noble metal: the standard reduction potential for the Rg3+/Rg couple is expected to be +1.9 V, more than the +1.52 V for the Au3+/Au couple that is the highest among the stable metals; this is exceeded only by darmstadtium, roentgenium, and copernicium among the superheavy metals. The [Rg(H2O)2]+ cation is expected to be the softest among the metal cations. Due to relativistic stabilisation of the 7s subshell, roentgenium is expected to have a full s-subshell and a partially filled d-subshell, instead of the free s-electron and full d-subshell of copper, silver, and gold.
4.2. Group 12
On the group 12 metals (zinc, cadmium and mercury), Smith[43] observed that, "Textbook writers have always found difficulty in dealing with these elements." There is an abrupt and significant reduction in physical metallic character from group 11 to group 12.[44] Their chemistry is that of main group elements.[45] A 2003 survey of chemistry books showed that they were treated as either transition metals or main group elements on about a 50/50 basis.[5][46] The IUPAC Red Book notes that although the group 3−12 elements are commonly referred to as the transition elements, the group 12 elements are not always included.[47] The group 12 elements do not satisfy the IUPAC Gold Book definition of a transition metal.[48][49]



Zinc is a soft metal (MH 2.5) with poor mechanical properties.[50] It has a crystalline structure (BCN 6+6) that is slightly distorted from the ideal. Many zinc compounds are markedly covalent in character.[51] The oxide and hydroxide of zinc in its preferred oxidation state of +2, namely ZnO and Zn(OH)2, are amphoteric;[52] it forms anionic zincates in strongly basic solutions.[53] Zinc forms Zintl phases such as LiZn, NaZn13 and BaZn13.[54] Highly purified zinc, at room temperature, is ductile.[55] It reacts with moist air to form a thin layer of carbonate that prevents further corrosion.[56]
Cadmium is a soft, ductile metal (MH 2.0) that undergoes substantial deformation, under load, at room temperature.[57] Like zinc, it has a crystalline structure (BCN 6+6) that is slightly distorted from the ideal. The halides of cadmium, with the exception of the fluoride, exhibit a substantially covalent nature.[58] The oxides of cadmium in its preferred oxidation state of +2, namely CdO and Cd(OH)2, are weakly amphoteric; it forms cadmates in strongly basic solutions.[59] Cadmium forms Zintl phases such as LiCd, RbCd13 and CsCd13.[54] When heated in air to a few hundred degrees, cadmium represents a toxicity hazard due to the release of cadmium vapour; when heated to its boiling point in air (just above 1000 K; 725 C; 1340 F; cf steel ~2700 K; 2425 C; 4400 F),[60] the cadmium vapour oxidizes, 'with a reddish-yellow flame, dispersing as an aerosol of potentially lethal CdO particles.'[57] Cadmium is otherwise stable in air and in water, at ambient conditions, protected by a layer of cadmium oxide.
Mercury is a liquid at room temperature. It has the weakest metallic bonding of all, as indicated by its bonding energy (61 kJ/mol) and melting point (−39 °C) which, together, are the lowest of all the metallic elements.[61][62] Solid mercury (MH 1.5)[63] has a distorted crystalline structure,[64] with mixed metallic-covalent bonding,[65] and a BCN of 6. "All of the [Group 12] metals, but especially mercury, tend to form covalent rather than ionic compounds."[66] The oxide of mercury in its preferred oxidation state (HgO; +2) is weakly amphoteric, as is the congener sulfide HgS.[67] It forms anionic thiomercurates (such as Na2HgS2 and BaHgS3) in strongly basic solutions.[68][69] It forms or is a part of Zintl phases such as NaHg and K8In10Hg.[70] Mercury is a relatively inert metal, showing little oxide formation at room temperature.[71]
Copernicium is expected to be a liquid at room temperature, although experiments have so far not succeeded in determining its boiling point with sufficient precision to prove this. Like its lighter congener mercury, many of its singular properties stem from its closed-shell d10s2 electron configuration as well as strong relativistic effects. Its cohesive energy is even less than that of mercury and is likely only higher than that of flerovium. Solid copernicium is expected to crystallise in a close-packed body-centred cubic structure and have a density of about 14.7 g/cm3, decreasing to 14.0 g/cm3 on melting, which is similar to that of mercury (13.534 g/cm3). Copernicium chemistry is expected to be dominated by the +2 oxidation state, in which it would behave like a post-transition metal similar to mercury, although the relativistic stabilisation of the 7s orbitals means that this oxidation state involves giving up 6d rather than 7s electrons. A concurrent relativistic destabilisation of the 6d orbitals should allow higher oxidation states such as +3 and +4 with electronegative ligands, such as the halogens. Copernicium should be the most noble metal in the periodic system, with a very high standard reduction potential of +2.1 V expected for the Cn2+/Cn couple. In fact, bulk copernicium may even be an insulator with a band gap of 6.4±0.2 V, which would make it similar to the noble gases such as radon, though copernicium has previously been predicted to be a semiconductor or a noble metal instead. Copernicium oxide (CnO) is expected to be predominantly basic.
4.3. Group 13




Aluminium sometimes is[72] or is not[73] counted as a post-transition metal. It has a well shielded [Ne] noble gas core rather than the less well shielded [Ar]3d10, [Kr]4d10 or [Xe]4f145d10 core of the post-transition metals. The small radius of the aluminium ion combined with its high charge make it a strongly polarizing species, prone to covalency.[74]
Aluminium in pure form is a soft metal (MH 3.0) with low mechanical strength.[75] It has a close-packed structure (BCN 12) showing some evidence of partially directional bonding.[76][77] It has a low melting point and a high thermal conductivity. Its strength is halved at 200 °C, and for many of its alloys is minimal at 300 °C.[78] The latter three properties of aluminium limit its use to situations where fire protection is not required,[79] or necessitate the provision of increased fire protection.[80][81] It bonds covalently in most of its compounds;[82] has an amphoteric oxide; and can form anionic aluminates.[53] Aluminium forms Zintl phases such as LiAl, Ca3Al2Sb6, and SrAl2.[83] A thin protective layer of oxide confers a reasonable degree of corrosion resistance.[84] It is susceptible to attack in low pH (<4) and high (> 8.5) pH conditions,[85][86] a phenomenon that is generally more pronounced in the case of commercial purity aluminium and aluminium alloys.[87] Given many of these properties and its proximity to the dividing line between metals and nonmetals, aluminium is occasionally classified as a metalloid.[88] Despite its shortcomings, it has a good strength-to-weight ratio and excellent ductility; its mechanical strength can be improved considerably with the use of alloying additives; its very high thermal conductivity can be put to good use in heat sinks and heat exchangers;[89] and it has a high electrical conductivity.[90] At lower temperatures, aluminium increases its deformation strength (as do most materials) whilst maintaining ductility (as do face-centred cubic metals generally).[91] Chemically, bulk aluminium is a strongly electropositive metal, with a high negative electrode potential.[92][93]
Gallium is a soft, brittle metal (MH 1.5) that melts at only a few degrees above room temperature.[94] It has an unusual crystalline structure featuring mixed metallic-covalent bonding and low symmetry[94] (BCN 7 i.e. 1+2+2+2).[95] It bonds covalently in most of its compounds,[96] has an amphoteric oxide;[97] and can form anionic gallates.[53] Gallium forms Zintl phases such as Li2Ga7, K3Ga13 and YbGa2.[98] It is slowly oxidized in moist air at ambient conditions; a protective film of oxide prevents further corrosion.[99]
Indium is a soft, highly ductile metal (MH 1.0) with a low tensile strength.[100][101] It has a partially distorted crystalline structure (BCN 4+8) associated with incompletely ionised atoms.[102] The tendency of indium '...to form covalent compounds is one of the more important properties influencing its electrochemical behavior'.[103] The oxides of indium in its preferred oxidation state of +3, namely In2O3 and In(OH)3 are weakly amphoteric; it forms anionic indates in strongly basic solutions.[104] Indium forms Zintl phases such as LiIn, Na2In and Rb2In3.[105] Indium does not oxidize in air at ambient conditions.[101]
Thallium is a soft, reactive metal (MH 1.0), so much so that it has no structural uses.[106] It has a close-packed crystalline structure (BCN 6+6) but an abnormally large interatomic distance that has been attributed to partial ionisation of the thallium atoms.[107] Although compounds in the +1 (mostly ionic) oxidation state are the more numerous, thallium has an appreciable chemistry in the +3 (largely covalent) oxidation state, as seen in its chalcogenides and trihalides.[108] It is the only one of the Group 13 elements to react with air at room temperature, slowly forming the amphoteric oxide Tl2O3.[109][110][111] It forms anionic thallates such as Tl3TlO3, Na3Tl(OH)6, NaTlO2, and KTlO2,[110] and is present as the Tl− thallide anion in the compound CsTl.[112] Thallium forms Zintl phases, such as Na2Tl, Na2K21Tl19, CsTl and Sr5Tl3H.[113]
Nihonium is expected to have a hexagonal close-packed crystalline structure, albeit based on extrapolation from those of the lighter group 13 elements: its density is expected to be around 16 g/cm3. With a standard electrode potential of +0.6 V for the Nh+/Nh couple, it should be between copper and silver in inertness. The relativistic stabilisation of the 7s electrons is very high and hence nihonium should predominantly form the +1 oxidation state; nevertheless, as for copernicium, the +3 oxidation state should be reachable with highly electronegative ligands, with NhF−4 likely being of similar stability to AgF−4 (which is a strong oxidising agent, fuming in moist air and reacting with glass). Because of the shell closure at flerovium caused by spin-orbit coupling, nihonium is also one 7p electron short of a closed shell and would hence form a −1 oxidation state; in both the +1 and −1 oxidation states, nihonium should show more similarities to astatine than thallium. The Nh+ ion is expected to also have some similarities to the Ag+ ion, particularly in its propensity for complexation. Nihonium oxide (Nh2O) is expected to be amphoteric.
4.4. Group 14



Germanium is a hard (MH 6), very brittle semi-metallic element.[114] It was originally thought to be a poorly conducting metal[115] but has the electronic band structure of a semiconductor.[116] Germanium is usually considered to be a metalloid rather than a metal.[117] Like carbon (as diamond) and silicon, it has a covalent tetrahedral crystalline structure (BCN 4).[118] Compounds in its preferred oxidation state of +4 are covalent.[119] Germanium forms an amphoteric oxide, GeO2[120] and anionic germanates, such as Mg2GeO4.[121] It forms Zintl phases such as LiGe, K8Ge44 and La4Ge3.[122]
Tin is a soft, exceptionally[123] weak metal (MH 1.5);[124] a 1-cm thick rod will bend easily under mild finger pressure.[123] It has an irregularly coordinated crystalline structure (BCN 4+2) associated with incompletely ionised atoms.[102] All of the Group 14 elements form compounds in which they are in the +4, predominantly covalent, oxidation state; even in the +2 oxidation state tin generally forms covalent bonds.[125] The oxides of tin in its preferred oxidation state of +2, namely SnO and Sn(OH)2, are amphoteric;[126] it forms stannites in strongly basic solutions.[53] Below 13 °C (55.4 °F) tin changes its structure and becomes 'grey tin', which has the same structure as diamond, silicon and germanium (BCN 4). This transformation causes ordinary tin to crumble and disintegrate since, as well as being brittle, grey tin occupies more volume due to having a less efficient crystalline packing structure. Tin forms Zintl phases such as Na4Sn, BaSn, K8Sn25 and Ca31Sn20.[127] It has good corrosion resistance in air on account of forming a thin protective oxide layer. Pure tin has no structural uses.[128] It is used in lead-free solders, and as a hardening agent in alloys of other metals, such as copper, lead, titanium and zinc.[129]
Lead is a soft metal (MH 1.5, but hardens close to melting) which, in many cases,[130] is unable to support its own weight.[131] It has a close-packed structure (BCN 12) but an abnormally large inter-atomic distance that has been attributed to partial ionisation of the lead atoms.[107][132] It forms a semi-covalent dioxide PbO2; a covalently bonded sulfide PbS; covalently bonded halides;[133] and a range of covalently bonded organolead compounds such as the lead(II) mercaptan Pb(SC2H5)2, lead tetra-acetate Pb(CH3CO2)4, and the once common, anti-knock additive, tetra-ethyl lead (CH3CH2)4Pb.[134] The oxide of lead in its preferred oxidation state (PbO; +2) is amphoteric;[135] it forms anionic plumbates in strongly basic solutions.[53] Lead forms Zintl phases such as CsPb, Sr31Pb20, La5Pb3N and Yb3Pb20.[136] It has reasonable to good corrosion resistance; in moist air it forms a mixed gray coating of oxide, carbonate and sulfate that hinders further oxidation.[137]
Flerovium is expected to be a gaseous metal due to spin-orbit coupling "tearing" apart the 7p subshell, so that its 7s27p1/22 valence configuration forms a quasi-closed shell similar to those of mercury and copernicium. Indeed, experimental evidence suggests that it has a boiling point of around −60 °C, which is by far the lowest of all the metals. Solid flerovium should have a face-centered cubic structure and be a rather dense metal, with a density of around 14 g/cm3. Flerovium is expected from its standard electrode potential of +0.9 V for the Fl2+/Fl couple to have similar nobility to palladium. Flerovium oxide (FlO) is expected to be amphoteric, forming anionic flerovates in basic solutions.
4.5. Group 15



Arsenic is a moderately hard (MH 3.5) and brittle semi-metallic element. It is commonly regarded as a metalloid, or by some other authors as either a metal or a non-metal. It exhibits poor electrical conductivity which, like a metal, decreases with temperature. It has a relatively open and partially covalent crystalline structure (BCN 3+3). Arsenic forms covalent bonds with most other elements. The oxide in its preferred oxidation state (As2O3, +3) is amphoteric,[138] as is the corresponding oxoacid in aqueous solution (H3AsO3) and congener sulfide (As2S3). Arsenic forms a series of anionic arsenates such as Na3AsO3 and PbHAsO4, and Zintl phases such as Na3As, Ca2As and SrAs3.
Antimony is a soft (MH 3.0) and brittle semi-metallic element. It is commonly regarded as a metalloid, or by some other authors as either a metal or a non-metal. It exhibits poor electrical conductivity which, like a metal, decreases with temperature. It has a relatively open and partially covalent crystalline structure (BCN 3+3). Antimony forms covalent bonds with most other elements. The oxide in its preferred oxidation state (Sb2O3, +3) is amphoteric. Antimony forms a series of anionic antimonites and antimonates such as NaSbO2 and AlSbO4, and Zintl phases such as K5Sb4, Sr2Sb3 and BaSb3.
Bismuth is a soft metal (MH 2.5) that is too brittle for any structural use.[139] It has an open-packed crystalline structure (BCN 3+3) with bonding that is intermediate between metallic and covalent.[140] For a metal, it has exceptionally low electrical and thermal conductivity.[141] Most of the ordinary compounds of bismuth are covalent in nature.[142] The oxide, Bi2O3 is predominantly basic but will act as a weak acid in warm, very concentrated KOH.[143] It can also be fused with potassium hydroxide in air, resulting in a brown mass of potassium bismuthate.[144] The solution chemistry of bismuth is characterised by the formation of oxyanions;[145] it forms anionic bismuthates in strongly basic solutions.[146] Bismuth forms Zintl phases such as NaBi,[147] Rb7In4Bi6[148] and Ba11Cd8Bi14.[149] Bailar et al.[150] refer to bismuth as being, 'the least "metallic" metal in its physical properties' given its brittle nature (and possibly) 'the lowest electrical conductivity of all metals.'[151]
Moscovium is expected to be a quite reactive metal, with a similar standard reduction potential to that of aluminium (−1.5 V for the Mc+/Mc couple). This increased reactivity is consistent with the quasi-closed shell of flerovium and the beginning of a new series of elements with the filling of the loosely bound 7p3/2 subshell, and is rather different from the relative nobility of bismuth. Like thallium, moscovium should have a common +1 oxidation state and a less common +3 oxidation state, although their relative stabilities may change depending on the complexing ligands or the degree of hydrolysis. Moscovium(I) oxide (Mc2O) should be quite basic, like that of thallium, while moscovium(III) oxide (Mc2O3) should be amphoteric, like that of bismuth.
4.6. Group 16
Selenium is a soft (MH 2.0) and brittle semi-metallic element. It is commonly regarded as a nonmetal, but is sometimes considered a metalloid or even a heavy metal. Selenium has a hexagonal polyatomic (CN 2) crystalline structure. It is a semiconductor with a band gap of 1.7 eV, and a photoconductor meaning its electrical conductivity increases a million-fold when illuminated. Selenium forms covalent bonds with most other elements, noting it can form ionic selenides with highly electropositive metals. The common oxide of selenium (SeO3) is strongly acidic. Selenium forms a series of anionic selenites and selenates such as Na2SeO3, Na2Se2O5, and Na2SeO4,[152] as well as Zintl phases such as Cs4Se16.[153]
Tellurium is a soft (MH 2.25) and brittle semi-metallic element. It is commonly regarded as a metalloid, or by some authors either as a metal or a non-metal. Tellurium has a polyatomic (CN 2) hexagonal crystalline structure. It is a semiconductor with a band gap of 0.32 to 0.38 eV. Tellurium forms covalent bonds with most other elements, noting it has an extensive organometallic chemistry and that many tellurides can be regarded as metallic alloys. The common oxide of tellurium (TeO2) is amphoteric. Tellurium forms a series of anionic tellurites and tellurates such as Na2TeO3, Na6TeO6, and Rb6Te2O9 (the last containing tetrahedral TeO2−4 and trigonal bipyramidal TeO4−5 anions),[152] as well as Zintl phases such as NaTe3.[153]
Polonium is a radioactive, soft metal with a hardness similar to lead.[154] It has a simple cubic crystalline structure characterised (as determined by electron density calculations) by partially directional bonding,[155] and a BCN of 6. Such a structure ordinarily results in very low ductility and fracture resistance[156] however polonium has been predicted to be a ductile metal.[157] It forms a covalent hydride;[158] its halides are covalent, volatile compounds, resembling those of tellurium.[159] The oxide of polonium in its preferred oxidation state (PoO2; +4) is predominantly basic, but amphoteric if dissolved in concentrated aqueous alkali, or fused with potassium hydroxide in air.[160] The yellow polonate(IV) ion PoO2−3 is known in aqueous solutions of low Cl‒ concentration and high pH.[161][162] Polonides such as Na2Po, BePo, ZnPo, CdPo and HgPo feature Po2− anions;[163] except for HgPo these are some of the more stable of the polonium compounds.[164][165]
Livermorium is expected to be less reactive than moscovium: the standard reduction potential of the Lv2+/Lv couple is expected to be around +0.1 V. It should be most stable in the +2 oxidation state; the 7p3/2 electrons are expected to be so weakly bound that the first two ionisation potentials of livermorium should lie between those of the reactive alkaline earth metals magnesium and calcium. The +4 oxidation state should only be reachable with the most electronegative ligands. Livermorium(II) oxide (LvO) should be basic and livermorium(IV) oxide (LvO2) should be amphoteric, analogous to polonium.
4.7. Group 17
Astatine is a radioactive element that has never been seen; a visible quantity would immediately be vaporised due to its intense radioactivity.[166] It may be possible to prevent this with sufficient cooling.[167] Astatine is commonly regarded as a nonmetal,[168] less commonly as a metalloid[169] and occasionally as a metal. Unlike its lighter congener iodine, evidence for diatomic astatine is sparse and inconclusive.[170] In 2013, on the basis of relativistic modelling, astatine was predicted to be a monatomic metal, with a face-centered cubic crystalline structure.[167] As such, astatine could be expected to have a metallic appearance; show metallic conductivity; and have excellent ductility, even at cryogenic temperatures.[171] It could also be expected to show significant nonmetallic character, as is normally the case for metals in, or in the vicinity of, the p-block. Astatine oxyanions AtO−, AtO−3 and AtO−4 are known,[172] oxyanion formation being a tendency of nonmetals.[173] The hydroxide of astatine At(OH) is presumed to be amphoteric.[174][175] Astatine forms covalent compounds with nonmetals,[176] including hydrogen astatide HAt and carbon tetraastatide CAt4.[177][178] At− anions have been reported to form astatides with silver, thallium, palladium and lead.[179] Pruszyński et al. note that astatide ions should form strong complexes with soft metal cations such as Hg2+, Pd2+, Ag+ and Tl3+; they list the astatide formed with mercury as Hg(OH)At.[180]
Tennessine, despite being in the halogen column of the periodic table, is expected to go even further towards metallicity than astatine due to its small electron affinity. The −1 state should not be important for tennessine and its major oxidation states should be +1 and +3, with +3 more stable: Ts3+ is expected to behave similarly to Au3+ in halide media. As such, tennessine oxide (Ts2O3) is expected to be amphoteric, similar to gold oxide and astatine(III) oxide.
4.8. Group 18
Oganesson is expected to be a very poor "noble gas" and may even be metallised by its large atomic radius and the weak binding of the easily removed 7p3/2 electrons: certainly it is expected to be a quite reactive element that is solid at room temperature and has some similarities to tin, as one effect of the spin–orbit splitting of the 7p subshell is a "partial role reversal" of groups 14 and 18. Due to the immense polarisability of oganesson, it is expected that not only oganesson(II) fluoride but also oganesson(IV) fluoride should be predominantly ionic, involving the formation of Og2+ and Og4+ cations. Oganesson(II) oxide (OgO) and oganesson(IV) oxide (OgO2) are both expected to be amphoteric, similar to the oxides of tin.
5. Related Groupings
5.1. B-Subgroup Metals
Superficially, the B-subgroup metals are the metals in Groups IB to VIIB of the periodic table, corresponding to groups 11 to 17 using current IUPAC nonmenclature. Practically, the group 11 metals (copper, silver and gold) are ordinarily regarded as transition metals (or sometimes as coinage metals, or noble metals) whereas the group 12 metals (zinc, cadmium, and mercury) may or may not be treated as B-subgroup metals depending on if the transition metals are taken to end at group 11 or group 12. The 'B' nomenclature (as in Groups IB, IIB, and so on) was superseded in 1988 but is still occasionally encountered in more recent literature.[181][182]
The B-subgroup metals show nonmetallic properties; this is particularly apparent in moving from group 12 to group 16.[183] Although the group 11 metals have normal close-packed metallic structures[184] they show an overlap in chemical properties. In their +1 compounds (the stable state for silver; less so for copper)[185] they are typical B-subgroup metals. In their +2 and +3 states their chemistry is typical of transition metal compounds.[186]
Pseudo metals and hybrid metals
The B-subgroup metals can be subdivided into pseudo metals and hybrid metals. The pseudo metals (groups 12 and 13, including boron) are said to behave more like true metals (groups 1 to 11) than non-metals. The hybrid metals As, Sb, Bi, Te, Po, At — which other authors would call metalloids — partake about equally the properties of both. The pseudo metals can be considered related to the hybrid metals through the group 14 carbon column.[187]
5.2. Base Metals
Mingos[188] writes that while the p-block metals are typical, that are not strongly reducing and that, as such, they are base metals requiring oxidizing acids to dissolve them.
5.3. Borderline Metals
Parish[189] writes that, 'as anticipated', the borderline metals of groups 13 and 14 have non-standard structures. Gallium, indium, thallium, germanium, and tin are specifically mentioned in this context. The group 12 metals are also noted as having slightly distorted structures; this has been interpreted as evidence of weak directional (i.e. covalent) bonding.[190]
5.4. Chemically Weak Metals
Rayner-Canham and Overton[191] use the term chemically weak metals to refer to the metals close to the metal-nonmetal borderline. These metals behave chemically more like the metalloids, particularly with respect to anionic species formation. The nine chemically weak metals identified by them are beryllium, magnesium, aluminum, gallium, tin, lead, antimony, bismuth, and polonium.[192]5.5
5.5. Frontier Metals
Vernon[193] uses the term "frontier metal" to refer to the class of chemically weak metals adjacent to the dividing line between metals. He notes that several of them "are further distinguished by a series of…knight's move relationships, formed between one element and the element one period down and two groups to its right."[194] For example, copper(I) chemistry resembles indium(I) chemistry: "both ions are found mostly in solid-state compounds such as CuCl and InCl; the fluorides are unknown for both ions while the iodides are the most stable."[194] The name frontier metal is adapted from Russell and Lee,[195] who wrote that, "…bismuth and group 16 element polonium are generally considered to be metals, although they occupy 'frontier territory' on the periodic table, adjacent to the nonmetals."
5.6. Fusible Metals
Cardarelli,[196] writing in 2008, categorizes zinc, cadmium, mercury, gallium, indium, thallium, tin, lead, antimony and bismuth as fusible metals. Nearly 100 years earlier, Louis (1911)[197] noted that fusible metals were alloys containing tin, cadmium, lead, and bismuth in various proportions, "the tin ranging from 10 to 20%."
5.7. Heavy Metals (of Low Melting Point)
Van Wert[198] grouped the periodic table metals into a. the light metals; b. the heavy brittle metals of high melting point, c. the heavy ductile metals of high melting point; d. the heavy metals of low melting point (Zn, Cd, Hg; Ga, In, Tl; Ge, Sn; As, Sb, Bi; and Po), and e. the strong, electropositive metals. Britton, Abbatiello and Robins[199] speak of 'the soft, low melting point, heavy metals in columns lIB, IlIA, IVA, and VA of the periodic table, namely Zn, Cd, Hg; Al, Ga, In, Tl; [Si], Ge, Sn, Pb; and Bi. The Sargent-Welch Chart of the Elements groups the metals into: light metals, the lanthanide series; the actinide series; heavy metals (brittle); heavy metals (ductile); and heavy metals (low melting point): Zn, Cd, Hg, [Cn]; Al, Ga, In, Tl; Ge, Sn, Pb, [Fl]; Sb, Bi; and Po.[200][201]
5.8. Less typical metals
Habashi[202] groups the elements into eight major categories: [1] typical metals (alkali metals, alkaline earth metals, and aluminium); [2] lanthanides (Ce–Lu); [3] actinides (Th–Lr); [4] transition metals (Sc, Y, La, Ac, groups 4–10); [5] less typical metals (groups 11–12, Ga, In, Tl, Sn and Pb); [6] metalloids (B, Si, Ge, As, Se, Sb, Te, Bi and Po); [7] covalent nonmetals (H, C, N, O, P, S and the halogens); and [8] monatomic nonmetals (that is, the noble gases).
5.9. Metametals
The metametals are zinc, cadmium, mercury, indium, thallium, tin and lead. They are ductile elements but, compared to their metallic periodic table neighbours to the left, have lower melting points, relatively low electrical and thermal conductivities, and show distortions from close-packed forms.[203] Sometimes beryllium[204] and gallium[205] are included as metametals despite having low ductility.
5.10. Ordinary Metals
Abrikosov[206] distinguishes between ordinary metals, and transition metals where the inner shells are not filled. The ordinary metals have lower melting points and cohesive energies than those of the transition metals.[207] Gray[208] identifies as ordinary metals: aluminium, gallium, indium, thallium, nihonium, tin, lead, flerovium, bismuth, moscovium, and livermorium. He adds that, 'in reality most of the metals that people think of as ordinary are in fact transition metals...'.
5.11. Other Metals
As noted, the metals falling between the transition metals and the metalloids on the periodic table are sometimes called other metals (see also, for example, Taylor et al.).[209] 'Other' in this sense has the related meanings of, 'existing besides, or distinct from, that already mentioned'[210] (that is, the alkali and alkaline earth metals, the lanthanides and actinides, and the transition metals); 'auxiliary'; 'ancillary, secondary'.[211] According to Gray[212] there should be a better name for these elements than 'other metals'.
5.12. P-Block Metals
The p-block metals are the metals in groups 13‒16 of the periodic table. Usually, this includes aluminium, gallium, indium and thallium; tin and lead; and bismuth. Germanium, antimony and polonium are sometimes also included, although the first two are commonly recognised as metalloids. The p-block metals tend to have structures that display low coordination numbers and directional bonding. Pronounced covalency is found in their compounds; the majority of their oxides are amphoteric.[213]
Aluminium is an undisputed p-block element by group membership and its [Ne] 3s2 3p1 electron configuration, but aluminium does not literally come after transition metals unlike p-block metals from period 4 and on. The epithet "post-transition" in reference to aluminium is a misnomer, and aluminium normally has no d electrons unlike all other p-block metals.
5.13. Peculiar Metals
Slater[214] divides the metals 'fairly definitely, though not perfectly sharply' into the ordinary metals and the peculiar metals, the latter of which verge on the nonmetals. The peculiar metals occur towards the ends of the rows of the periodic table and include 'approximately:' gallium, indium, and thallium; carbon, silicon '(both of which have some metallic properties, though we have previously treated them as nonmetals),' germanium and tin; arsenic, antimony, and bismuth; and selenium '(which is partly metallic)' and tellurium. The ordinary metals have centro-symmetrical crystalline structures[215] whereas the peculiar metals have structures involving directional bonding. More recently, Joshua observed that the peculiar metals have mixed metallic-covalent bonding.[216]
5.14. Poor Metals
Farrell and Van Sicien[217] use the term poor metal, for simplicity, 'to denote one with a significant covalent, or directional character.' Hill and Holman[218] observe that, 'The term poor metals is not widely used, but it is a useful description for several metals including tin, lead and bismuth. These metals fall in a triangular block of the periodic table to the right of the transition metals. They are usually low in the activity (electrochemical) series and they have some resemblances to non-metals.' Reid et al.[219] write that 'poor metals' is, '[A]n older term for metallic elements in Groups 13‒15 of the periodic table that are softer and have lower melting points than the metals traditionally used for tools.'
5.15. Semimetals
In modern use, the term 'semimetal' sometimes refers, loosely or explicitly, to metals with incomplete metallic character in crystalline structure, electrical conductivity or electronic structure. Examples include gallium,[220] ytterbium,[221] bismuth,[222] mercury[223] and neptunium.[224] Metalloids, which are in-between elements that are neither metals nor nonmetals, are also sometimes instead called semimetals. The elements commonly recognised as metalloids are boron, silicon, germanium, arsenic, antimony and tellurium. In old chemistry, before the publication in 1789 of Lavoisier's 'revolutionary'[225] Elementary Treatise on Chemistry,[226] a semimetal was a metallic element with 'very imperfect ductility and malleability'[227] such as zinc, mercury or bismuth.
5.16. Transition Metals
Historically, the transition metal series "includes those elements of the Periodic Table which 'bridge the gap' between the very electropositive alkali and allkaline earth metals and the electronegative non-metals of the groups: nitrogen-phosphorus, oxygen-sulfur, and the halogens."[228] Cheronis, Parsons and Ronneberg[229] wrote that, "The transition metals of low melting point form a block in the Periodic Table: those of Groups II 'b' [zinc, cadmium, mercury], III 'b' [aluminium, gallium, indium, thallium], and germanium, tin and lead in Group IV. These metals all have melting points below 425 °C."[230]
The content is sourced from: https://handwiki.org/wiki/Chemistry:Post-transition_metal
References
- Roher 2001, pp. 2‒3
- Messler 2006, p. 347
- Physical properties: "The lighter alkaline earths possess fairly high electrical and thermal conductivities and sufficient strength for structural use. The heavier elements are poor conductors and are too weak and reactive for structural use."[10] Chemical: The lighter alkaline earths show covalent bonding tendencies (Be predominantly; Mg considerably) whereas compounds of the heavier alkaline earths are predominantly ionic in nature; the heavier alkaline earths have more stable hydrides and less stable carbides.[11]
- A first IUPAC definition states "[T]he elements of groups 3–12 are the d-block elements. These elements are also commonly referred to as the transition elements, though the elements of group 12 are not always included". Depending on the inclusion of group 12 as transition metals, the post-transition metals therefore may or may not include the group 12 elements—zinc, cadmium, and mercury. A second IUPAC definition for transition metals states "An element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Based on this definition one could argue group 12 should be split with mercury and probably also copernicium as transition metals, and zinc and cadmium as post-transition metals. Of relevance is the synthesis of mercury(IV) fluoride, which seemingly establishes mercury as a transition metal. This conclusion has been challenged by Jensen[12] with the argument that HgF4 only exists under highly atypical non-equilibrium conditions (at 4 K) and should best be considered as an exception. Copernicium has been predicted to have (a) an electron configuration similar to that of mercury; and (b) a predominance of its chemistry in the +4 state, and on that basis would be regarded as a transition metal. However, in recent years, doubt has been cast on the synthesis of HgF4 and the possible existence of copernicium(IV), so that group 12 would have only post-transition metals.
- Jensen 2003, p. 952
- Cox 2004, p. 17
- Atkins & de Paula 2011, p. 352
- Greenwood & Earnshaw 1998, pp. 222–3
- Steele 1966, p. 193
- Johnson 1970
- The scandide contraction refers to the first row transition metals; the d-block contraction is a more general term.
- Huheey & Huheey 1972, p. 229; Mason 1988
- Cox 2004, pp. 20, 186, 188
- Science Education 1948, p. 120
- Deming 1940, p. 704–715
- Moh's hardness values are taken from Samsanov,[22] unless otherwise noted; bulk coordination number values are taken from Darken and Gurry,[23] unless otherwise noted.
- Russell & Lee 2005, p. 302
- Steele 1966, p. 67
- Deming 1940, pp. 705–7; Karamad, Tripkovic & Rossmeisl 2014
- Cheemalapati, Keleher & Li 2008, p. 226
- Liu & Pecht 2004, p. 54
- Donohue 1982, p. 222
- Vanderah 1992, p. 52
- Lidin 1996, p. 110
- Slabon et al. 2012
- Larson et al. 2006, p. 035111-2
- Schumann 2008, p. 52
- Braunović 2014, p. 244
- Donohue 1982, p. 222
- Banthorpe, Gatforde & Hollebone 1968, p. 61; Dillard & Goldberg 1971, p. 558
- Steiner & Campbell 1955, p. 394
- Lidin 1996, p. 5
- Klassen & Hoppe 1982; Darriet, Devalette & Lecart 1977; Sofin et al. 2002
- Goodwin et al. 2005, p. 341
- Köhler & Whangbo 2008
- Arndt & Ganino 2012, p. 115
- Goffer 2007, p. 176
- Sidgwick 1950, p. 177
- Pauling 1988, p. 698
- Lidin 1996, p. 21–22
- Miller et al. 2011, p. 150
- Fishcher-Bünher 2011, p. 150
- Smith 1990, p. 113
- Sorensen 1991, p. 3
- King 1995, pp. xiii, 273–288; Cotton et al. 1999, pp. ix, 598; Massey 2000, pp. 159–176
- The group 12 metals have been treated as transition metals for reasons of historical precedent, to compare and contrast properties, to preserve symmetry, or for basic teaching purposes.[53]
- IUPAC 2005, p. 51
- Crichton 2012, p. 11
- The IUPAC Gold Book defines a transition metal as 'An element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell.[56]
- Schweitzer 2003, p. 603
- Hutchinson 1964, p. 562
- Greenwood & Earnshaw 1998, p. 1209; Gupta CK 2002, p. 590
- Rayner-Canham & Overton 2006, p. 30
- Kneip 1996, p. xxii
- Russell & Lee 2005, p. 339
- Sequeira 2013, p. 243
- Russell & Lee 2005, p. 349
- Borsari 2005, p. 608
- Dirkse 1986, pp. 287–288, 296; Ivanov-Emin, Misel'son & Greksa 1960
- Wanamaker & Pennington 1921, p. 56
- Rayner-Canham 2006, p. 570; Chambers & Holliday 1975, p. 58; Wiberg, Holleman & Wiberg 2001, p. 247; Aylward & Findlay 2008, p. 4
- Francium may have a comparably low bonding energy but its melting point of around 8°C is significantly higher than that of mercury, at −39°C.
- Poole 2004, p. 821
- Mittemeijer 2010, p. 138
- Russell & Lee 2005, pp. 1–2; 354
- Rayner-Canham 2006, p. 567
- Moeller 1952, pp. 859, 866
- Cooney & Hall 1966, p. 2179
- Mercury also forms partially anionic oxomercurates, such as Li2HgO2 and CdHgO4, by heating mixtures of HgO with the relevant cation oxides, including under oxygen pressure (Müller-Buschbaum 1995; Deiseroth 2004, pp. 173, 177, 185–186).
- Deiseroth 2008, pp. 179‒180; Sevov 1993
- Russell & Lee 2005, p. 354
- Whitten et al. 2014, p. 1045
- Cox 2004, p. 186
- Kneen, Rogers & Simpson 2004, p. 370; Cox 2004, p. 199
- Gerard & King 1968, p. 16; Dwight 1999, p. 2
- Russell & Lee 2005, pp. 1–2; 359
- The partially directional bonding in aluminium improves its shear strength but means that ultrahigh-purity aluminium cannot maintain work hardening at room temperature.[81]
- Lyons 2004, p. 170
- Cobb 2009, p. 323
- Polemear 2006, p. 184
- Without the use of thermal insulation and detailed structural design attention,[85] aluminium's low melting point and high thermal conductivity mitigate against its use, for example, in military ship construction—should a ship burn, the low melting point results in structural collapse; the high thermal conductivity helps spread the fire.[86] Its use in the construction of cargo ships is limited as little or no economic advantage is gained over steel, once the cost and weight of fitting thermal insulation is taken into account.[87]
- Cooper 1968, p. 25; Henderson 2000, p. 5
- Kauzlarich 2005, pp. 6009–10
- Dennis & Such 1993, p. 391
- Cramer & Covino 2006, p. 25
- Aluminium can be attacked, for example, by alkaline detergents[92] (including those used in dishwashers);[93] by wet concrete,[94] and by highly acidic foods such as tomatoes, rhubarb or cabbage.[95] It is not attacked by nitric acid.[96]
- Russell & Lee 2005, p. 360
- See the list of metalloid lists for references
- Clegg & Dovaston 2003, p. 5/5
- Aluminium wire is used in electrical transmission lines for the distribution of power but, on account of its low breaking strength, is refinforced with a central core of galvanised steel wire.[99]
- Kent 1993, pp. 13–14
- Steele 1966, p. 60
- In the absence of protective measures, the relatively high electropositivity of aluminium renders it susceptible to galvanic corrosion when in physical or electrical contact with other metals such as copper or steel, especially when exposed to saline media, such as sea water or wind-blown sea spray.[102]
- Russell & Lee 2005, p. 387
- Driess 2004, p. 151; Donohue 1982, p. 237
- Walker, Enache & Newman 2013, p. 38
- Atkins et al. 2006, p. 123
- Corbett 1996, p. 161
- Eranna 2012, p. 67
- Chandler 1998, p. 59
- Russell & Lee 2005, p. 389
- Evans 1966, p. 129–130
- Liang, King & White 1968, p. 288
- Busev 1962, p. 33; Liang, King & White 1968, p. 287; Solov'eva et al. 1973, p. 43; Greenwood & Earnshaw 1998, p. 226; Leman & Barron 2005, p. 1522
- Kneip 1996, p. xxii; Corbett 1996, pp. 153, 158
- Russell & Lee 2005, p. 390
- Wells 1985, p. 1279–80
- Howe 1968a, p. 709; Taylor & Brothers 1993, p. 131; Lidin 1996, p. 410; Tóth & Győri 2005, pp. 4, 6–7
- Chambers & Holliday 1975, p. 144
- Bashilova & Khomutova 1984, p. 1546
- Ropp 2012, p. 484
- King & Schleyer 2004, p. 19
- Corbett 1996, p. 153; King 2004, p. 199
- Wiberg, Holleman & Wiberg 2001, p. 894
- Haller 2006, p. 3
- Russell & Lee 2005, p. 399
- Ryan 1968, p. 65
- Wiberg, Holleman & Wiberg 2001, p. 895
- Abd-El-Aziz et al. 2003, p. 200
- Cooper 1968, pp. 28–9
- Ropp 2012, p. 405
- Corbett 1996, p. 143
- Russell & Lee 2005, p. 405
- Charles, Crane and Furness write that, 'Most metals, except perhaps lead and tin, can be alloyed to give [yield] strengths that lie in the upper two-thirds of the low-strength range…'[133]
- Rayner-Canham 2006, pp. 306, 340
- Wiberg, Holleman & Wiberg 2001, p. 247
- Corbett 1996, p. 143; Cotton et al. 1999, pp. 99, 122; Kauzlarich 2005, p. 6009
- Russell & Lee 2005, pp. 402, 405
- Russell & Lee 2005, p. 402, 407
- Alhassan & Goodwin 2005, p. 532
- Schweitzer 2003, p. 695
- Mackay & Mackay 1989, p. 86; Norman 1997, p. 36
- Hutchinson 1959, p. 455; Wells 1984, p. 1188; Liu, Knowles & Chang 1995, p. 125; Bharara & Atwood 2005, pp. 2, 4
- Durrant & Durrant 1970, p. 670; Lister 1998, p. A12; Cox 2004, p. 204
- Patnaik 2003, p. 474
- Corbett 1996, pp. 143, 147; Cotton et al. 1999, p. 122; Kauzlarich 2005, p. 6009
- Russell & Lee 2005, pp. 411, 13
- As2O3 is usually regarded as being amphoteric but a few sources say it is (weakly)[147] acidic. They describe its "basic" properties (its reaction with concentrated hydrochloric acid to form arsenic trichloride) as being alcoholic, in analogy with the formation of covalent alkyl chlorides by covalent alcohols (e.g., R-OH + HCl → RCl + H2O)[148]
- Russell & Lee 2005, p. 428
- Eagleson 1994, p. 282
- Russell & Lee 2005, p. 427
- Sidgwick 1937, p. 181
- Howe 1968, p. 62
- Durrant & Durrant 1970, p. 790
- Wiberg, Holleman & Wiberg 2001, p. 771; McQuarrie, Rock & Gallogly 2010, p. 111
- Ropp 2012, p. 328
- Miller, Lee & Choe 2002, p. 14; Aleandri & Bogdanović 2008, p. 326
- Bobev & Sevov 2002
- Xia & Bobev 2006
- Bailar et al. 1984, p. 951
- Which metal has the lowest electrical conductivity is debatable but bismuth is certainly in the lowest cohort; Hoffman[161] refers to bismuth as 'a poor metal, on the verge of being a semiconductor.'
- Greenwood & Earnshaw 2002, pp. 781–3
- Greenwood & Earnshaw 2002, pp. 762–5
- Beamer & Maxwell 1946, pp. 1, 31
- Russell & Lee 2005, p. 431
- Halford 2006,p. 378
- Legut, Friák & Šob 2010
- Wiberg, Holleman & Wiberg 2001, pp. 594; Petrii 2012, p. 754
- Bagnall 1966, p. 83
- Bagnall 1966, pp. 42, 61; Wiberg, Holleman & Wiberg 2001, pp. 767–68
- Schwietzer & Pesterfield pp. 241, 243
- Bagnall[172] writes that the fusion of polonium dioxide with a potassium chlorate/hydroxide mixture yields a bluish solid which, '...presumably contains some potassium polonate.'
- Wiberg, Holleman & Wiberg 2001, pp. 283, 595
- Greenwood & Earnshaw 1998, p. 766
- Bagnall[175] noted that the rare-earth polonides have the greatest thermal stability of any polonium compound.
- Emsley 2011, p. 58
- Hermann, Hoffmann & Ashcroft 2013, p. 11604–1
- Hawkes 2010; Holt, Rinehart & Wilson c. 2007; Hawkes 1999, p. 14; Roza 2009, p. 12
- Harding, Johnson & Janes 2002, p. 61
- Merinis, Legoux & Bouissières 1972; Kugler & Keller 1985, pp. 110, 116, 210–211, 224; Takahashi & Otozai 1986; Zuckerman & Hagen 1989, pp. 21–22 (21); Takahashi, Yano & Baba 1992
- Russell & Lee 2005, p. 299
- Eberle1985, pp. 190, 192,
- Brown et al. 2012, p. 264
- Wiberg 2001, p. 283
- Eagleson refers to the OH compound of astatine as hypoastatous acid HAtO;[185] Pimpentel and Spratley give the formula for hypoastatous acid as HOAt.[186]
- Messler & Messler 2011, p. 38
- Fine 1978, p. 718; Emsley 2011, p. 57
- In hydrogen astatide the negative charge is predicted to be on the hydrogen atom,[189] implying that this compound should instead be referred to as astatine hydride (AtH).
- Berei K & Vasáros 1985, p. 214
- Pruszyński et al. 2006, pp. 91, 94
- Zubieta & Zuckerman 2009, p. 260: 'The compounds AsSn and SbSn, which are classified as alloys of two B subgroup metals, exhibit superconducting properties with a transition temperature of about 4 K.'; Schwartz 2010, p. 32: 'The metals include the alkali and alkaline earths, beryllium, magnesium, copper, silver, gold and the transition metals. These metals exhibit those characteristics generally associated with the metallic state. The B subgroups comprise the remaining metallic elements. These elements exhibit complex structures and significant departures from typically metallic properties. Aluminum, although considered under the B subgroup metals, is somewhat anomalous as it exhibits many characteristics of a true metal.'
- Greenwood and Earnshaw[193] refer to the B-subgroup metals as post-transition elements: 'Arsenic and antimony are classed as metalloids or semi-metals and bismuth is a typical B sub-group (post-transition-element) metal like tin and lead.'
- Phillips & Williams 1965, pp. 4‒5; Steele 1966, p. 66
- Phillips & Williams 1965, p. 33
- Wiberg, Holleman & Wiberg 2001, pp. 1253, 1268
- Steele 1966, p. 67
- Harrington 1946, pp. 143, 146-147
- Mingos 1998, pp. 18–19
- Parish 1977, pp. 201–202
- Aluminium is identified by Parish, along with germanium, antimony and bismuth, as being a metal on the boundary line between metals and non-metals; he suggests that all these elements are 'probably better classed as metalloids.'[201]
- Rayner-Canham & Overton 2006, p. 29‒30
- Pauling,[203] in contrast, refers to the strong metals in Groups 1 and 2 (that form ionic compounds with 'the strong nonmetals in the upper right corner of the periodic table.').
- Vernon 2020
- Rayner-Canham 2006, pp. 212 − 215
- Russell & Lee 2005, p. 419
- Cardarelli 2008, p. 1181
- Louis 1911, p. 11–12
- Van Wert 1936, pp. 16, 18
- Britton, Abbatiello & Robins 1972, p. 704
- Sargent-Welch 2008
- Hawkes,[212] attempting to address the question of what is a heavy metal, commented that, 'Being a heavy metal has little to do with density, but rather concerns chemical properties'. He observed that, 'It may mean different things to different people, but as I have used, heard and interpreted the term over the last half-century, it refers to metals with insoluble sulfides and hydroxides, whose salts produce colored solutions in water, and whose complexes are usually colored.' He goes on to note that, 'The metals I have seen referred to as heavy metals comprise a block of all the metals in Groups 3 to 16 that are in periods 4 and greater. It may also be stated as the transition metals and post-transition metals.
- Habashi 2010
- Wiberg, Holleman & Wiberg 2001, p. 143
- Klemm 1950
- Miller GJ, Lee C & Choe W 2002, p. 22
- Abrikosov 1988, p. 31
- Cremer 1965, p. 514
- Gray 2009, p. 9
- Taylor et al. 2007, p. 148
- Oxford English Dictionary 1989, 'other'
- Roget's 21st Century Thesaurus
- Gray 2010
- Parish 1977, pp. 178, 189–190, 192–3
- Slater 1939, p. 444‒445
- On manganese, Slater says, '[It] is a very peculiar and anomalous exception to the general order of the elements. It is the only definite metal, far from the nonmetals in the table, which has a complicated structure.'[226]
- Joshua 1991, p. 45
- Farrell & Van Sicien 2007, p. 1442
- Hill & Holman 2000, p. 40
- Reid 2011, p. 143
- Pashaey & Seleznev 1973, p. 565
- Johansen & Mackintosh 1970, pp. 121–4; Divakar, Mohan & Singh 1984, p. 2337; Dávila et al. 2002, p. 035411-3
- Jezequel & Thomas1997
- Savitsky 1961, p. 107
- Hindman 1968, p. 434: 'The high values obtained for the [electrical] resistivity indicate that the metallic properties of neptunium are closer to the semimetals than the true metals. This is also true for other metals in the actinide series.'; Dunlap et al. 1970, pp. 44, 46: '...α-Np is a semimetal, in which covalency effects are believed to also be of importance...For a semimetal having strong covalent bonding, like α-Np...'
- Strathern 2000, p. 239
- Roscoe & Schormlemmer 1894, p. 4
- Murray 1809, p. 300
- Young et al. 1969, p. 228
- Cheronis, Parsons & Ronneberg 1942, p. 570
- In fact, both aluminium (660.32) and germanium (938.25) have melting points greater than 425°C.
- Subba Rao & Shafer 1979, p. 170
- Collings 1986, p. 5
- Temkin 2012, pp. 1, 726
- Russell & Lee 2005, p. 165
- Cotton et al. 1999, pp. 111–113; Greenwood & Earnshaw 2002, p. 111–113
- Jensen 2008
- Samsanov 1968
- Darken & Gurry 1953, pp. 50–53
- Young et al. 1969; Geffner 1969; Jensen 2003
- IUPAC 2006–, transition element entry
- Ogata, Li & Yip 2002; Russell & Lee 2005, p. 360; Glaeser 1992, p. 224
- Holl 1989, p. 90
- Ramroth 2006, p. 6; US Dept. of Transportation, Maritime Administration 1987, pp. 97, 358
- Noble 1985, p. 21
- Hinton & Dobrota 1978, p. 37
- Holman & Stone 2001, p. 141
- Hurd 2005, p. 4-15
- Vargel 2004, p. 580
- Hill & Holman 2000, p. 276
- Liptrot 2001, p. 181
- Davis 1999, p. 75–7
- Charles, Crane & Furness 1997, pp. 49, 57
- Wiberg 2001, pp. 750, 975; Silberberg 2006, p. 314
- Sidgwick 1950, p. 784; Moody 1991, pp. 248–9, 319
- Hoffman 2004
- Bagnall 1962, p. 211
- Bagnall 1966, p. 47
- Eagleson 1994, p. 95
- Pimpentel 1971, p. 827
- Thayer 2010, p. 79
- Greenwood & Earnshaw 1998, p. 548
- Parish 1977, pp. 178
- Pauling 1988, p. 173
- Hawkes 1997
- Slater 1939, p. 448


