Breast cancer (BC) is a serious global challenge, and depression is one of the risk factors and comorbidities of BC. Recently, the research on the comorbidity of BC and depression has focused on the dysfunction of the hypothalamic–pituitary–adrenal axis and the persistent stimulation of the inflammatory response. However, the further mechanisms for comorbidity remain unclear. Epoxide metabolism has been shown to have a regulatory function in the comorbid mechanism with scattered reports. Hence, this article reviews the role of epoxide metabolism in depression and BC. The comprehensive review discloses the imbalance in epoxide metabolism and its downstream effect shared by BC and depression, including overexpression of inflammation, upregulation of toxic diols, and disturbed lipid metabolism. These downstream effects are mainly involved in the construction of the breast malignancy microenvironment through liver regulation.
- depression
- epoxide metabolism
- comorbidity
- tumor microenvironment
- breast cancer
1. Introduction
Cancer is one of the malignant diseases with the highest mortality in the world, and its incidence continues to grow rapidly [1,2]. The leading cause of cancer-related mortality among the female population is breast cancer (BC) [3]. Since 2020, BC has been the major cause of cancer incidence worldwide, accounting for 11.7% of all cancer cases [3,4]. BC is also the fifth highest cause of cancer deaths around the world [3]. In addition, BC is an obstacle to improving life expectancy in each country, causing a heavy economic burden and health and social challenges globally [3]. BC is a highly heterogeneous disease whose development is associated with genetic, dietary, and environmental factors [5]. Various types of BC can be broadly classified as hormone receptor status (estrogen receptor, ER, or progesterone receptor, PR), human epidermal growth factor receptor status (HER2), and triple-negative status (TNBC) [5, 6, 7, 8]. The current mainstream treatment options include conventional chemotherapy, monoclonal antibodies, and coupled systemic administration [5]. Although increased levels of diagnosis of BC over the years have led to increased survival rates, the side effects of treatment, the impact of stress, and the unsatisfactory quality of survival have still attracted public concern [9]. BC has a high rate of physical and mental comorbidity, mainly due to chronic stress [10]. Depression, as a vital risk factor and comorbidity of BC, has plagued women with BC for decades. In the absence of the precise management of individuals, families, and professional domains, female BC patients are placed under mental stress, which eventually brings a heavier physical burden. Studies have illustrated that depression is an independent predictor of higher frequency hospitalization, longer hospitalization, lower quality of life, and lower treatment compliance [11]. Depression has also been demonstrated to be an important predictor for the diagnosis of advanced BC patients, and the suicide rate of BC patients has highly correlated with clinical symptoms of depression phenotypes [12]. It was reported that comorbid depression is associated with poor prognosis and increased mortality in cancer patients [13]. A study demonstrated that the prevalence of depression in BC patients is 15% during and after medical cancer treatment. The treatment of anxiety and depression are associated with decreased neurocognitive function and reduced hippocampal volume following chemotherapy [9,14, 15, 16, 17]. What is more, in the context of the current global prevalence of infectious diseases, BC patients are prone to emotional disturbances and cognitive dysfunction due to the impact of work and employment [18]. A meta-analysis demonstrates that negative emotions significantly increase the risk for the incidence of BC [19]. Thus, the comorbidity of BC and depression is an inescapable biomedical problem.
Hitherto, most studies addressing the comorbidity of BC and depression have focused on four aspects: inflammation and oxidative/nitrosative stress, reduced immune monitoring, abnormal activation of the autonomic nervous system, and the hypothalamic–pituitary renal axis (HPA) [20]. In fact, the imbalance of peripheral dopamine (DA) and kynurenine (KYN) are proposed to positively predict depression in BC patients [21]. Moreover, the persistent activation of the HPA and sympathetic nervous system is believed to promote BC growth. Unfortunately, due to the dispersion of information, the bridging mechanism between depression and BC is still unclear since the etiology and final effect of the comorbidity have only been partly discussed. Epoxide metabolism is an important metabolic process that mediates inflammation, tumor, and immune surveillance, which mainly occurs in the liver, kidney, and blood vessels [22].
Epoxide metabolism is noted to play a significant regulatory role in BC. Soluble epoxide hydrolase (sEH) is an essential intermediate enzyme in epoxide metabolism and has a vital effect on the pathogenesis of depression and BC [22, 23]. Several studies have indicated that upregulation of sEH is closely related to neurological disorders [24]. A decrease in sEH level is also found in BC tissues, whereas an increase in sEH level inhibited BC proliferation. Other scholars have shown that sEH can promote BC cell proliferation by hydrolyzing toxic epoxides, which is inconsistent with previous studies [25, 26]. Therefore, sEH-mediated epoxide metabolism might be a crucial area for investigation and one of the critical comorbid mechanisms of BC and depression. However, the presented evidence is controversial. According to the study, epoxide metabolism mainly occurs in the liver, and sEH may have different effects on different subtypes of BC. Furthermore, epoxide metabolism is involved in mediating immune responses and regulating lipid homeostasis in the tumor microenvironment (TME) [27]. Researchers have demonstrated that the levels of plasma interleukin 6 (IL-6) in patients with BC and depression are higher and are also regulated by sEH [28,29]. The epoxide metabolism mediated by sEH might be related to a deeper mechanism, which is the key point of the controversy.
2.Depression Is an Important Risk Factor and Comorbidity of BC
Depression, one of the reported risk factors for cancer, is known as a comorbidity of BC. Researchers have found that BC survivors experience a high rate of depression, and the incidence of depression during and after treatment is 15% [9, 30]. Current research has underlined the neurohormonal signaling system as the major shared mechanism of BC and depression. The sympathetic nervous system (SNS) and HPA are two stress responses that affect the nervous system and contribute to BC development [31]. When depression occurs, chronic stressors activate the HPA axis, resulting in adrenaline and catecholamines release. Following the HPA axis activation, adrenaline activates BC-adrenergic receptors, accumulates myeloid-derived suppressor cells (MDSCs), and promotes BC development [32]. Cortisol secreted by the adrenal cortex promotes BC cell development by activating the glucocorticoid receptor (GR) signaling pathway, serum/glucocorticoid-regulated kinase 1 (SGK1), and mitogen-activated protein kinase phosphatase 1 (MKP1)/dual-specific phosphatase 1 (DUSP1) [33]. At the same time, cortisol leads to a reduction in tumor immunosurveillance by suppressing immune function with decreased natural killer (NK) cell activity and T cell proliferation [34] (Figure 1).
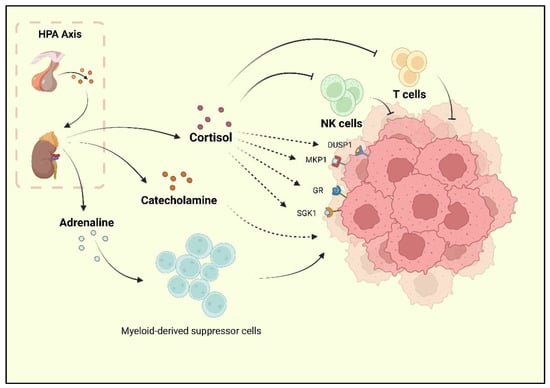
Further, depression is associated with BC partly due to the increase in macrophage activity induced by depressive phenotypes. The M1 macrophages are an important factor in inflammation in patients with severe neurological disorders [35]. Research on major depressive disorder found elevated levels of circulating cytokines in peripheral blood mononuclear cells (PBMCs), as well as increased levels of NF-kB in PBMCs [36]. Adipocytes and BC tumor cells release chemokines (e.g., C-C motif chemokine ligand 2 (CCL2), C-C motif chemokine ligand 5 (CCL-5), or colony-stimulating factor (CSF-1)) to promote the migration of monocytes and macrophages into the BC microenvironment [37,38]. These macrophages contain M1 and M2 phenotypes, while the M1 macrophages are always transformed into M2 within the BC microenvironment, and so are the monocytes [39, 40, 41, 42]. Adipocytes in the breast stroma are an important source of interleukin 10 (IL-10), which also contributes to the polarization of macrophages to the M2 phenotype in BC [43, 44, 45]. Clinical studies have also indicated that IL-10 is an independent factor in poor prognosis in TNBC, ER-negative, or PR-negative cases [46,47].
Chronic systemic inflammation induced by prolonged stress in depression has clearly been shown to be an initiating factor in carcinogenesis [48]. IL-6, one of the proinflammatory cytokines, is a signaling promoter and pathological product of depression [49]. Studies have shown that high levels of IL-6 are related to the chronic course of depression, and the severity of depression in patients with high expression of IL-6 is increased as well. The research results of elderly patients with depression show higher levels of IL-6 than in healthy elderly people [50]. Likewise, IL-6 also plays an instrumental procancer role in BC. Clinical evidence indicates that IL-6 induction is associated with a poor prognosis for a patient with BC, with plasma IL-6 levels showing a positive correlation with pathological grade. A preclinical study derived that the IL-6/ IL-6R/ gp130 pathway promotes the growth and metastasis of BC, while inhibiting the pathway is not conducive to the development of BC. Therefore, IL-6 may contribute to BC and depression in comorbid states. Additionally, TNF-α is a pathogenic cytokine in depression. One study found that the levels of proinflammatory cytokines TNF-α and IL-6 in patients with major depression increased significantly [51]. Meanwhile, anti-TNF-α drugs are found to be antidepressants [52]. The dual effect of TNF-α on BC is discussed as well [53]. The immune response further suggests that chronic inflammation is an important basis for depression and BC comorbidity. The fact of the hormone regulation and cytokine effect have been widely mentioned, but the intermediate stage of the pathogenesis of the comorbidity is still unclear.
3. Different Status of sEH Mediates Epoxide Metabolism in BC
4. Depression-Associated sEH Promotes Liver Dysfunction and Breast Cancer
References
References
- Bray, F.; Msc, M.L.; Weiderpass, E.; Soerjomataram, I. The ever‐increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. https://doi.org/10.1002/cncr.33587.
- American Cancer Society. Cancer Facts & Figures 2021; American Cancer Society: Atlanta , 2021.
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. https://doi.org/10.3322/caac.21660.
- Huang, J.; Chan, P.S.; Lok, V.; Chen, X.; Ding, H.; Jin, Y.; Yuan, J.; Lao, X.-Q.; Zheng, Z.-J.; Wong, M.C. Global incidence and mortality of breast cancer: A trend analysis. Aging 2021, 13, 5748–5803. https://doi.org/10.18632/aging.202502.
- Barzaman, K.; Karami, J.; Zarei, Z.; Hosseinzadeh, A.; Kazemi, M.H.; Moradi-Kalbolandi, S.; Safari, E.; Farahmand, L. Breast cancer: Biology, biomarkers, and treatments. Immunopharmacol. 2020, 84, 106535. https://doi.org/10.1016/j.intimp.2020.106535.
- Liedtke, C.; Mazouni, C.; Hess, K.R.; André, F.; Tordai, A.; Mejia, J.A.; Symmans, W.F.; Gonzalez-Angulo, A.M.; Hennessy, B.; Green, M.; et al. Response to Neoadjuvant Therapy and Long-Term Survival in Patients With Triple-Negative Breast Cancer. Clin. Oncol. 2008, 26, 1275–1281. https://doi.org/10.1200/jco.2007.14.4147.
- Apaya, M.K.; Shiau, J.-Y.; Liao, G.-S.; Liang, Y.-J.; Chen, C.-W.; Yang, H.-C.; Chu, C.-H.; Yu, J.-C.; Shyur, L.-F. Integrated omics-based pathway analyses uncover CYP epoxygenase-associated networks as theranostic targets for metastatic triple negative breast cancer. Exp. Clin. Cancer Res. 2019, 38, 1–22. https://doi.org/10.1186/s13046-019-1187-y.
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. Clin. Investig. 2011, 121, 2750–2767. https://doi.org/10.1172/jci45014.
- Puigpinós-Riera, R.; Graells-Sans, A.; Serral, G.; Continente, X.; Bargalló, X.; Domènech, M.; Espinosa-Bravo, M.; Grau, J.; Macià, F.; Manzanera, R.; et al. Anxiety and depression in women with breast cancer: Social and clinical determinants and influence of the social network and social support (DAMA cohort). Cancer Epidemiology 2018, 55, 123–129. https://doi.org/10.1016/j.canep.2018.06.002.
- Kissane, D.W.; Grabsch, B.; Love, A.; Clarke, D.M.; Bloch, S.; Smith, G.C. Psychiatric Disorder in Women with Early Stage and Advanced Breast Cancer: A Comparative Analysis. New Zealand J. Psychiatry 2004, 38, 320–326. https://doi.org/10.1080/j.1440-1614.2004.01358.x.
- Pelletier, G.; Verhoef, M.J.; Khatri, N.; Hagen, N. Quality of Life in Brain Tumor Patients: The Relative Contributions of Depression, Fatigue, Emotional Distress, and Existential Issues. Neuro-Oncology 2002, 57, 41–49. https://doi.org/10.1023/a:1015728825642.
- Desai, M.; Bruce, M.L.; Kasl, S.V. The Effects of Major Depression and Phobia on Stage at Diagnosis of Breast Cancer. J. Psychiatry Med. 1999, 29, 29–45. https://doi.org/10.2190/0c63-u15v-5nur-tvxe.
- Satin, J.R.; Linden, W.; Phillips, M.J. Depression as a predictor of disease progression and mortality in cancer patients. Cancer 2009, 115, 5349–5361. https://doi.org/10.1002/cncr.24561.
- Inagaki, M.; Yoshikawa, E.; Matsuoka, Y.; Sugawara, Y.; Nakano, T.; Akechi, T.; Wada, N.; Imoto, S.; Murakami, K.; Uchitomi, Y.; et al. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer 2006, 109, 146–156. https://doi.org/10.1002/cncr.22368.
- Dietrich, J.; Han, R.; Yang, Y.; Mayer-Pröschel, M.; Noble, M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. Biol. 2006, 5, 22–22. https://doi.org/10.1186/jbiol50.
- Janelsins, M.C.; Roscoe, J.A.; Berg, M.J.; Thompson, B.D.; Gallagher, M.J.; Morrow, G.R.; Heckler, C.E.; Jean-Pierre, P.; Opanashuk, L.A.; Gross, R.A. IGF-1 Partially Restores Chemotherapy-Induced Reductions in Neural Cell Proliferation in Adult C57BL/6 Mice. Cancer Investig. 2009, 28, 544–553. https://doi.org/10.3109/07357900903405942.
- Christie, L.-A.; Acharya, M.M.; Parihar, V.K.; Nguyen, A.; Martirosian, V.; Limoli, C.L. Impaired Cognitive Function and Hippocampal Neurogenesis following Cancer Chemotherapy. Cancer Res. 2012, 18, 1954–1965. https://doi.org/10.1158/1078-0432.ccr-11-2000.
- Chapman, B.; Swainston, J.; Grunfeld, E.A.; Derakshan, N. COVID-19 Outbreak Effects on Job Security and Emotional Functioning Amongst Women Living With Breast Cancer. Psychol. 2020, 11, 582014. https://doi.org/10.3389/fpsyg.2020.582014.
- Xu, C.; Ganesan, K.; Liu, X.; Ye, Q.; Cheung, Y.; Liu, D.; Zhong, S.; Chen, J. Prognostic Value of Negative Emotions on the Incidence of Breast Cancer: A Systematic Review and Meta-Analysis of 129,621 Patients with Breast Cancer. Cancers 2022, 14, 475. https://doi.org/10.3390/cancers14030475.
- Bortolato, B.; Hyphantis, T.N.; Valpione, S.; Perini, G.; Maes, M.; Morris, G.; Kubera, M.; Köhler, C.A.; Fernandes, B.S.; Stubbs, B.; et al. Depression in cancer: The many biobehavioral pathways driving tumor progression. Cancer Treat. Rev. 2016, 52, 58–70. https://doi.org/10.1016/j.ctrv.2016.11.004.
- Perez-Tejada, J.; Labaka, A.; Vegas, O.; Larraioz, A.; Pescador, A.; Arregi, A. Anxiety and depression after breast cancer: The predictive role of monoamine levels. J. Oncol. Nurs. 2021, 52, 101953. https://doi.org/10.1016/j.ejon.2021.101953.
- Newman, J.W.; Morisseau, C.; Hammock, B.D. Epoxide hydrolases: Their roles and interactions with lipid metabolism. Lipid Res. 2005, 44, 1–51. https://doi.org/10.1016/j.plipres.2004.10.001.
- Morisseau, C. Role of epoxide hydrolases in lipid metabolism. Biochime 2013, 95, 91–95. https://doi.org/10.1016/j.biochi.2012.06.011.
- Hashimoto, K. Role of Soluble Epoxide Hydrolase in Metabolism of PUFAs in Psychiatric and Neurological Disorders. Pharmacol. 2019, 10, 36. https://doi.org/10.3389/fphar.2019.00036.
- Markaverich, B.M.; Crowley, J.R.; Alejandro, M.A.; Shoulars, K.; Casajuna, N.; Mani, S.; Reyna, A.; Sharp, J. Leukotoxin Diols from Ground Corncob Bedding Disrupt Estrous Cyclicity in Rats and Stimulate MCF-7 Breast Cancer Cell Proliferation. Heal. Perspect. 2005, 113, 1698–1704. https://doi.org/10.1289/ehp.8231.
- Markaverich, B.; Mani, S.; Alejandro, M.A.; Mitchell, A.; Markaverich, D.; Brown, T.; Velez-Trippe, C.; Murchison, C.; O'Malley, B.; Faith, R. A novel endocrine-disrupting agent in corn with mitogenic activity in human breast and prostatic cancer cells.. Heal. Perspect. 2002, 110, 169–177. https://doi.org/10.1289/ehp.02110169.
- Fagundes, C.P.; Glaser, R.; Hwang, B.S.; Malarkey, W.B.; Kiecolt-Glaser, J.K. Depressive symptoms enhance stress-induced inflammatory responses. Brain, Behav. Immun. 2012, 31, 172–176. https://doi.org/10.1016/j.bbi.2012.05.006.
- Ibrahim, S.A.; Gadalla, R.; El-Ghonaimy, E.A.; Samir, O.; Mohamed, H.T.; Hassan, H.; Greve, B.; El-Shinawi, M.; Mohamed, M.M.; Götte, M. Syndecan-1 is a novel molecular marker for triple negative inflammatory breast cancer and modulates the cancer stem cell phenotype via the IL-6/STAT3, Notch and EGFR signaling pathways. Cancer 2017, 16, 1–19. https://doi.org/10.1186/s12943-017-0621-z.
- Weng, Y.-S.; Tseng, H.-Y.; Chen, Y.-A.; Shen, P.-C.; Al Haq, A.T.; Chen, L.-M.; Tung, Y.-C.; Hsu, H.-L. MCT-1/miR-34a/IL-6/IL-6R signaling axis promotes EMT progression, cancer stemness and M2 macrophage polarization in triple-negative breast cancer. Cancer 2019, 18, 1–15. https://doi.org/10.1186/s12943-019-0988-0.
- Wang, F.; Liu, J.; Liu, L.; Wang, F.; Ma, Z.; Gao, D.; Zhang, Q.; Yu, Z. The status and correlates of depression and anxiety among breast-cancer survivors in Eastern China: A population-based, cross-sectional case–control study. BMC Public Heal. 2014, 14, 326–326. https://doi.org/10.1186/1471-2458-14-326.
- Eng, J.W.-L.; Kokolus, K.M.; Reed, C.B.; Hylander, B.L.; Ma, W.W.; Repasky, E.A. A nervous tumor microenvironment: The impact of adrenergic stress on cancer cells, immunosuppression, and immunotherapeutic response. Cancer Immunol. Immunother. 2014, 63, 1115–1128. https://doi.org/10.1007/s00262-014-1617-9.
- Gosain, R.; Gage-Bouchard, E.; Ambrosone, C.; Repasky, E.; Gandhi, S. Stress reduction strategies in breast cancer: Review of pharmacologic and non-pharmacologic based strategies. Immunopathol. 2020, 42, 719–734. https://doi.org/10.1007/s00281-020-00815-y.
- Kach, J.; Conzen, S.D.; Szmulewitz, R.Z. Targeting the glucocorticoid receptor in breast and prostate cancers. Transl. Med. 2015, 7, 305ps19. https://doi.org/10.1126/scitranslmed.aac7531.
- Van Der Pompe, G.; Antoni, M.H.; Mulder, C.L.; Heijnen, C.; Goodkin, K.; De Graeff, A.; Garssen, B.; De Vries, M.J. Psychoneuroimmunology and the course of breast cancer: An overview the impact of psychosocial factors on progression of breast cancer through immune and endocrine mechanisms. Psycho-Oncology 1994, 3, 271–288. https://doi.org/10.1002/pon.2960030404.
- Prinz, M.; Priller, J. Microglia and brain macrophages in the molecular age: From origin to neuropsychiatric disease. Rev. Neurosci. 2014, 15, 300–312. https://doi.org/10.1038/nrn3722.
- Pace, T.W.; Mletzko, T.C.; Alagbe, O.; Musselman, D.L.; Nemeroff, C.B.; Miller, A.H.; Heim, C.M. Increased Stress-Induced Inflammatory Responses in Male Patients With Major Depression and Increased Early Life Stress. J. Psychiatry 2006, 163, 1630–1633. https://doi.org/10.1176/ajp.2006.163.9.1630.
- Arendt, L.M.; McCready, J.; Keller, P.J.; Baker, D.D.; Naber, S.P.; Seewaldt, V.; Kuperwasser, C. Obesity Promotes Breast Cancer by CCL2-Mediated Macrophage Recruitment and Angiogenesis. Cancer Res. 2013, 73, 6080–6093. https://doi.org/10.1158/0008-5472.can-13-0926.
- Santander, A.M.; Lopez-Ocejo, O.; Casas, O.; Agostini, T.; Sanchez, L.; Lamas-Basulto, E.; Carrio, R.; Cleary, M.P.; Gonzalez-Perez, R.R.; Torroella-Kouri, M. Paracrine Interactions between Adipocytes and Tumor Cells Recruit and Modify Macrophages to the Mammary Tumor Microenvironment: The Role of Obesity and Inflammation in Breast Adipose Tissue. Cancers 2015, 7, 143–178. https://doi.org/10.3390/cancers7010143.
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Immunol. 2010, 11, 889–896. https://doi.org/10.1038/ni.1937.
- Dirkx, A.E.M.; Egbrink, M.G.A.O.; Wagstaff, J.; Griffioen, A.W. Monocyte/macrophage infiltration in tumors: Modulators of angiogenesis. Leukoc. Biol. 2006, 80, 1183–1196. https://doi.org/10.1189/jlb.0905495.
- Junjeong, C.; Jones, G.; Haerin, J.; Seung, K.J. The role of tumor-associated macrophage in breast cancer biology. Histopathol. 2017, 33, 133–145. https://doi.org/10.14670/HH-11-916.
- Lewis, C.E.; Pollard, J.W. Distinct Role of Macrophages in Different Tumor Microenvironments. Cancer Res. 2006, 66, 605–612. https://doi.org/10.1158/0008-5472.can-05-4005.
- Alhallak, I.; Wolter, K.G.; Munoz, A.C.; A Simmen, F.; Ward, R.J.; A Petty, S.; Li, L.-X.; Simmen, R.C. Breast adipose regulation of premenopausal breast epithelial phenotype involves interleukin 10. Mol. Endocrinol. 2021, 67, 173–188. https://doi.org/10.1530/jme-21-0100.
- Qiu, X.; Zhao, T.; Luo, R.; Qiu, R.; Li, Z. Tumor-Associated Macrophages: Key Players in Triple-Negative Breast Cancer. Oncol. 2022, 12, 772615. https://doi.org/10.3389/fonc.2022.772615.
- Lucas, C.; Workman, C.J.; Beyaz, S.; LoCascio, S.; Zhao, G.; Vignali, D.A.A.; Sykes, M. LAG-3, TGF-β, and cell-intrinsic PD-1 inhibitory pathways contribute to CD8 but not CD4 T-cell tolerance induced by allogeneic BMT with anti-CD40L. Blood 2011, 117, 5532–5540. https://doi.org/10.1182/blood-2010-11-318675.
- Chu, P.-Y.; Wang, S.-M.; Chen, P.-M.; Tang, F.-Y.; Chiang, E.-P.I. Expression of MTDH and IL-10 Is an Independent Predictor of Worse Prognosis in ER-Negative or PR-Negative Breast Cancer Patients. Clin. Med. 2020, 9, 3153. https://doi.org/10.3390/jcm9103153.
- Chen, K.-Y.; Chien, W.-C.; Liao, J.-M.; Tsai, C.-W.; Chang, W.-S.; Su, C.-H.; Hsu, S.-W.; Wang, H.-C.; Bau, D.-T. Contribution of Interleukin-10 Genotype to Triple Negative Breast Cancer Risk. Anticancer Res. 2021, 41, 2451–2457. https://doi.org/10.21873/anticanres.15020.
- Kiecolt-Glaser, J.K.; Derry, H.M.; Fagundes, C.P. Inflammation: Depression Fans the Flames and Feasts on the Heat. J. Psychiatry 2015, 172, 1075–1091. https://doi.org/10.1176/appi.ajp.2015.15020152.
- Lamers, F.; Milaneschi, Y.; Smit, J.H.; Schoevers, R.A.; Wittenberg, G.; Penninx, B.W. Longitudinal Association Between Depression and Inflammatory Markers: Results From the Netherlands Study of Depression and Anxiety. Psychiatry 2019, 85, 829–837. https://doi.org/10.1016/j.biopsych.2018.12.020.
- Ng, A.; Tam, W.W.; Zhang, M.W.; Ho, C.S.; Husain, S.F.; McIntyre, R.S.; Ho, R. IL-1β, IL-6, TNF- α and CRP in Elderly Patients with Depression or Alzheimer’s disease: Systematic Review and Meta-Analysis. Rep. 2018, 8, 1–12. https://doi.org/10.1038/s41598-018-30487-6.
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A Meta-Analysis of Cytokines in Major Depression. Psychiatry 2010, 67, 446–457. https://doi.org/10.1016/j.biopsych.2009.09.033.
- Uzzan, S.; Azab, A. Anti-TNF-α Compounds as a Treatment for Depression. Molecules 2021, 26, 2368. https://doi.org/10.3390/molecules26082368.
- Cruceriu, D.; Baldasici, O.; Balacescu, O.; Berindan-Neagoe, I. The dual role of tumor necrosis factor-alpha (TNF-α) in breast cancer: Molecular insights and therapeutic approaches. Oncol. 2020, 43, 1–18. https://doi.org/10.1007/s13402-019-00489-1.
- Panigrahy, D.; Kaipainen, A.; Greene, E.R.; Huang, S. Cytochrome P450-derived eicosanoids: The neglected pathway in cancer. Cancer Metastasis Rev. 2010, 29, 723–735. https://doi.org/10.1007/s10555-010-9264-x.
- Panigrahy, D.; Edin, M.L.; Lee, C.; Huang, S.; Bielenberg, D.R.; Butterfield, C.E.; Barnés, C.M.; Mammoto, A.; Mammoto, T.; Luria, A.; et al. Epoxyeicosanoids stimulate multiorgan metastasis and tumor dormancy escape in mice. Clin. Investig. 2012, 122, 178–191. https://doi.org/10.1172/jci58128.
- Luo, J.; Yao, J.F.; Deng, X.F.; Zheng, X.D.; Jia, M.; Wang, Y.Q.; Huang, Y.; Zhu, J.H. 14, 15-EET induces breast cancer cell EMT and cisplatin resistance by up-regulating integrin αvβ3 and activating FAK/PI3K/AKT signaling. Exp. Clin. Cancer Res. 2018, 37, 1–11. https://doi.org/10.1186/s13046-018-0694-6.
- Zhu, P.; Peck, B.; Licea-Perez, H.; Callahan, J.F.; Booth-Genthe, C. Development of a semi-automated LC/MS/MS method for the simultaneous quantitation of 14,15-epoxyeicosatrienoic acid, 14,15-dihydroxyeicosatrienoic acid, leukotoxin and leukotoxin diol in human plasma as biomarkers of soluble epoxide hydrolase activity in vivo. Chromatogr. B 2011, 879, 2487–2493. https://doi.org/10.1016/j.jchromb.2011.06.042.
- Hildreth, K.; Kodani, S.D.; Hammock, B.D.; Zhao, L. Cytochrome P450-derived linoleic acid metabolites EpOMEs and DiHOMEs: A review of recent studies. Nutr. Biochem. 2020, 86, 108484. https://doi.org/10.1016/j.jnutbio.2020.108484.
- Moghaddam, M.F.; Grant, D.F.; Cheek, J.M.; Greene, J.F.; Williamson, K.C.; Hammock, B.D. Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Med. 1997, 3, 562–566. https://doi.org/10.1038/nm0597-562.
- Rody, A.; Karn, T.; Liedtke, C.; Pusztai, L.; Ruckhaeberle, E.; Hanker, L.; Gaetje, R.; Solbach, C.; Ahr, A.; Metzler, D.; et al. A clinically relevant gene signature in triple negative and basal-like breast cancer. Breast Cancer Res. 2011, 13, R97–R97. https://doi.org/10.1186/bcr3035.
- Sørlie, T.; Wang, Y.; Xiao, C.; Johnsen, H.; Naume, B.; Samaha, R.R.; Børresen-Dale, A.-L. Distinct molecular mechanisms underlying clinically relevant subtypes of breast cancer: Gene expression analyses across three different platforms. BMC Genom. 2006, 7, 127–127. https://doi.org/10.1186/1471-2164-7-127.
- Paquet, E.; Hallett, M.T. Absolute Assignment of Breast Cancer Intrinsic Molecular Subtype. JNCI: J. Natl. Cancer Inst. 2014, 107, 357. https://doi.org/10.1093/jnci/dju357.
- Badve, S.; Dabbs, D.J.; Schnitt, S.J.; Baehner, F.L.; Decker, T.; Eusebi, V.; Fox, S.B.; Ichihara, S.; Jacquemier, J.; Lakhani, S.R.; et al. Basal-like and triple-negative breast cancers: A critical review with an emphasis on the implications for pathologists and oncologists. Pathol. 2010, 24, 157–167. https://doi.org/10.1038/modpathol.2010.200.
- Phuong, N.T.T.; Kim, J.W.; Kim, J.-A.; Jeon, J.S.; Lee, J.-Y.; Xu, W.J.; Yang, J.W.; Kim, S.K.; Kang, K.W. Role of the CYP3A4-mediated 11,12-epoxyeicosatrienoic acid pathway in the development of tamoxifen-resistant breast cancer. Oncotarget 2017, 8, 71054–71069. https://doi.org/10.18632/oncotarget.20329.
- Lee, J.W.; Park, S.H. Association between depression and nonalcoholic fatty liver disease: Contributions of insulin resistance and inflammation. Affect. Disord. 2020, 278, 259–263. https://doi.org/10.1016/j.jad.2020.09.073.
- Bhardwaj, P.; Brown, K.A. Obese Adipose Tissue as a Driver of Breast Cancer Growth and Development: Update and Emerging Evidence. Oncol. 2021, 11, 638918. https://doi.org/10.3389/fonc.2021.638918.
- Ng, C.H.; Lim, W.H.; Lim, X.C.; Xiao, J.; Tan, D.J.H.; Syn, N.; Ho, C.S.H.; Kow, A.W.C.; Tan, E.X.X.; Fung, J.; et al. A meta‐analysis on the incidence of donor‐related depression after liver transplant. Int. 2021, 34, 2061–2070. https://doi.org/10.1111/tri.13975.
- Cho, I.Y.; Chang, Y.; Sung, E.; Kang, J.-H.; Wild, S.H.; Byrne, C.D.; Shin, H.; Ryu, S. Depression and increased risk of non-alcoholic fatty liver disease in individuals with obesity. Epidemiology Psychiatr. Sci. 2021, 30, e23. https://doi.org/10.1017/s204579602000116x.
- Orrù, M.G.; Pariante, C.M. Depression and liver diseases. Liver Dis. 2005, 37, 564–565. https://doi.org/10.1016/j.dld.2005.04.003.
- Sierksma, A.S.; Hove, D.L.V.D.; Steinbusch, H.W.; Prickaerts, J. Major depression, cognitive dysfunction and Alzheimer's disease: Is there a link?. J. Pharmacol. 2010, 626, 72–82. https://doi.org/10.1016/j.ejphar.2009.10.021.
- Caraci, F.; Copani, A.; Nicoletti, F.; Drago, F. Depression and Alzheimer's disease: Neurobiological links and common pharmacological targets. J. Pharmacol. 2009, 626, 64–71. https://doi.org/10.1016/j.ejphar.2009.10.022.
- Guo, P.; Chen, S.; Wang, H.; Wang, Y.; Wang, J. A Systematic Analysis on the Genes and Their Interaction Underlying the Comorbidity of Alzheimer's Disease and Major Depressive Disorder. Aging Neurosci. 2022, 13, 789698 https://doi.org/10.3389/fnagi.2021.789698.
- Baba, H.; Nakano, Y.; Maeshima, H.; Satomura, E.; Kita, Y.; Suzuki, T.; Arai, H. Metabolism of Amyloid-β Protein May Be Affected in Depression. Clin. Psychiatry 2011, 73, 115–120. https://doi.org/10.4088/jcp.10m06766.
- Kita, Y.; Baba, H.; Maeshima, H.; Nakano, Y.; Suzuki, T.; Arai, H. Serum amyloid β protein in young and elderly depression: A pilot study. Psychogeriatrics 2009, 9, 180–185. https://doi.org/10.1111/j.1479-8301.2009.00293.x.
- Pandolfo, G.; Iannuzzo, F.; Genovese, G.; Bruno, A.; Pioggia, G.; Baldari, S.; Gangemi, S. Mental Illness and Amyloid: A Scoping Review of Scientific Evidence over the Last 10 Years (2011 to 2021). Brain Sci. 2021, 11, 1352. https://doi.org/10.3390/brainsci11101352.
- Qiu, W.Q.; Zhu, H.; Dean, M.; Liu, Z.; Vu, L.; Fan, G.; Li, H.; Mwamburi, M.; Steffens, D.C.; Au, R. Amyloid-associated depression and ApoE4 allele: Longitudinal follow-up for the development of Alzheimer's disease. J. Geriatr. Psychiatry 2015, 31, 316–322. https://doi.org/10.1002/gps.4339.
- Estrada, L.D.; Ahumada, P.; Cabrera, D.; Arab, J.P. Liver Dysfunction as a Novel Player in Alzheimer’s Progression: Looking Outside the Brain. Aging Neurosci. 2019, 11, 174. https://doi.org/10.3389/fnagi.2019.00174.
- Zhao, Y.; Li, D.; Zhao, J.; Song, J.; Zhao, Y. The role of the low-density lipoprotein receptor–related protein 1 (LRP-1) in regulating blood-brain barrier integrity. Neurosci. 2016, 27, 623–634. https://doi.org/10.1515/revneuro-2015-0069.
- Daskalopoulos, E.P.; Malliou, F.; Rentesi, G.; Marselos, M.; Lang, M.A.; Konstandi, M. Stress is a critical player inCYP3A,CYP2C, andCYP2Dregulation: Role of adrenergic receptor signaling pathways. J. Physiol. Metab. 2012, 303, E40–E54. https://doi.org/10.1152/ajpendo.00545.2011.
- Bromek, E.; Daniel, W.A. The regulation of liver cytochrome P450 expression and activity by the brain serotonergic system in different experimental models. Expert Opin. Drug Metab. Toxicol. 2021, 17, 413–424. https://doi.org/10.1080/17425255.2021.1872543.
- Bromek, E.; Wójcikowski, J.; Daniel, W.A. Involvement of the paraventricular (PVN) and arcuate (ARC) nuclei of the hypothalamus in the central noradrenergic regulation of liver cytochrome P450. Pharmacol. 2013, 86, 1614–1620. https://doi.org/10.1016/j.bcp.2013.09.006.
- Sadakierska-Chudy, A.; Haduch, A.; Rysz, M.; Gołembiowska, K.; Daniel, W.A. The role of brain noradrenergic system in the regulation of liver cytochrome P450 expression. Pharmacol. 2013, 86, 800–807. https://doi.org/10.1016/j.bcp.2013.07.017.
- Wójcikowski, J.; Daniel, W.A. The brain dopaminergic system as an important center regulating liver cytochrome P450 in the rat. Expert Opin. Drug Metab. Toxicol. 2009, 5, 631–645. https://doi.org/10.1517/17425250902973703.
- Danek, P.; Kuban, W.; Daniel, W. The Effect of Chronic Iloperidone Treatment on Cytochrome P450 Expression and Activity in the Rat Liver: Involvement of Neuroendocrine Mechanisms. J. Mol. Sci. 2021, 22, 8447. https://doi.org/10.3390/ijms22168447.
- Rifkind, A.B.; Lee, C.; Chang, T.K.; Waxman, D.J. Arachidonic acid metabolism by human cytochrome P450s 2C8, 2C9, 2E1, and 1A2: Regioselective oxygenation and evidence for a role for CYP2C enzymes in arachidonic acid epoxygenation in human liver microsomes. Biochem. Biophys. 1995, 320, 380–389. https://doi.org/10.1016/0003-9861(95)90023-3.
- Qin, X.-H.; Wu, Z.; Dong, J.-H.; Zeng, Y.-N.; Xiong, W.-C.; Liu, C.; Wang, M.-Y.; Zhu, M.-Z.; Chen, W.-J.; Zhang, Y.; et al. Liver Soluble Epoxide Hydrolase Regulates Behavioral and Cellular Effects of Chronic Stress. Cell Rep. 2019, 29, 3223–3234.e6. https://doi.org/10.1016/j.celrep.2019.11.006.
- Zhang, J.; Tan, Y.; Chang, L.; Hammock, B.D.; Hashimoto, K. Increased expression of soluble epoxide hydrolase in the brain and liver from patients with major psychiatric disorders: A role of brain—Liver axis. Affect. Disord. 2020, 270, 131–134. https://doi.org/10.1016/j.jad.2020.03.070.
- Jung, J.Y.; Park, S.K.; Oh, C.-M.; Chung, P.-W.; Ryoo, J.-H. Non-Alcoholic Fatty Liver Disease and Its Association with Depression in Korean General Population. Korean Med. Sci. 2019, 34, e199. https://doi.org/10.3346/jkms.2019.34.e199.
- Harris, T.R.; Bettaieb, A.; Kodani, S.; Dong, H.; Myers, R.; Chiamvimonvat, N.; Haj, F.G.; Hammock, B.D. Inhibition of soluble epoxide hydrolase attenuates hepatic fibrosis and endoplasmic reticulum stress induced by carbon tetrachloride in mice. Appl. Pharmacol. 2015, 286, 102–111. https://doi.org/10.1016/j.taap.2015.03.022.
- Yao, L.; Cao, B.; Cheng, Q.; Cai, W.; Ye, C.; Liang, J.; Liu, W.; Tan, L.; Yan, M.; Li, B.; et al. Inhibition of soluble epoxide hydrolase ameliorates hyperhomocysteinemia-induced hepatic steatosis by enhancing β-oxidation of fatty acid in mice. J. Physiol. Liver Physiol. 2019, 316, G527–G538. https://doi.org/10.1152/ajpgi.00148.2018.
- Mello, A.; Hsu, M.-F.; Koike, S.; Chu, B.; Cheng, J.; Yang, J.; Morisseau, C.; Torok, N.J.; Hammock, B.D.; Haj, F.G. Soluble Epoxide Hydrolase Hepatic Deficiency Ameliorates Alcohol-Associated Liver Disease. Mol. Gastroenterol. Hepatol. 2021, 11, 815–830. https://doi.org/10.1016/j.jcmgh.2020.10.002.
- Liu, Y.; Dang, H.; Li, D.; Pang, W.; Hammock, B.D.; Zhu, Y. Inhibition of Soluble Epoxide Hydrolase Attenuates High-Fat-Diet–Induced Hepatic Steatosis by Reduced Systemic Inflammatory Status in Mice. PLoS ONE 2012, 7, e39165. https://doi.org/10.1371/journal.pone.0039165.
- Warner, J.; Hardesty, J.; Zirnheld, K.; McClain, C.; Warner, D.; Kirpich, I. Soluble Epoxide Hydrolase Inhibition in Liver Diseases: A Review of Current Research and Knowledge Gaps. Biology 2020, 9, 124. https://doi.org/10.3390/biology9060124.
- Rodríguez-Iglesias, N.; Nadjar, A.; Sierra, A.; Valero, J. Susceptibility of Female Mice to the Dietary Omega-3/Omega-6 Fatty-Acid Ratio: Effects on Adult Hippocampal Neurogenesis and Glia. J. Mol. Sci. 2022, 23, 3399. https://doi.org/10.3390/ijms23063399.
- Simopoulos, A. Importance of the Ratio of Omega-6/Omega-3 Essential Fatty Acids: Evolutionary Aspects. World Rev. Nutr. Diet. 2003, 92, 1–22. https://doi.org/10.1159/000073788.
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Pharmacother. 2002, 56, 365–379. https://doi.org/10.1016/s0753-3322(02)00253-6.
- Fabian, C.J.; Kimler, B.F.; Hursting, S.D. Omega-3 fatty acids for breast cancer prevention and survivorship. Breast Cancer Res. 2015, 17, 1–11. https://doi.org/10.1186/s13058-015-0571-6.
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Rev. Food Sci. Technol. 2018, 9, 345–381. https://doi.org/10.1146/annurev-food-111317-095850.
- Chen, X.; Wu, S.; Chen, C.; Xie, B.; Fang, Z.; Hu, W.; Chen, J.; Fu, H.; He, H. Omega-3 polyunsaturated fatty acid supplementation attenuates microglial-induced inflammation by inhibiting the HMGB1/TLR4/NF-κB pathway following experimental traumatic brain injury. Neuroinflammation 2017, 14, 1–12. https://doi.org/10.1186/s12974-017-0917-3.
- Simopoulos, A.P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: Nutritional implications for chronic diseases. Pharmacother. 2006, 60, 502–507. https://doi.org/10.1016/j.biopha.2006.07.080.
- Sellem, F.; Pesando, D.; Bodennec, G.; Girard, J.-P.; Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. https://doi.org/10.3390/nu8030128.
- Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.; Stanton, C. Health Implications of High Dietary Omega-6 Polyunsaturated Fatty Acids. Nutr. Metab. 2012, 2012, 1–16. https://doi.org/10.1155/2012/539426.
- Simopoulos, A.P. The Importance of the Omega-6/Omega-3 Fatty Acid Ratio in Cardiovascular Disease and Other Chronic Diseases. Biol. Med. 2008, 233, 674–688. https://doi.org/10.3181/0711-mr-311.
- Kang, J.X. The importance of omega-6/omega-3 fatty acid ratio in cell function. The gene transfer of omega-3 fatty acid desaturase. World Rev. Nutr. Dietetics. 2003, 92, 23–36.
- Simopoulos, A.P. Evolutionary Aspects of Diet: Essential Fatty Acids. World Rev. Nutr. https://doi.org/10.1385/1-59259-119-1:3.
- Ramos-Lopez, O.; Martinez-Urbistondo, D.; Vargas-Nuñez, J.A.; Martinez, J.A. The Role of Nutrition on Meta-inflammation: Insights and Potential Targets in Communicable and Chronic Disease Management. Obes. Rep. 2022, 22, 1–31. https://doi.org/10.1007/s13679-022-00490-0.
- Trovato, F.M.; Martines, G.F.; Catalano, D. Addressing Western dietary pattern in obesity and NAFLD. Nutrire 2018, 43, 11. https://doi.org/10.1186/s41110-018-0067-0.
- Yang, J.; Solaimani, P.; Dong, H.; Hammock, B.; Hankinson, O. Treatment of mice with 2,3,7,8-Tetrachlorodibenzo-p-dioxin markedly increases the levels of a number of cytochrome P450 metabolites of omega-3 polyunsaturated fatty acids in the liver and lung. Toxicol. Sci. 2013, 38, 833–836. https://doi.org/10.2131/jts.38.833.
- Fan, R.; Kim, J.; You, M.; Giraud, D.; Toney, A.M.; Shin, S.-H.; Kim, S.-Y.; Borkowski, K.; Newman, J.W.; Chung, S. α-Linolenic acid-enriched butter attenuated high fat diet-induced insulin resistance and inflammation by promoting bioconversion of n-3 PUFA and subsequent oxylipin formation. Nutr. Biochem. 2019, 76, 108285–108285. https://doi.org/10.1016/j.jnutbio.2019.108285.
- Ansari, M.H.; Ahmad, S.; Ahmad, F.; Ahmad, M.; Osman, S.M. Co‐occurrence of Coronaric and Vernolic Acids in Compositae Seed Oils. 1987, 89, 116–118. https://doi.org/10.1002/lipi.19870890307.
- Powell, R.G.; Smith, C.R.; Wolff, I.A. cis-5,cis-9,cis-12-octadecatrienoic and some unusual oxygenated acids inXeranthemum annuum seed oil. Lipids 1967, 2, 172–177. https://doi.org/10.1007/bf02530918.
- Kato, T.; Yamaguchi, Y.; Uyehara, T.; Yokoyama, T.; Namai, T.; Yamanaka, S. Self defensive substances in rice plant against rice blast disease. Tetrahedron Lett. 1983, 24, 4715–4718. https://doi.org/10.1016/s0040-4039(00)86236-x.
- Deol, S.; Fahrmann, J.; Yang, J.; Evans, J.R.; Rizo, A.; Grapov, D.; Salemi, M.; Wanichthanarak, K.; Fiehn, O.; Phinney, B.; et al. Omega-6 and omega-3 oxylipins are implicated in soybean oil-induced obesity in mice. Rep. 2017, 7, 1–13. https://doi.org/10.1038/s41598-017-12624-9.
- Burns, J.L.; Nakamura, M.T.; Ma, D.W.L. Differentiating the biological effects of linoleic acid from arachidonic acid in health and disease. Prostaglandins, Leukot. Essent. Fat. Acids 2018, 135, 1–4. https://doi.org/10.1016/j.plefa.2018.05.004.
- Bassett, J.K.; Hodge, A.M.; English, D.R.; MacInnis, R.J.; Giles, G.G. Plasma phospholipids fatty acids, dietary fatty acids, and breast cancer risk. Cancer Causes Control 2016, 27, 759–773. https://doi.org/10.1007/s10552-016-0753-2.
- Goodstine, S.L.; Zheng, T.; Holford, T.R.; Ward, B.A.; Carter, D.; Owens, P.H.; Mayne, S.T. Dietary (n-3)/(n-6) fatty acid ratio: Possible relationship to premenopausal but not postmenopausal breast cancer risk in U.S. women.. Nutr. 2003, 133, 1409–1414. https://doi.org/10.1093/jn/133.5.1409.
- Murff, H.J.; Shu, X.-O.; Li, H.; Yang, G.; Wu, X.; Cai, H.; Wen, W.; Gao, Y.-T.; Zheng, W. Dietary polyunsaturated fatty acids and breast cancer risk in Chinese women: A prospective cohort study. J. Cancer 2010, 128, 1434–1441. https://doi.org/10.1002/ijc.25703.
- Serna-Marquez, N.; Diaz-Aragon, R.; Reyes-Uribe, E.; Cortes-Reynosa, P.; Salazar, E.P. Linoleic acid induces migration and invasion through FFAR4- and PI3K-/Akt-dependent pathway in MDA-MB-231 breast cancer cells. Med Oncol. 2017, 34, 1–12. https://doi.org/10.1007/s12032-017-0969-3.
- Gonzalez-Reyes, C.; Marcial-Medina, C.; Cervantes-Anaya, N.; Cortes-Reynosa, P.; Salazar, E.P. Migration and invasion induced by linoleic acid are mediated through fascin in MDA-MB-231 breast cancer cells. Cell. Biochem. 2017, 443, 1–10. https://doi.org/10.1007/s11010-017-3205-8.
- Cao, Q.; Hersl, J.; La, H.; Smith, M.; Jenkins, J.; Goloubeva, O.; Dilsizian, V.; Tkaczuk, K.; Chen, W.; Jones, L. A pilot study of FDG PET/CT detects a link between brown adipose tissue and breast cancer. BMC Cancer 2014, 14, 126. https://doi.org/10.1186/1471-2407-14-126.
- Villegas-Comonfort, S.; Castillo-Sanchez, R.; Serna-Marquez, N.; Cortes-Reynosa, P.; Salazar, E.P. Arachidonic acid promotes migration and invasion through a PI3K/Akt-dependent pathway in MDA-MB-231 breast cancer cells. Prostaglandins, Leukot. Essent. Fat. Acids 2014, 90, 169–177. https://doi.org/10.1016/j.plefa.2014.01.007.
- Naughton, S.S.; Mathai, M.L.; Hryciw, D.H.; McAinch, A.J. Linoleic acid and the pathogenesis of obesity. Prostaglandins Other Lipid Mediat. 2016, 125, 90–99. https://doi.org/10.1016/j.prostaglandins.2016.06.003.
- Liu, J.; Han, L.; Zhu, L.; Yu, Y. Free fatty acids, not triglycerides, are associated with non-alcoholic liver injury progression in high fat diet induced obese rats. Lipids Health Dis. 2016, 15, 1–9. https://doi.org/10.1186/s12944-016-0194-7.
- Zhang, J.; Zhao, Y.; Xu, C.; Hong, Y.; Lu, H.; Wu, J.; Chen, Y. Association between serum free fatty acid levels and nonalcoholic fatty liver disease: A cross-sectional study. Rep. 2014, 4, srep05832. https://doi.org/10.1038/srep05832.
- Schuster, S.; Johnson, C.D.; Hennebelle, M.; Holtmann, T.; Taha, A.Y.; Kirpich, I.A.; Eguchi, A.; Ramsden, C.E.; Papouchado, B.G.; McClain, C.J.; et al. Oxidized linoleic acid metabolites induce liver mitochondrial dysfunction, apoptosis, and NLRP3 activation in mice. Lipid Res. 2018, 59, 1597–1609. https://doi.org/10.1194/jlr.m083741.
- Hillers-Ziemer, L.E.; Kuziel, G.; Williams, A.E.; Moore, B.N.; Arendt, L.M. Breast cancer microenvironment and obesity: Challenges for therapy. Cancer Metastasis Rev. 2022, 41, 627–647. https://doi.org/10.1007/s10555-022-10031-9.
- Kim, D.-G.; Krenz, A.; Toussaint, L.E.; Maurer, K.J.; Robinson, S.-A.; Yan, A.; Torres, L.; Bynoe, M.S. Non-alcoholic fatty liver disease induces signs of Alzheimer’s disease (AD) in wild-type mice and accelerates pathological signs of AD in an AD model. Neuroinflammation 2016, 13, 1–18. https://doi.org/10.1186/s12974-015-0467-5.
- Zhang, C.; Duan, J.; Yang, H.; Sun, C.; Zhong, W.; Tao, J.; Guan, X.; Jiang, H.; Hammock, B.D.; Hwang, S.H.; et al. COX‐2/sEH dual inhibitor PTUPB alleviates bleomycin‐induced pulmonary fibrosis in mice via inhibiting senescence. FEBS J. 2019, 287, 1666–1680. https://doi.org/10.1111/febs.15105.
- Lee, Y.-S.; Lee, H.S.; Chang, S.W.; Lee, C.U.; Kim, J.S.; Jung, Y.K.; Kim, J.H.; Seo, Y.S.; Yim, H.J.; Lee, C.H.; et al. Underlying nonalcoholic fatty liver disease is a significant factor for breast cancer recurrence after curative surgery. Medicine 2019, 98, e17277. https://doi.org/10.1097/md.0000000000017277.
- Bilici, A.; Ozguroglu, M.; Mihmanlı, I.; Turna, H.; Adaletli, I. A case–control study of non-alcoholic fatty liver disease in breast cancer. Med Oncol. 2007, 24, 367–371. https://doi.org/10.1007/s12032-007-0034-8.
- Esquivel-Velázquez, M.; Ostoa-Saloma, P.; Palacios-Arreola, M.I.; Nava-Castro, K.E.; Castro, J.I.; Morales-Montor, J. The Role of Cytokines in Breast Cancer Development and Progression. Interf. Cytokine Res. 2015, 35, 1–16. https://doi.org/10.1089/jir.2014.0026.
- Lengacher, C.A.; Reich, R.R.; Paterson, C.L.; Shelton, M.; Shivers, S.; Ramesar, S.; Pleasant, M.L.; Budhrani-Shani, P.; Groer, M.; Post-White, J.; et al. A Large Randomized Trial: Effects of Mindfulness-Based Stress Reduction (MBSR) for Breast Cancer (BC) Survivors on Salivary Cortisol and IL-6. Res. Nurs. 2018, 21, 39–49. https://doi.org/10.1177/1099800418789777.
- Danforth, D. The Role of Chronic Inflammation in the Development of Breast Cancer. Cancers 2021, 13, 3918. https://doi.org/10.3390/cancers13153918.
- Ruffell, B.; Au, A.; Rugo, H.S.; Esserman, L.J.; Hwang, E.S.; Coussens, L.M. Leukocyte composition of human breast cancer. Natl. Acad. Sci. USA 2011, 109, 2796–2801. https://doi.org/10.1073/pnas.1104303108.
- Wang, J.; Cai, D.; Ma, B.; Wu, G.; Wu, J. Skewing the Balance of Regulatory T-Cells and T-Helper 17 Cells in Breast Cancer Patients. Int. Med. Res. 2011, 39, 691–701. https://doi.org/10.1177/147323001103900301.
- Mohammed, Z.M.A.; Going, J.; Edwards, J.; Elsberger, B.; Doughty, J.C.; McMillan, D. The relationship between components of tumour inflammatory cell infiltrate and clinicopathological factors and survival in patients with primary operable invasive ductal breast cancer. Br. J. Cancer 2012, 107, 864–873. https://doi.org/10.1038/bjc.2012.347.
This entry is adapted from the peer-reviewed paper 10.3390/molecules27217269
