1. Supramolecular Combination Therapy Based on Metal-Organic Frameworks
MOF is a rising category of supramolecular host materials, the benefits of which include a facile surface functionalization process, high encapsulation efficiency, regulated drug release, and excellent biocompatibility
[1]. Therefore, MOF has been extensively developed in recent years, as has the medication delivery system built upon it.
MOFs are porous materials created by means of the pathway that metal cations or clusters are coordinated with organic ligands
[2][3]. Several macromolecular MOF materials have been created successively with clear structure and function because of the advancement of supramolecular host–guest chemistry. By causing biological macromolecules to self-assemble in a mixture of metal ions and organic ligands, these MOF materials can encapsulate biological macromolecules into their interior spaces. Moreover, due to the adaptability of coordination chemistry, these biological macromolecules, as guest molecules, can be successfully integrated into the lumen of host molecules, namely, MOFs, thus producing a host–guest system (Biomacromolecules@MOFs) with stable structure. So far, compared with cyclodextrin, crown ether and calixarene, less research has been conducted upon host–guest systems for biological macromolecules relying on MOFs.
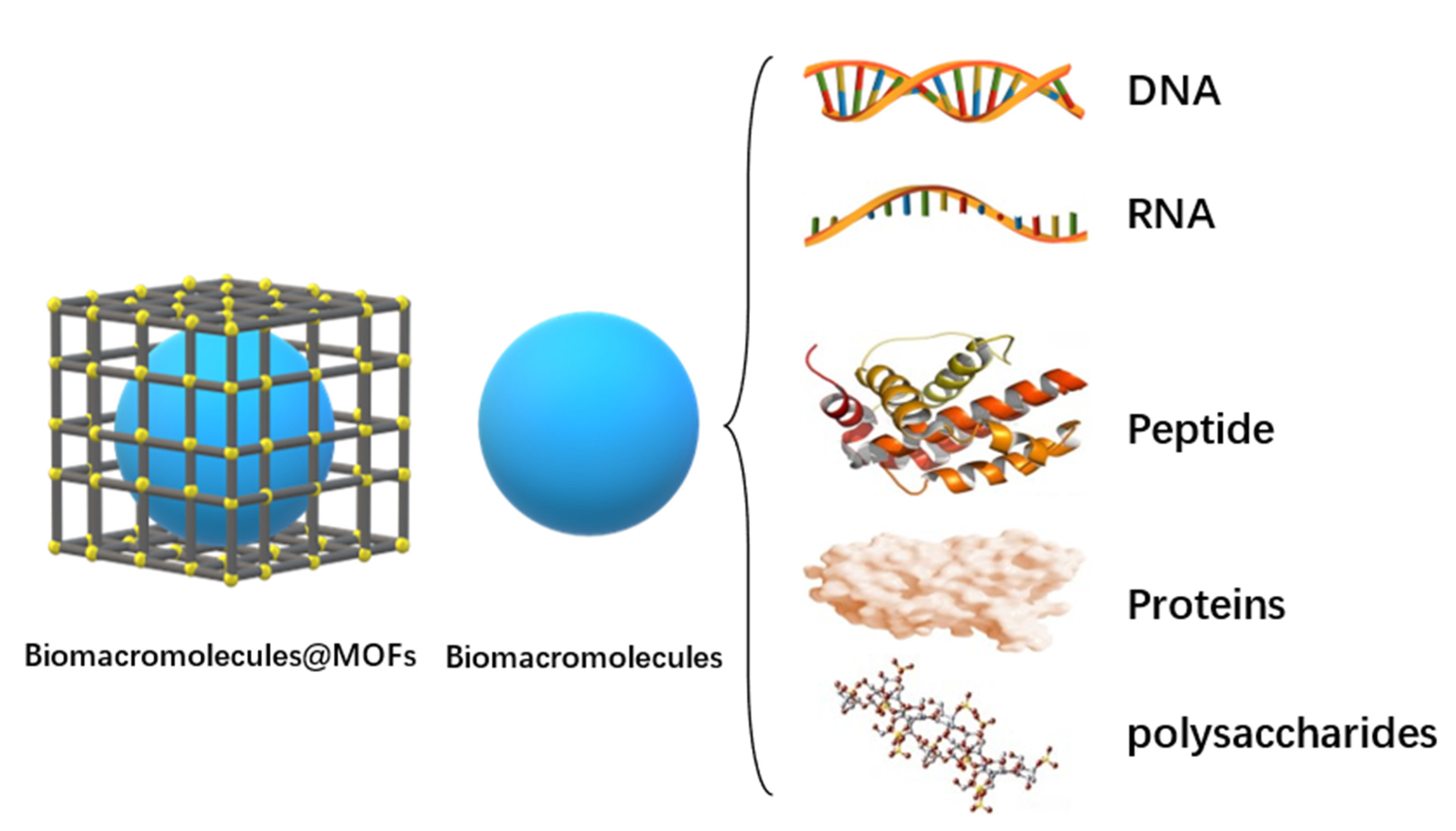
As shown in Figure 1, MOFs can combine with biomacromolecules to form “cage” structures on the outside and synthesize Biomacromolecules@MOFs molecules. By controlling the release of biomacromolecules in the human body, immunotherapy, starvation therapy, antioxidant therapy, gene therapy, multi-channel collaborative therapy, and other purposes are realized. Biomacromolecules in MOFs cavities can be DNA, RNA, peptides, proteins, polysaccharides, and other biological macromolecules, depending on their efficacy.
Figure 1. Morphology diagram of biomacromolecules@MOFs and biomacromolecules types.
There are two ways to synthesize biomacromolecules@MOFs. The first is pore encapsulation, which involves inserting biomacromolecules into the cavity of prefabricated MOFs. This method requires biomacromolecules to be consistent with the 3D shape of the cavity of MOFs. The second is in situ encapsulation. In situ encapsulation, which serves as an alternative to pore encapsulation, can absorb a wider variety of biomacromolecules, regardless of their size. The basic idea is that biomolecules are evenly encased in MOF materials after being self-assembled around biomacromolecules by MOF precursors, and eventually results in host–guest supramolecules.
Using pore encapsulation, Hu et al. synthesized a MOF material, Mg(H
2TBAPy)(H
2O)
3·C
4H
8O
2 (TDL-Mg)
[4], and then successfully loaded 5-fluorouracil (5-FU) into TDL-MG using the abundant π binding sites provided by TDL-MG. Multidirectional hydrogen bonds, numerous π-interactions, and the synergy of restricted channels produced appropriate host–guest interactions. Within 72 h, the microthermal technique released 76% of the load, which is a medically acceptable rate. Drug release is achieved by regulating the temperature.
Four coordinated water molecules and two distinct carboxyl oxygen atoms from H2TBAPy2 make up the center of Mg(II), which has an octahedral hexacoordinated shape
[5].
In addition to pore encapsulation, Cui et al. prepared microcapsules (MBMs) which have characteristics of remarkable drug loading capacity, satisfactory drug release effects, and high magnetic resonance imaging capability by solvothermal method using competitive coordination method based on Fe-MOF
[6].
In the experiment, a small amount of (NH4)6Mo7O24·4H2O and thiacetamide were added in the synthesis of MBMs to achieve the purpose of hollow structure competition with iron site and BTC ligand. In the subsequent experiments, 5-FU was loaded in this cavity structure, which achieved good drug loading and drug release ability. MBMs could release drugs over time without the need for special methods, and accordingly, the form of drug release over time cannot be controlled.
For in situ encapsulation, Chen et al.
[7] used horseradish peroxidase (HRP) as a biomacromolecule model. The MOF structure was constructed with 2-methylimidazole (HmIM) and zinc acetate to form biomacromolecules@MOFs biohybrid. However, the negative charge contributed by carboxyl groups on amino acid residues of biological macromolecules plays an important role in the process of forming MOFs around biological macromolecules. HRP with positive surface charge could not induce the formation of MOFs.
In addition to its use in cancer treatment, MOF can also be used to fight antibiotic resistance. Liao and co-workers used amide bonds to construct a zirconium-based MOF nanocomposite named UiO-66-NH-COMoS
2 (UNMS NCs)
[8]. The antibacterial mechanism of UNMS NCs can be controlled by photothermal and photodynamic regulation of peroxidase (POD)-mimic enzymatic activity. Due to their advantages of high specific surface area and pore structure, UNMS NCs capture bacteria mainly through electrostatic interactions to improve antimicrobial efficiency. Moreover, UNMS NCs can produce good thermal and photodynamic properties under 808 nm laser irradiation. A large amount of •OH produced by high specific surface area can produce significant antibacterial effect. Additionally, 808 nm laser-induced high temperature can effectively accelerate the oxidation of glutathione (GSH) and destroy the intercellular protection system of bacteria, thus effectively improving the antibacterial efficiency against ampicillin-resistant Escherichia coli (AREC) and methicillin-resistant Staphylococcus aureus (MRSA).
2. Supramolecular Combination Therapy Based on Supramolecular Coordination Complexes
From the above statement, it is not difficult to find that the metal-to-metal connections in the MOF structure are mostly linear “short rod-like” structures and diversely arranged to form infinite coordination polymers or networks. Contrarily, discrete two-dimensional or three-dimensional frameworks can be produced if the geometric combination permits convergent arrangement of nodes and connectors, which is the supramolecular coordination complex (SCCs) mentioned above
[9]. The so-called edge- and face-directed approaches are two popular synthesis techniques to produce SCC
[10]. The face-directed synthesis of SCC, developed by Fujita
[11] and also known as the “panel method”, is constructed using planar multidentate ligand molecules that coordinate with (convergent oriented) vacancies at metal nodes to form supramolecular polyhedrons or faces of polygons. Stang et al. are credited with inventing the edge-directed synthesis technique, which uses the “banana-shaped” pyridine ligand of double-ester to form the edge of SCCs, mainly binding to metal nodes of Pd(II) and Pt(II)
[12][13].
Recently, Yu et al. used a multi-component coordination self-assembly method to prepare M from 20 molecular units of three distinct classes, 4-(4′-pyridylethynyl)phenyl (HPPB), disodium terephthalate (DSTP), and chemotherapy drugs Cis-(PEt
3)
2Pt(OTf)
2 (CPT), at a ratio of 2:6:12, respectively
[14]. Subsequently, the host–guest compound (M@OEP) was synthesized through the host–guest interaction among M and OEP. N
3-PEG-b-PLBG was utilized as a polymer covering to encapsulate M@OEP into its hydrophobic cavity and provide MNPs containing 30.6% M@OEP in order to boost the stability and solubility of M. The circulating RGD (cRGDfK) function was bonded to the surface of MNPs via copper-free click reaction between cRGDfK-DBCO and azide groups of the polymer shield in order to provide the nanoparticles the capacity to target cancer cells overexpressing αVβ3 integrin. Recent research has revealed that photosensitizers and chemotherapeutic drugs may be distributed to lysosomes and endosomes, indicating that photochemotherapy could act synergistically in treating tumor cells.
3. Supramolecular Combination Therapy Based on Hierarchical Self-Assembly
As mentioned above, the synthesis of SCCs adopts convergent metal binding sites to form discrete and independent coordination complexes, while MOFs adopts divergent metal binding sites to form a frame structure that can be infinitely extended. If discrete SCCs are connected in a special way, a new class of supramolecular complexes can be obtained.
Hierarchical self-assembly (HSA) formed through multiple non-interfering mutual effects is the foundation for the formation of varieties of complicated biotic structures, such as double-stranded DNA, 3D folded polypeptide, and bioactive cytomembranes, and is essential for cell survival
[15]. Numerous noncovalent interactions, such as metal coordination, h-bonding, hydrophobic interaction, electrostatic interaction, and so on, play a significant role in the formation of HSA. When SCC is used as the original for the synthesis of HSA, metal-coordinated ligands confer considerable advantages over classical covalent methods, such as fewer steps and rapid and easy preparation of defect-free end products. The perpendicularity of metal ligands with non-covalent interactions has resulted in some unique hierarchical structures where metal acyclic cores may be built via ligand-driven self-assembly. The final hierarchy is obtained by using a second different interaction.
Using the HSA synthesis method, Wu et al. successively synthesized two new supramolecular polymers using copper(I)-phenanthroline complexes
[16][17]. In 2018, Wu et al. used a pillararene [5] dimer and a tris-[2]pseudorotaxane metallacycle containing hexacyanos on its perimeter to create a novel cross-linked supramolecular polymer.
Recently, on the basis of the above studies, Wu et al. created another redox supramolecular polymer using a well-defined rhomboidal bis-[2]pseudorotaxanes metallacycle based on coordination-driven self-assembly and host–guest interaction employing hierarchical orthogonal design strategy using Cu(I)-phenanthroline complex as a new host for linkage. In the supramolecular polymer produced by this scheme, the host–guest interaction was like the covalent bond end sealing process, and reactive redox stimulation used Cu(I)N4[2] pseudonaphthenes as the active site. The unstable tetrahedral complex Cu(II)N4 may be able to retain sufficient stability in redox processes with the aid of supramolecular polymerization. The first step in the complex preparation process is to construct [2]pseudonaphthenes with a dipyridinium (120°) donor D using a transitional Cu(I) template strategy consisting of a 120° macroloop 1 containing a fenyl and ligand 2 with a cyanosite. Subsequently, Cu(I) was used as template for the threading process of D. Finally, in order to create the novel bis-[2]pseudorotaxanes rhombohedral metallacycle R, donor building block D and matching 60° receiver A were mixed in 1 and self-assembled by coordination drive. Compared to the 2017 study, the rhombohedral metallacycle has a smaller skeleton size than the hexagonal metallacycle M, which was previously assembled using a 120° receptor. Based on the theories of the above two studies, the prepared polymers can be stimulated by redox reactions to achieve drug release when used for drug delivery.
Combined with the above narration, it is not difficult to find that cyclodextrin, pillararenes, and cucurbiturils have similar macrocyclic structures. When they are applied to construct drug delivery systems, if drugs are placed in their cavities, the differences among the three are mainly reflected in the size of the cavities, which affects the selection of drug molecules to a certain extent. They bind to the guest molecule with a non-covalent bond. Cyclodextrins mainly use hydrophobic interaction and van der Waals forces to form stable structures; pillararenes use hydrophobic interaction, hydrogen bonds, and electrostatic interactions to form stable structures; cucurbiturils use hydrogen bonds, van der waals forces, and the interaction between ion dipoles and CB[n] channels to form stable structures
[18]. Because these three have the function of transforming the optical properties of drugs, they show excellent advantages in the combination therapy involving phototherapy. The advantages of metal coordination schemes over macrocyclic schemes are mainly reflected in their high programmability. The strong directionality between the metal and the ligand drives the overall structure of the complex via self-assembly. Moreover, a variety of metal elements may show different effects on different treatment regimens when applied to drug delivery.
This entry is adapted from the peer-reviewed paper 10.3390/polym14224855