There are nearly 5000 parasitic plant species belonging to 20 families of angiosperms, and many of them cause significant losses in crops used for food and industrial purposes. Alongside the world’s major plant pests, and mistletoes are more pronounced. Striga, Orobanche, and Cuscuta have been reviewed extensively, but attention could be called to mistletoe, which has emerged as a growing global problem and requires better management and control. The mistletoe is a hemiparasitic plant that clings to trees and depletes them of nutrients and materials, and, in many cases, heightened infection can result in tree death. Efficient seed-dispersing mechanisms and/or frugivorous avians, as well as highly diversified haustorial structures, contribute to their enhanced tropism. These pests severely affect tree plantations across the globe. In addition to being a keystone resource of biodiversity and, to a somewhat minimal extent, their medicinal assets, they have also raised serious concerns regarding the commercial fruit and timber farming communities. In spite of the efforts of these and some research communities, conventional mistletoe management approaches have not succeeded in mitigating the mistletoe problem. On the contrary, most of these approaches, such as deliberate fires, herbicide use, pruning, pollarding, plastic wrapping and so on, overlook damages caused to the environment and public health. To counter this, newer approaches followed, exploring hyperparasitism from biological entities that thrive on the mistletoes and testing their worth as mistletoe biocontrol agents (MBCAs). However, no MBCA formulation has yet been translated to the market. Despite some silvicultural trials in small-scale settings, other biotechnological interventions were limited to time-consuming and laborious tree-breeding strategies. Beyond these, transgenic approaches and smart solutions have not yet been explored in the 21st century. In the face of emerging new host records and enhanced tropism, mistletoe seems to be outperforming management initiatives and losing the necessary pace of development and advancement.
- mistletoe
- biocontrol
- transcriptome
- parasitic plant
- resistance
- seed dispersal
1. The Biology of Mistletoe
Mistletoes occur in the order Santalales, occupying the Loranthaceae (approx. 1000 species) and Viscaceae (approx. 550 species) [1]. Notably, the most notoriously damaging species in the are the honey-suckled (Dendrophthoe spp.), the showy (Helixanthera spp. and Psittacanthus spp.), and the red mistletoes (Tapinanthus spp.), while, among the Viscaceae are the Dwarf (Arceuthobium spp.), the American (Phoradendron spp.), and the European mistletoes (Viscum spp.) [2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29]. Mistletoes are characterized as hemiparasitic plants because of their reduced photosynthetic efficiency and the absence of a true rooting feature [30][31]. A false root-like appendage, known as a haustorium, attaches them to their host plants (mostly trees) and draws water and nutrients from them [32][33][34] (Figure 1). Generally, these haustorial connections lack a retranslocation system, meaning that the hemiparasites directly and exclusively associate with the host xylem, but exploitation of the host phloem is never reported [35][36][37]. By transpiring almost nine times as quickly as their hosts, mistletoes suppress their host’s ability to maintain water potential, thus causing early stomatal closure and reduced carbon assimilation [31][38][39][40][41][42]. Hosts find it difficult to maintain their water, carbohydrate, and mineral profiles, especially under drought and soil infertility conditions [43][44][45][46]. In addition, climate change may add to the host detriments since mistletoe may spread to new geographical regions, possibly infecting new hosts and increasing in infectivity[39][43][44]. Mistletoe seeds are dispersed predominantly by fruit-eating birds[47][48]. Some birds have coevolved with mistletoes exhibiting fruiting displays [49]. Mistletoes attract a narrow range of avian dispersers that have anatomical adaptations and dietary preferences specific to mistletoe fruits [33][49][50]. Some Viscaceae mistletoe in the genera Arceuthobium and Korthalsella are equipped with explosive dispersal mechanisms in their seeds [33]. Seed dispersal by these modes has possibly allowed mistletoe to spread to nearby potential host trees, as well as those in islands and continents far off [51][52][53][54]. Seed dispersal on a compatible tree host marks the start of the mistletoe life cycle and parasitism (Figure 2).
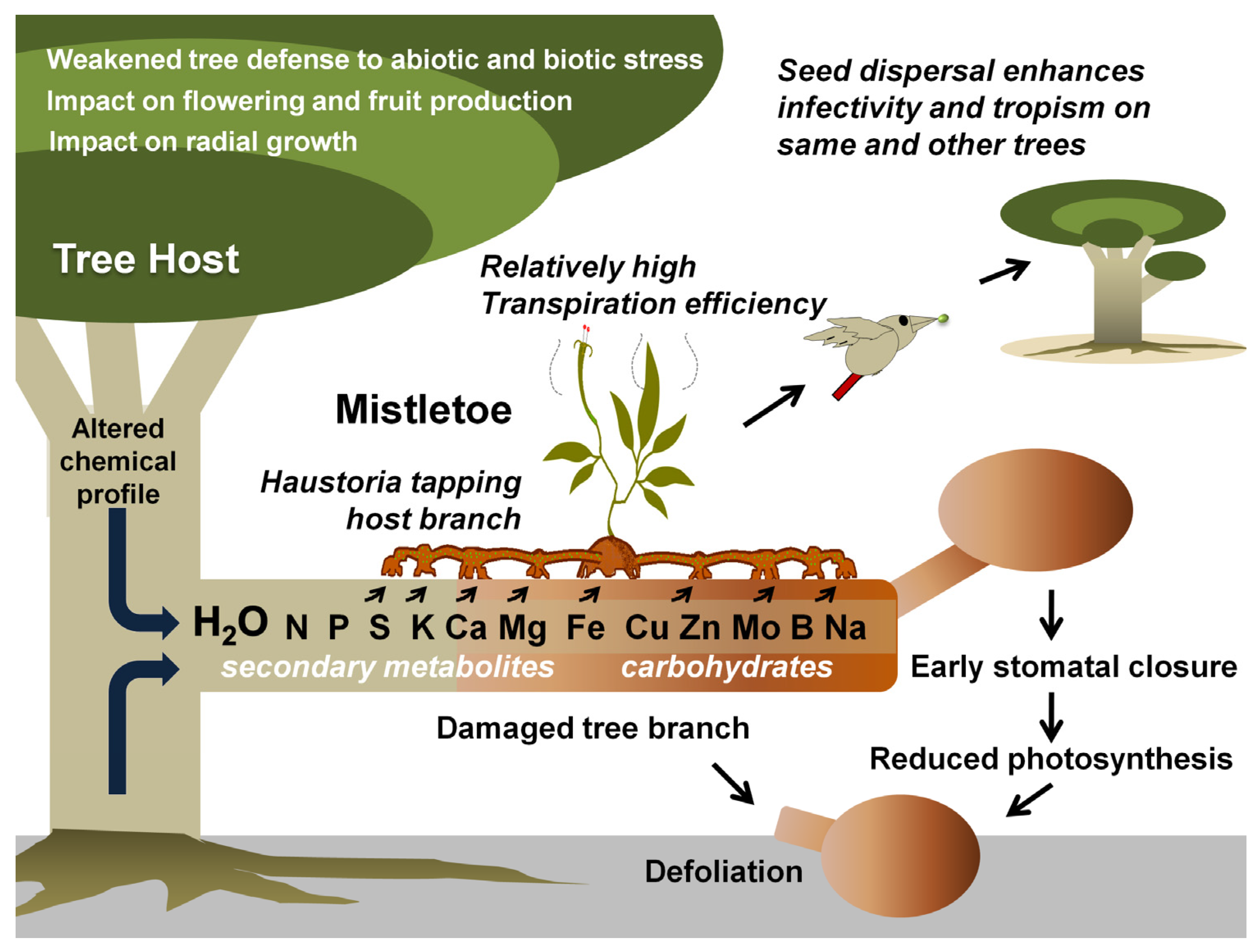
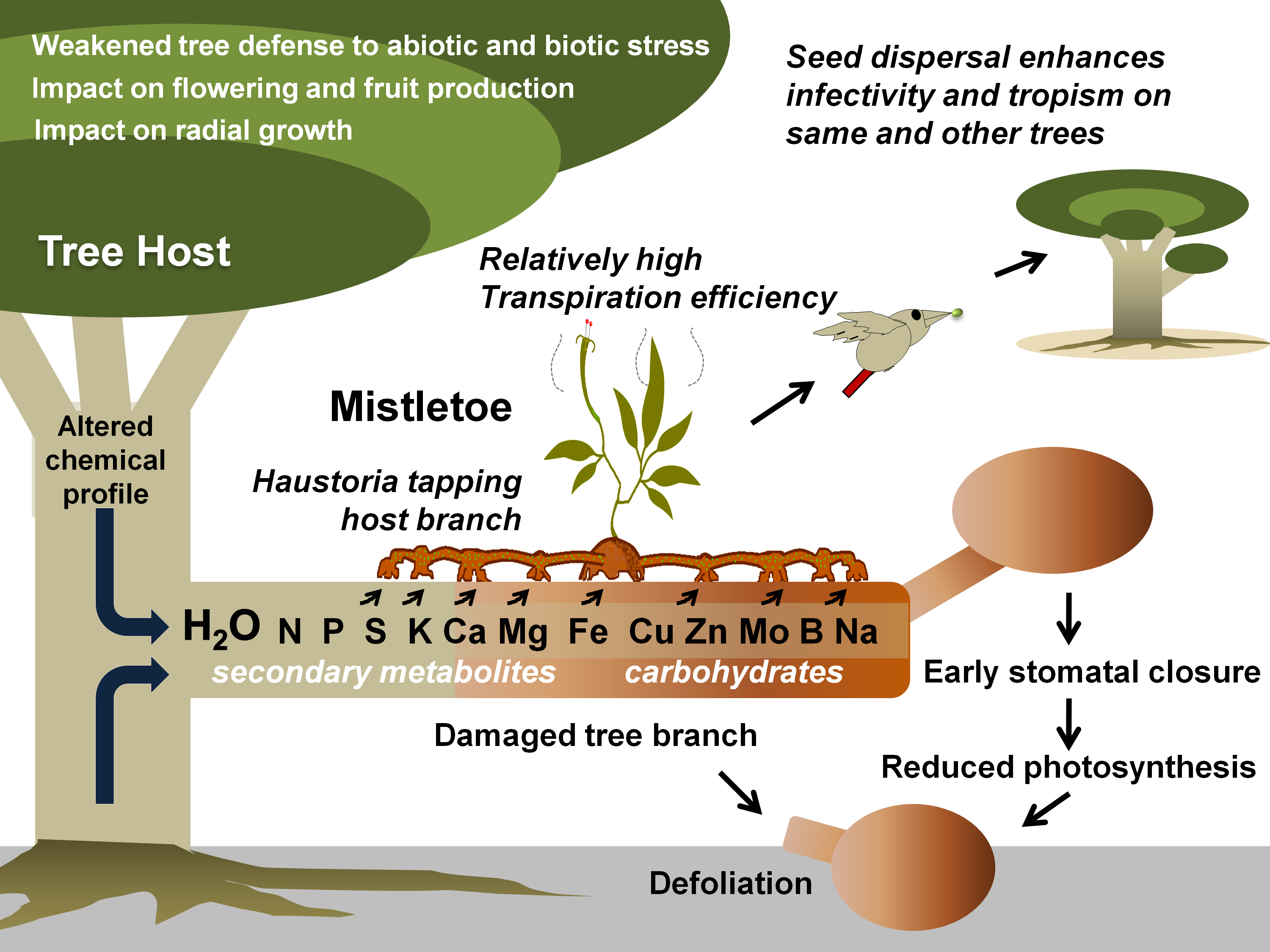
Figure 1. Mistletoe parasitism and impacts on a tree host. Specific mistletoe may parasitize its host by exploiting its branch(es) for water and various other resources, thereby limiting its photosynthetic potential, optimal growth, response to various stresses, and fruit or timber production. Gradually, infective spread (by haustorial connections and endophytic expansion) and growing infection instances (ensured by mistletoe seed dispersal) may lead to early senescence of the tree(s). 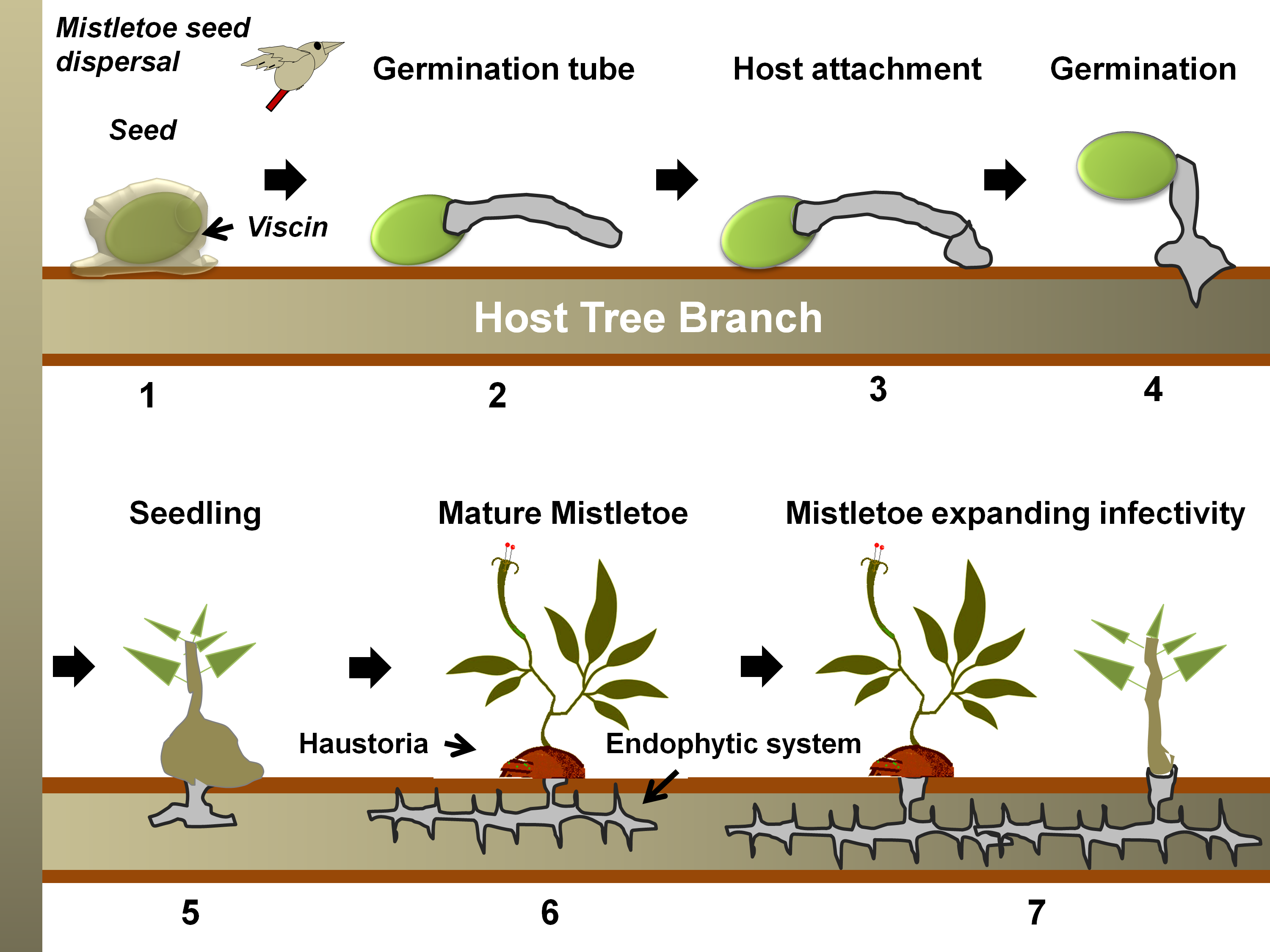
Figure 2. A generalized life cycle of a mistletoe parasite on a tree host. Following seed dispersal, a sticky material, viscin, over the seed coat ensures firm attachment to the host branch (in 1) [55][56]. Hypocotyl(s) from the seed then emerges and grows towards the bark (in 2), which eventually, while attaching to the bark (in 3), penetrates through it (in 4) [57]. Once the hypocotyl connects to the host xylem (in 5), it exploits the host for water, minerals, carbohydrates, and secondary metabolites (in 6) [51]. The endophytic system may expand as sinkers (or epicortical roots) along the host cambium, from which sporadically new shoots may emerge (in 7) and facilitate the heightening of infectivity [32].
2. Conventional Control Strategies and Integrated Pest Management Approaches
2.1. Physical Methods
2.2. Chemical Methods
2.3. Silvicultural Practices
3. Mistletoe Control through Biotechnological Interventions and Smart Management
3.1. Mistletoe Biocontrol Agents (MBCAs)
3.2. Requirements of an Effective MBCA Selection Program

3.3. Challenges with MBCAs
3.4. Inducing Host Plant Resistance to Mistletoes and/or Herbicides
3.5. Hunting for the Genetic Basis to Hosts’ Inherent Resistance to Mistletoes: Background Studies
3.6. Mistletoe Community Restructuring, Disturbances, and Biological Interactions
3.7. Transcriptomic/Metabolomic Profiling, Transgenic Trees, and Translational Research Pipeline
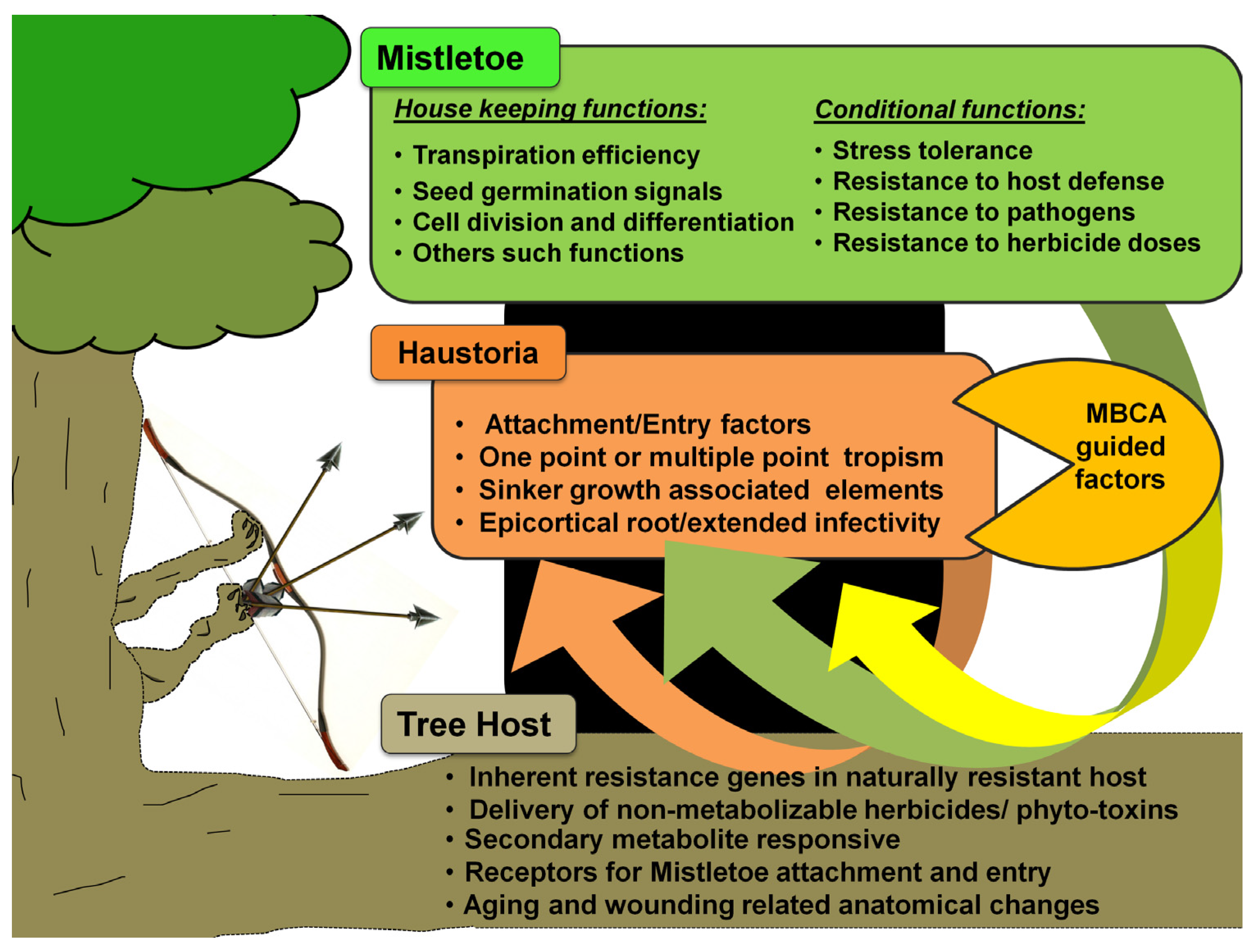
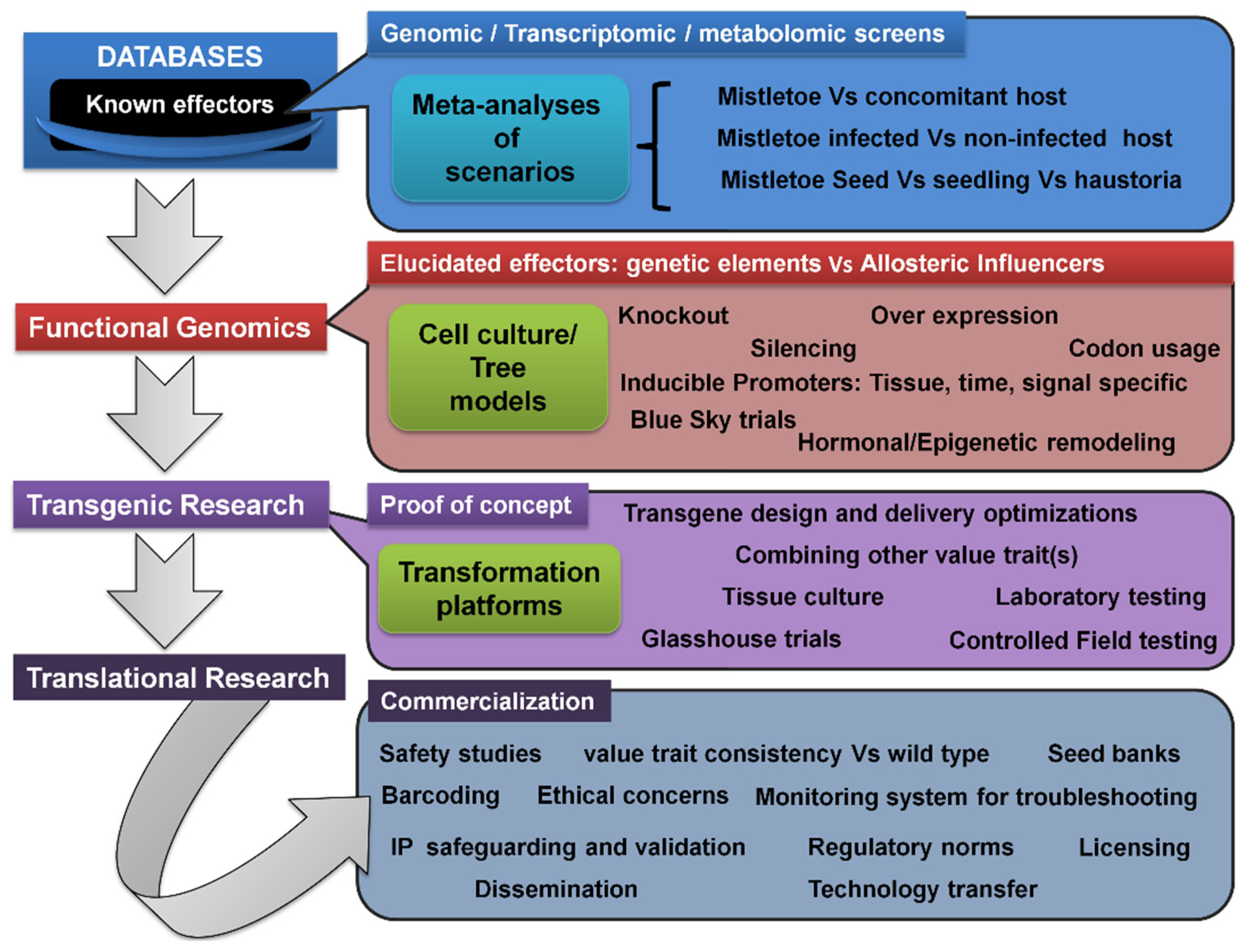
3.8. Role of Epigenetic Signaling in Mistletoe Parasitism: Moving beyond the Genetic Basis
3.9. Smart Mistletoe Management: How the 21st Century Can Mitigate the Mistletoe Problem
Considering the aforementioned promising approaches, and not neglecting the ecological value of the mistletoes (in being a keystone resource for biodiversity), the genetic engineering of hosts to develop, induce, and enhance specific resistance will be greatly rewarding. Even though its benefits include being environmentally safe and with no use of chemical agents, due to the additional labor, costs, complicated management, as well as potential to wipe out the parasite seed banks on hosts, this approach has remained unrealized [135]. Such transgenic trees would satisfy both (i) the conservation of the biodiversity and medicinal value aspects of mistletoes and (ii) aims to foresee a fruit and timber business that is unaffected by mistletoe. Successful examples of the transgenic approach are known in relation to other parasitic weeds, such as Striga, Orobanche, and Cuscuta . In this regard, herein, it was intended to supply blueprints for transgenic and translational research into developing and commercializing stable mistletoe-resistant tree cultivars.
Herein, ecological significance and medicinal value of mistletoes do not be underestimated, both in traditional and modern treatment practices. However, here it was emphasized the development and implementation of more feasible management solutions for highly damaging mistletoes that affect commercial tree plantations. The risks surely outweigh the usefulness of mistletoes in such settings, as others pointed out long ago [124]. Thus, tree decline, combined with mistletoe, cannot be overlooked in itself as a major factor negatively impacting biodiversity and commerce. Hopefully, the biotechnological and smart management approaches discussed here, if operationalized in the future, should serve as a paradigm shift in mistletoe management.
This entry is adapted from the peer-reviewed paper 10.3390/biology11111645
References
- Choi, S.-U.; Kim, S.T.; Han, D.G.; Hwang, Y.-H.; Lee, K.Y.; Kim, D.U.; Cho, K.H.; Park, S.Y.; Kim, H.-C.; Kim, S.-B.; et al. Comparative Assessment of Biological Activities of Mistletoes for Cosmetic Applications: Viscum Album Var. Coloratum (Kom.) Ohwi and Loranthus Tanakae Franch. & Sav. J. Cosmet. Sci. 2019, 70, 235–245.
- Mudgal, G.; Mudgal, B. Evidence for unusual choice of host and haustoria by Dendrophthoe falcata (Lf) Ettingsh, a leafy mistletoe. Arch. Phytopathol. Plant Prot. 2011, 44, 186–190.
- Mudgal, G.; Mudgal, B.; Gururani, M.A.; Jelli, V. Pseudaulacaspis cockerelli (Cooley) hyperparasitizing Dendrophthoe falcata (Lf) Ettingsh. Arch. Phytopathol. Plant Prot. 2011, 44, 282–286.
- Singh, S.R.; Piloo, N.; Senjam, P.; Hemanta, L. Need of awareness programme to control the loranthus weed–Helixanthera ligustrina. Agri-India Today 2021, 1, 10–13.
- Arce-Acosta, I.; Suzán-Azpiri, H.; García-Rubio, O. Biotic factors associated with the spatial distribution of the mistletoe Psittacanthus calyculatus in a tropical deciduous forest of central Mexico. Bot. Sci. 2016, 94, 89–96.
- Fadini, R.F.; Fischer, E.; Castro, S.J.; Araujo, A.C.; Ornelas, J.F.; de Souza, P.R. Bat and bee pollination in Psittacanthus mistletoes, a genus regarded as exclusively hummingbird-pollinated. Ecology 2018, 99, 1239–1241.
- Infante, S.D.; Lara, C.; del Coro Arizmendi, M.; Eguiarte, L.E.; Ornelas, J.F. Reproductive ecology and isolation of Psittacanthus calyculatus and P. auriculatus mistletoes (Loranthaceae). PeerJ 2016, 4, e2491.
- Pérez-Crespo, M.J.; Lara, C.; Ornelas, J.F. Uncorrelated mistletoe infection patterns and mating success with local host specialization in Psittacanthus calyculatus (Loranthaceae). Evol. Ecol. 2016, 30, 1061–1080.
- Teodoro, G.S.; van den Berg, E.; Santos, M.d.C.N.; de Freitas Coelho, F. How does a Psittacanthus robustus Mart. population structure relate to a Vochysia thyrsoidea Pohl. host population? Flora-Morphol. Distrib. Funct. Ecol. Plants 2010, 205, 797–801.
- Aliero, B.; Samaila, A. The occurrence of Parasitic mistletoe (Tapinanthus spp) on Parkia biglobosa (Jacq.) Benth (African Locust Bean Tree) in Yauri Local Government Area, Kebbi State. Niger. J. Basic Appl. Sci. 2000, 9, 5–10.
- Edagbo, D.E.; Ajiboye, T.O.; Borokini, T.I.; Ighere, D.A.; Alowonle, A.A.; Clement, M. The Influence of African Mistletoe (Tapinanthus bangwensis) on the Conservation Status and Productivity of Irvingia gabonensis in Moor Plantation Area of Ibadan, Nigeria. Int. J. Curr. Res. 2012, 4, 484–487.
- Kwon-Ndung, E.; Ismaila, A. Prospects of host resistance in improved and domesticated species of Parkia biglobosa to African mistletoes (Tapinanthus spp.) in Central Nigeria. Electron. J. Environ. Agric. Food Chem. 2009, 8, 382–388.
- Ávalos, V.M.C.; Collazo, I.V.; Flores, H.J.M.; Castillo, J.V. Impacto de tierra de diatomeas sobre Arceuthobium globosum Hawksworth & Wiens subsp. grandicaule en Pinus pseudostrobus Lindl. Rev. Mex. Cienc. For. 2010, 1, 39–46.
- Gilbert, J.A. The Biology of Dwarf Mistletoes (Arceuthobium spp.) in Manitoba. Master Thesis, University of Manitoba, Winnipeg, MB, Canada, 1984.
- Hernández-Benítez, R.; Cano-Santana, Z.; Castellanos-Vargas, I. Incidencia de infestación de Arceuthobium globosum grandicaule (Hawks. y Wiens) en Pinus hartwegii (Lindl.). Cienc. For. En México 2005, 30, 79–86.
- Queijeiro-Bolanos, M.E.; Cano-Santana, Z.; Castellanos-Vargas, I. Does disturbance determine the prevalence of dwarf mistletoe (Arceuthobium, Santalales: Viscaceae) in Central Mexico? Rev. Chil. De Hist. Nat. 2013, 86, 181–190.
- Shaw, D.C.; Agne, M.C.J.B. Fire and dwarf mistletoe (Viscaceae: Arceuthobium species) in western North America: Contrasting Arceuthobium tsugense and Arceuthobium americanum. Botany 2017, 95, 231–246.
- Villier, J.A.d.; Reblin, J.S.; Logan, B.A. Needle properties of host white spruce (Picea glauca Voss) experiencing eastern dwarf mistletoe (Arceuthobium pusillum Peck) infections of differing severity. Botany 2017, 95, 295–305.
- Anselmo-Moreira, F.; Teixeira-Costa, L.; Ceccantini, G.; Furlan, C.M. Mistletoe effects on the host tree Tapirira guianensis: Insights from primary and secondary metabolites. Chemoecology 2019, 29, 11–24.
- Solís-Gracia, V.; Suzán-Azpiri, H. Análisis de la distribución espacial del muérdago (Phoradendron californicum) en el sur del Desierto Sonorense. Cactáceas Suculentas Mex. 2014, 59, 11–28.
- Wiens, D.; Hawksworth, F. New species of Phoradendron (Viscaceae) from Mexico and Guatemala and a synopsis of species in section Pauciflorae. Aliso J. Syst. Evol. Bot. 2002, 21, 33–43.
- Ahmad, S.; Mir, N.; Sultan, S. White-berry mistletoe (Viscum album L.): A hemiparasitic plant: Occurrence and ethnobotanical use in Kashmir. J. Pharmacogn. Phytochem. 2018, 7, 1831–1833.
- Alam, M. A critical review on the biology and control of Loranthaceae with a particular reference to Bangladesh. Bano Biggyan Patrika 1984, 13, 7–18.
- Barbu, C. Impact of mistletoe attack (Viscum album ssp. abietis) on the radial growth of silver fir. A case study in the North of Eastern Carpathians. Ann. For. Res. 2009, 52, 89–96.
- Bilgili, E.; Coskuner, K.A.; Öztürk, M. Leaf area–sapwood area relationship in Scots pine (Pinus sylvestris L.) under mistletoe (Viscum album ssp. austriacum) infection. Dendrobiology 2020, 84, 1–11.
- Dobbertin, M.; Rigling, A. Pine mistletoe (Viscum album ssp. austriacum) contributes to Scots pine (Pinus sylvestris) mortality in the Rhone valley of Switzerland. For. Pathol. 2006, 36, 309–322.
- Mutlu, S.; Osma, E.; Ilhan, V.; Turkoglu, H.I.; Atici, O. Mistletoe (Viscum album) reduces the growth of the Scots pine by accumulating essential nutrient elements in its structure as a trap. Trees 2016, 30, 815–824.
- Ozturk, M.; Coskuner, K.A.; Serdar, B.; Atar, F.; Bilgili, E. Impact of white mistletoe (Viscum album ssp. abietis) infection severity on morphology, anatomy and photosynthetic pigment content of the needles of cilicican fir (Abies cilicica). Flora 2022, 294, 152135.
- Ozturk, M.; Coskuner, K.A.; Usta, Y.; Serdar, B.; Bilgili, E. The effect of mistletoe (Viscum album) on branch wood and needle anatomy of Scots pine (Pinus sylvestris). IAWA J. 2019, 40, 352–365.
- Nickrent, D.L. Parasitic angiosperms: How often and how many? Taxon 2020, 69, 5–27.
- Glatzel, G.; Geils, B. Mistletoe ecophysiology: Host–parasite interactions. Botany 2008, 87, 10–15.
- Calvin, C.L.; Wilson, C.A. Comparative morphology of epicortical roots in Old and New World Loranthaceae with reference to root types, origin, patterns of longitudinal extension and potential for clonal growth. Flora-Morphol. Distrib. Funct. Ecol. Plants 2006, 201, 51–64.
- Barlow, B. MIstletoes in Australia. Available online: http://www.anbg.gov.au/mistletoe/haustoria.html (accessed on 12 January 2020).
- Wilson, C.A.; Calvin, C.L. An origin of aerial branch parasitism in the mistletoe family, Loranthaceae. Am. J. Bot. 2006, 93, 787–796.
- Smith, S.; Stewart, G.R. Effect of Potassium Levels on the Stomatal Behavior of the Hemi-Parasite Striga hermonthica. Plant Physiol. 1990, 94, 1472–1476.
- Lo Gullo, M.A.; Glatzel, G.; Devkota, M.; Raimondo, F.; Trifilò, P.; Richter, H. Mistletoes and mutant albino shoots on woody plants as mineral nutrient traps. Ann. Bot. 2012, 109, 1101–1109.
- Karunaichamy, K.S.T.K.; Paliwal, K.; Arp, P.A. Biomass and nutrient dynamics of mistletoe (Dendrophthoe falcata) and neem (Azadirachta indica) seedlings. Curr. Sci. 1999, 76, 840–842.
- Schulze, E.D.; Turner, N.; Glatzel, G. Carbon, water and nutrient relations of two mistletoes and their hosts: A hypothesis. Plant Cell Environ. 1984, 7, 293–299.
- Zweifel, R.; Bangerter, S.; Rigling, A.; Sterck, F.J. Pine and mistletoes: How to live with a leak in the water flow and storage system? J. Exp. Bot. 2012, 63, 2565–2578.
- Urban, J.; Gebauer, R.; Nadezhdina, N.; Čermák, J. Transpiration and stomatal conductance of mistletoe (Loranthus europaeus) and its host plant, downy oak (Quercus pubescens). Biologia 2012, 67, 917–926.
- Glatzel, G. Mineral nutrition and water relations of hemiparasitic mistletoes: A question of partitioning. Experiments with Loranthus europaeus on Quercus petraea and Quercus robur. Oecologia 1983, 56, 193–201.
- Yang, D.; Goldstein, G.; Wang, M.; Zhang, W.-W.; Wang, A.-Y.; Liu, Y.-Y.; Hao, G.-Y. Microenvironment in the canopy rivals the host tree water status in controlling sap flow of a mistletoe species. Tree Physiol. 2017, 37, 501–510.
- Rigling, A.; Eilmann, B.; Koechli, R.; Dobbertin, M. Mistletoe-induced crown degradation in Scots pine in a xeric environment. Tree Physiol. 2010, 30, 845–852.
- Dobbertin, M.; Hilker, N.; Rebetez, M.; Zimmermann, N.E.; Wohlgemuth, T.; Rigling, A. The upward shift in altitude of pine mistletoe (Viscum album ssp. austriacum) in Switzerland—The result of climate warming? Int. J. Biometeorol. 2005, 50, 40–47.
- Doležal, J.; Lehečková, E.; Sohar, K.; Altman, J. Oak decline induced by mistletoe, competition and climate change: A case study from central Europe. Preslia 2016, 88, 323–346.
- Matula, R.; Svátek, M.; Pálková, M.; Volařík, D.; Vrška, T. Mistletoe infection in an oak forest is influenced by competition and host size. PLoS ONE 2015, 10, e0127055.
- Aukema, J.E.; Martinez del Rio, C. Mistletoes as parasites and seed-dispersing birds as disease vectors: Current understanding, challenges, and opportunities. In Seed Dispersal and Frugivory: Ecology, Evolution, and Conservation; CABI International: Wallingford, UK, 2002; pp. 99–110.
- Aukema, J.E.; Martínez del Rio, C. Where does a fruit-eating bird deposit mistletoe seeds? Seed deposition patterns and an experiment. Ecology 2002, 83, 3489–3496.
- Reid, N. Coevolution of mistletoes and frugivorous birds? Aust. J. Ecol. 1991, 16, 457–469.
- Green, A.K.; Ward, D.; Griffiths, M.E. Directed dispersal of mistletoe (Plicosepalus acaciae) by Yellow-vented Bulbuls (Pycnonotus xanthopygos). J. Ornithol. 2009, 150, 167–173.
- Mathiasen, R.L.; Nickrent, D.L.; Shaw, D.C.; Watson, D.M. Mistletoes: Pathology, systematics, ecology, and management. Plant Dis. 2008, 92, 988–1006.
- Watson, D.M. The Relative Contribution of Specialists and Generalists to Mistletoe Dispersal: Insights from a Neotropical Rain Forest. Biotropica 2013, 45, 195–202.
- Albert, S.; Rhumeur, A.; Rivière, J.L.; Chauvrat, A.; Sauroy-Toucouère, S.; Martos, F.; Strasberg, D. Rediscovery of the mistletoe Bakerella hoyifolia subsp. bojeri (Loranthaceae) on Reunion Island: Population status assessment for its conservation. Bot. Lett. 2017, 164, 229–236.
- Medel, R.; Vergara, E.; Silva, A.; Kalin-Arroyo, M. Effects of vector behavior and host resistance on mistletoe aggregation. Ecology 2004, 85, 120–126.
- Gill, L.S.; Hawksworth, F.G. The Mistletoes: A Literature Review; US Department of Agriculture: Washington, DC, USA, 1961.
- Berry, A.M.; Lichter, J.M.; Reid, M.S. New Methods for Mistletoe Control. Available online: http://slosson.ucdavis.edu/newsletters/Berry_199129111.pdf (accessed on 29 December 2019).
- Ko, S.M.; Kwon, Y.K.; Kim, J.H.; Song, I.-J.; Lee, H.-Y.; Choi, D.-W.; Liu, J.R.; Kim, S.W. Transcriptome analysis of mistletoe (Viscum album) haustorium development. Hortic. Environ. Biotechnol. 2014, 55, 352–361.
- Scharpf, R.F.; Smith, R.S.; Vogler, D. Management of Western Dwarf Mistletoe in Ponderosa and Jeffrey Pines in Forest Recreation Areas; Pacific Southwest Research Station, Forest Service; US Department of Agriculture: Washington, DC, USA, 1988; Volume 103, 11p.
- Beatty, J.S.; Mathiasen, R.L. Dwarf Mistletoes of Ponderosa Pine; US Department of Agriculture, Forest Service: Washington, DC, USA, 2003.
- Pearson, G.; Marsh, R. Timber Growing and Logging Practice in the Southwest and in the Black Hills Region. Available online: https://naldc.nal.usda.gov/download/CAT86200474/PDF (accessed on 22 October 2022).
- Knutson, D. Biological and chemical control of dwarf mistletoe. In Proceedings of the Symposium on Dwarf Mistletoe Control through Forest Management, Berkeley, CA, USA, 11–13 April 1978; Gen. Tech. Rep. PSW-31. Scharpf, R.F., Parmeter, J.R., Jr., Eds.; U.S. Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station: Berkeley, CA, USA, 1978; pp. 151–155.
- Milenkovic, M.; Karadzic, D.; Janjic, V.; Mihajlovic, L. Possibilities of mistletoe control. In Proceedings of the Peti Kongres o Korovima, Banja Koviljaca, Serbia, 18–21 June 1996.
- Watson, D. Reconnaissance and Recommendations for Mistletoe Management in Macadamia Orchards; Final Report; Hort Innovation: North Sydney, Australia, 2019; pp. 1–30.
- Reid, N.; Shamoun, S.F. Contrasting research approaches to managing mistletoes in commercial forests and wooded pastures. Botany 2008, 87, 1–9.
- Yan, C.-F.; Gessler, A.; Rigling, A.; Dobbertin, M.; Han, X.-G.; Li, M.-H. Effects of mistletoe removal on growth, N and C reserves, and carbon and oxygen isotope composition in Scots pine hosts. Tree Physiol. 2016, 36, 562–575.
- Walldén, B. Misteln vid dess nordgräns: Die Mistel an Ihrer Nordgrenze. Svensk Botanisk Tidskrift 1961, 55, 123.
- Weber, H. Untersuchungen zur Entwicklungsweise der Laubholzmistel Viscum album L.(Viscaceae) und über Zuwachsraten während ihrer ersten Stadien. Beiträge Zur Biol. Der Pflanz. 1993, 67, 319–331.
- Hoffman, J. Management Guide for Dwarf Mistletoe. Arceuthobium spp. Forest Health Protection and State Forestry Organizations. Available online: https://www.fs.usda.gov/Internet/FSE_DOCUMENTS/stelprdb5187427.pdf (accessed on 22 October 2022).
- Zielke, K.; Bancroft, B. Introduction to Silvicultural Systems (Web-Based Workbook). Available online: https://www.for.gov.bc.ca/hfd/pubs/ssintroworkbook/append2.htm (accessed on 20 July 2020).
- Parmeter, J.; Scharpf, R.F. Spread of Dwarf Mistletoe from Discrete Seed Sources into Young Stands of Ponderosa and Jeffrey Pines; US Forest Service: Washington, DC, USA; US Department of Agriculture, Pacific Southwest Forest and Range: Vallejo, CA, USA, 1972; Volume 269.
- Scharpf, R.F.; Parmeter, J. Seed Production and Dispersal by Dwarf Mistletoe in Overstory Jeffrey Pines in California; US Forest Service: Washington, DC, USA; US Department of Agriculture, Pacific Southwest Forest and Range: Berkeley CA, USA, 1971; Volume 247.
- Scharpf, R.F.; Parmeter, J.R., Jr. Spread of Dwarf Mistletoe into Jeffrey Pine Plantation-Trees Infected after 22 Years; U.S. Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station: Berkeley, CA, USA, 1976; 6p.
- Reid, N.; Yan, Z. Mistletoes and Other Phanerogams Parasitic on Eucalypts; CSIRO: Canberra, Australia, 2000; pp. 353–384.
- Kelly, P.; Reid, N.; Davies, I. Effects of experimental burning, defoliation, and pruning on survival and vegetative resprouting in mistletoes (Amyema miquelii and Amyema pendula). Int. J. Plant Sci. 1997, 158, 856–861.
- Conklin, D.A.; Geils, B.W. Survival and sanitation of dwarf mistletoe-infected ponderosa pine following prescribed underburning. West. J. Appl. For. 2008, 23, 216–222.
- Koonce, A.L.; Roth, L.F. The effects of prescribed burning on dwarf mistletoe in ponderosa pine. In Proceedings of the Sixth Conference on Fire and Forest Meteorology, Seattle, WA, USA, 22–24 April 1980; pp. 22–24.
- Ritter, S.; Hoffman, C.; Stewart, J.; Zimmerman, T. The influence of prescribed crown fire on lodgepole pine dwarf mistletoe (Arceuthobium americanum) populations 33 years post-fire. For. Pathol. 2018, 48, e12419.
- Alexander, M.E.; Hawksworth, F.G. Fire and dwarf mistletoes in North American coniferous forests. J. For. 1976, 74, 446–449.
- Kipfmueller, K.F.; Baker, W.L. Fires and dwarf mistletoe in a Rocky Mountain lodgepole pine ecosystem. For. Ecol. Manag. 1998, 108, 77–84.
- Manion, P. Tree Diseases Concepts, 2nd ed.; Prentice-Hall: Englewood Cliffs, NJ, USA, 1991.
- Knight, D.H. Parasites, lightning, and the vegetation mosaic in wilderness landscapes. In Landscape Heterogeneity and Disturbance; Springer: Berlin/Heidelberg, Germany, 1987; pp. 59–83.
- Conklin, D.A.; Armstrong, W.A. Effects of Three Prescribed Fires on Dwarf Mistletoe Infection in Southwestern Ponderosa Pine; US Department of Agriculture, Forest Service, Southwestern Region: Berkeley CA, USA, 2001.
- Haikerwal, A.; Reisen, F.; Sim, M.R.; Abramson, M.J.; Meyer, C.P.; Johnston, F.H.; Dennekamp, M. Impact of smoke from prescribed burning: Is it a public health concern? J. Air Waste Manag. Assoc. 2015, 65, 592–598.
- Afrin, S.; Garcia-Menendez, F. Potential impacts of prescribed fire smoke on public health and socially vulnerable populations in a Southeastern, U.S. state. Sci. Total Environ. 2021, 794, 148712.
- Cirocco, R.M.; Facelli, J.M.; Watling, J.R. High water availability increases the negative impact of a native hemiparasite on its non-native host. J. Exp. Bot. 2015, 67, 1567–1575.
- Griebel, A.; Metzen, D.; Pendall, E.; Nolan, R.H.; Clarke, H.; Renchon, A.A.; Boer, M.M. Recovery from Severe Mistletoe Infection After Heat- and Drought-Induced Mistletoe Death. Ecosystems 2022, 25, 1–16.
- Griebel, A.; Peters, J.M.R.; Metzen, D.; Maier, C.; Barton, C.V.M.; Speckman, H.N.; Boer, M.M.; Nolan, R.H.; Choat, B.; Pendall, E. Tapping into the physiological responses to mistletoe infection during heat and drought stress. Tree Physiol. 2021, 42, 523–536.
- Sidahmed, O.A. Incidence of mistletoe (Loranthus spp.) on citrus and guava trees in the central region of the Sudan. In Proceedings of the VIII African Symposium on Horticultural Crops 143, Wed Medani, Sudan, 20–24 March 1983; pp. 417–420.
- Knutson, D.M. The influence of urea fertilization on growth of mistle-toed pine seedlings. In Proceedings of the Second International Congress of plant pathology, Minneapolis, MN, USA, 5–12 September 1973; pp. 5–12.
- Kulkarni, H.D.; Srimathi, K. Plant pests of sandal (Santalum album L.). My For. 1988, 24, 29–38.
- Minko, G.; Fagg, P.C. Control of some mistletoe species on eucalypts by trunk injection with herbicides. Aust. For. 1989, 52, 94–102.
- Brown, A.; Greenham, C. Further investigations in the control of mistletoe by trunk injections. Aust. J. Exp. Agric. 1965, 5, 305–309.
- Brown, A. Mistletoe control on a large scale. J. Aust. Inst. Agric. Sci. 1959, 25, 282–286.
- Reid, N.; Fittler, J.; Kar, A.; Storey, A.; Cook, T. Effect of Spray Applications of Several Herbicides on the Mortality of Box Mistletoe (Amyema miquelii) and Host Saplings (Eucalyptus blakelyi and E. melliodora). In Ecosystem Management; University of New England: Armidale, Australia, 2008.
- Shamoun, S.; DeWald, L. Management strategies for dwarf mistletoes: Biological, chemical, and genetic approaches. In Mistletoes of North American Conifers; Geils, B.W., Tovar, J.C., Moody, B., Eds.; US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Washington, DC, USA, 2002; Volume 98, pp. 75–82.
- Watson, W.T.; Martinez-Trinidad, T. Strategies and treatments for leafy mistletoe (Phoradendron tomentosum Engelm ex. Gray) suppression on cedar elm (Ulmus crassifolia Nutt.). Arboric. Urban For. 2006, 32, 265.
- Quick, C.R. Experimental Herbicidal Control of Dwarfmistletoe on Some California Conifers; U.S. Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station: Berkeley, CA, USA, 1964; Volume 47, 9p.
- Hawksworth, F.G.; Wiens, D. Dwarf Mistletoes: Biology, Pathology, and Systematics; Diane Publishing: Washington, DC, USA; Department of Agriculture, Forest Service: Washington, DC, USA, 1996; p. 410.
- Livingston, W.; Brenner, M. Ethephon stimulates abscission of eastern dwarf mistletoe aerial shoots on black spruce. Plant Dis. 1983, 67, 909–910.
- Livingston, W.H.; Blanchette, R.A.; Brenner, M.L.; Zuzek, K.J. Effective use of ethylene-releasing agents to prevent spread of eastern dwarf mistletoe on black spruce. Can. J. For. Res. 1985, 15, 872–876.
- Livingston, W.H.; Brenner, M.L.; Blanchette, R.A. Altered concentrations of abscisic acid, indole-3-acetic acid, and zeatin riboside associated eastern dwarf mistletoe infections on black spruce. In Proceedings of the Biology of Dwarf Mistletoes, Symposium Proceedings, Fort Collins, CO, USA, 8 August 1984; Gen. Tech. Rep. RM-111. Hawksworth, F.G., Scharpf, R.F., Eds.; U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: Berkeley, CA, USA, 1984; pp. 53–60.
- Adams, D.H.; Frankel, S.J.; Lichter, J.M. Considerations when using ethephon for supressing dwarf and leafy mistletoe infestations in ornamental landscapes. J. Arboric. 1993, 19, 351–357.
- Watson, D.M.; Cook, M.E.; Fadini, R.R. Towards best-practice management of mistletoes in horticulture. Botany 2020, 98, 489–498.
- Winder, R.S.; Shamoun, S.F. Forest pathogens: Friend or foe to biodiversity? Can. J. Plant Pathol. 2006, 28, S221–S227.
- Robbins, K.; Johnson, D.W.; Hawksworth, F.G.; Nicholls, T.H. Aerial application of ethephon is ineffective in controlling lodgepole pine dwarf mistletoe. West. J. Appl. For. 1989, 4, 27–28.
- Baker, F.; Knowles, K.; Meyer, T.; French, D. Aerial applications of ethylene-releasing chemicals fail to promote abscission of dwarf mistletoe aerial shoots on Jack pine. For. Chron. 1989, 65, 194–195.
- Weber, H.C.; Forstreuter, W. Parasitismus von Blutenpflanzen; Wissenschaftliche Buchgesellschaft: Darmstadt, Germany, 1993.
- Parks, C.A.; Hoffman, J.T. Control of Western Dwarf Mistletoe with the Plant-Growth Regulator Ethephon; US Department of Agriculture, Forest Service, Pacific Northwest Research Station: Berkeley, CA, USA, 1991; Volume 506.
- Muir, J.; Geils, B. Management Strategies for Dwarf Mistletoe: Silviculture; Forest Service: Washington, DC, USA; USDA: Washington, DC, USA, 2002; pp. 83–94.
- Robinson, D.C.; Geils, B.W.; Muir, J.A. Spatial statistical model for the spread of dwarf mistletoe within and between stands. In Proceedings of the Second Forest Vegetation Simulator Conference, Fort Collins, CO, USA, 12–14 February 2002; Crookston, N.L., Havis, R.N., Eds.; pp. 178–185.
- Van Halder, I.; Castagneyrol, B.; Ordóñez, C.; Bravo, F.; del Río, M.; Perrot, L.; Jactel, H. Tree diversity reduces pine infestation by mistletoe. For. Ecol. Manag. 2019, 449, 117470.
- Fernández-Aparicio, M.; Delavault, P.; Timko, M.P. Management of Infection by Parasitic Weeds: A Review. Plants 2020, 9, 1184.
- Wyckhuys, K.A.G.; Hughes, A.C.; Buamas, C.; Johnson, A.C.; Vasseur, L.; Reymondin, L.; Deguine, J.P.; Sheil, D. Biological control of an agricultural pest protects tropical forests. Commun. Biol. 2019, 2, 10.
- Nzioki, H.S.; Oyosi, F.; Morris, C.E.; Kaya, E.; Pilgeram, A.L.; Baker, C.S.; Sands, D.C. Striga Biocontrol on a Toothpick: A Readily Deployable and Inexpensive Method for Smallholder Farmers. Front. Plant Sci. 2016, 7, 1121.
- Bale, J.; Van Lenteren, J.; Bigler, F. Biological control and sustainable food production. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 761–776.
- Charudattan, R. Biological control of weeds by means of plant pathogens: Significance for integrated weed management in modern agro-ecology. BioControl 2001, 46, 229–260.
- Hawksworth, F.G.; Geils, B.W. Biotic Associates; Diane Publishing: Washington, DC, USA; Department of Agriculture, Forest Service: Washington, DC, USA, 1996.
- Hawksworth, F.G.; Wicker, E.F.; Scharpf, R.F. Fungal Parasites of Dwarf Mistletoes ; Department of Agriculture, Forest Service: Washington, DC, USA, 1977; p. 14.
- Kuijt, J. Distribution of Dwarf Mistletoes and Their Fungus Hyperparasites in Western Canada; Department of Northern Affairs and National Resources: Ottawa, ON, Canada, 1963; Volume 186, pp. 134–148.
- Stevens, R.E.; Hawksworth, F.G. Insect-dwarf mistletoe associations: An update. In Proceedings of the Symposium: Biology of Dwarf Mistletoes, Fort Collins, CO, USA, 8 August 1984; pp. 94–101.
- Stevens, R.E.; Hawksworth, F.G. Insects and Mites Associated with Dwarf Mistletoes; Research Papers; Rocky Mountain Forest and Range Experiment Station: Fort Collins, CA, USA, 1970; p. 12.
- Room, P. The constitution and natural history of the fauna of the mistletoe Tapinanthus bangwensis (Engl. & K. Krause) growing on cocoa in Ghana. J. Anim. Ecol. 1972, 41, 519–535.
- Patrick, B.H.; Dugdale, J.S. Mistletoe moths-New Zealand’s loranthaceous mistletoes. In Proceedings of the A Workshop Hosted by Threatened Species Unit, Department of Conservation, Cass, Wellington, New Zealand, 17–20 July 1995; p. 125.
- Baloch, G.; Mohyuddin, A. The phytophagous fauna of a mistletoe (Loranthus longiflorus Desr.: Loranthaceae) in West Pakistan. Weed Res. 1969, 9, 62–64.
- Mark, W.R.; Hawksworth, F.G.; Oshima, N. Resin disease: A new disease of lodgepole pine dwarf mistletoe. Can. J. For. Res. 1976, 6, 415–424.
- Wicker, E.F.; Shaw, C.G. Fungal parasites of dwarf mistletoes. Mycologia 1968, 60, 372–383.
- Nguyen, J.; Fernandez, V.; Pontrelli, S.; Sauer, U.; Ackermann, M.; Stocker, R. A distinct growth physiology enhances bacterial growth under rapid nutrient fluctuations. Nat. Commun. 2021, 12, 3662.
- Burman, E.; Bengtsson-Palme, J. Microbial Community Interactions Are Sensitive to Small Changes in Temperature. Front. Microbiol. 2021, 12, 1–8.
- Hardy, D.E. Studies of fruitflies associated with mistletoe in Australia and Pakistan with notes and decriptions on genera related to Perilampsis Bezzi (Diptera: Tephritidae). Beiträge Zur Entomol. Contrib. Entomol. 1967, 17, 127–149.
- Mushtaque, M.; Baloch, G. Possibilities of biological control of mistletoes, Loranthus spp., using oligophagous insects from Pakistan. Entomophaga 1979, 24, 73–81.
- Garbelotto, M.; Lowell, N.; Chen, I.Y.; Osmundson, T.W. Evidence for inhibition of a fungal biocontrol agent by a plant microbiome. J. Plant Pathol. 2019, 101, 457–466.
- Perry, E. Broadleaf Mistletoe in Landscape Trees; University of California: Berkeley, CA, USA, 1995; p. 14.
- Hanover, J.W. Tree improvement for disease resistance in western United States and Canada. In Breeding Pest-Resistant Trees; Elsevier: Amsterdam, The Netherlands, 1966; pp. 53–56.
- Rubiales, D. Parasitic plants, wild relatives and the nature of resistance. New Phytol. 2003, 160, 459–461.
- Aly, R.; Dubey, N.K. Weed Management for Parasitic Weeds. In Recent Advances in Weed Management; Springer: Berlin/Heidelberg, Germany, 2014; pp. 315–345.
- Hawksworth, F.G. Biological factors of dwarf mistletoe in relation to control. In Proceedings of the Symposium on Dwarf Mistletoes Control through Forest Management, Berkeley, CA, USA, 11–13 April 1978; Gen. Tech. Rep. PSW-31. pp. 5–15.
- Roth, L.F. Genetic control of dwarf mistletoe. In Proceedings of the Symposium on Dwarf Mistletoes Control through Forest Management, Berkeley, CA, USA, 11–13 April 1978; pp. 69–72.
- Scharpf, R.F.; Roth, L.F. Resistance of Ponderosa Pine to Western Dwarf Mistletoe in Central Oregon; Res. Pap. PSW-RP-208; US Department of Agriculture, Pacific Southwest Forest and Range: Albany, CA, USA, 1992; Volume 208, 9p.
- Roth, L.F. Resistance of ponderosa pine to dwarf mistletoe. Silvae Genet. 1974, 23, 116–120.
- Downey, P.O. An inventory of host species for each aerial mistletoe species (Loranthaceae and Viscaceae) in Australia. Cunninghamia 1998, 5, 685–720.
- Arruda, R.; Carvalho, L.N.; Del-Claro, K. Host specificity of a Brazilian mistletoe, Struthanthus aff. polyanthus (Loranthaceae), in Cerrado Tropical Savanna. Flora-Morphol. Distrib. Funct. Ecol. Plants 2006, 201, 127–134.
- Atsatt, P. Host-parasite interactions in higher plants. In Physiological Plant Ecology III; Springer: Berlin/Heidelberg, Germany, 1983; Volume 12/C, pp. 519–535.
- Dean, W.; Midgley, J.; Stock, W. The distribution of mistletoes in South Africa: Patterns of species richness and host choice. J. Biogeogr. 1994, 21, 503–510.
- Downey, P.O.; Gill, A.M.; Banks, J.C. The Influence of Host Attributes on Mistletoe Colonization: An Example from Mulligan’s Flat Nature Reserve, A.C.T. Vic. Nat. 1997, 114, 105–111.
- Ghosh, S.K.; Balasundaram, M.; Mohamed, A.M. Studies in Host Parasite Relationship of Phanerogamic Parasites on Teak and Their Possible Control; Kerela Forest Reseach Institute: Kerala, India, 1984; p. 21.
- Halbritter, D.A.; Willett, D.S.; Gordon, J.M.; Stelinski, L.L.; Daniels, J.C. Behavioral Evidence for Host Transitions in Plant, Plant Parasite, and Insect Interactions. Environ. Entomol. 2018, 47, 646–653.
- Hoffmann, A.J.; Fuentes, E.R.; Cortes, I.; Liberona, F.; Costa, V. Tristerix tetrandrus (Loranthaceae) and its host-plants in the Chilean matorral: Patterns and mechanisms. Oecologia 1986, 69, 202–206.
- Norton, D.A.; Carpenter, M.A. Mistletoes as parasites: Host specificity and speciation. Trends Ecol. Evol. 1998, 13, 101–105.
- Okubamichael, D.Y.; Griffiths, M.E.; Ward, D. Host specificity in parasitic plants—Perspectives from mistletoes. AoB Plants 2016, 8, plw069.
- Rödl, T.; Ward, D. Host recognition in a desert mistletoe: Early stages of development are influenced by substrate and host origin. Funct. Ecol. 2002, 16, 128–134.
- Scharpf, R.F. Host resistance to dwarf mistletoe. In Proceedings of the Biology of Dwarf Mistletoes, Symposium Proceedings, Fort Collins, CO, USA, 8 August 1984; Hawksworth, F.G., Scharpf, R.F., Eds.; U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: Berkeley, CA, USA, 1984; pp. 70–76.
- Tainter, F.; French, D. The role of wound periderm in the resistance of eastern larch and jack pine to dwarf mistletoe. Can. J. Bot. 1971, 49, 501–504.
- Smith, R.; Wass, E.; Meagher, M. Evidence of resistance to hemlock dwarf mistletoe (Arceuthobium tsugense) in western hemlock (Tsuga heterophylla) clones. Eur. J. For. Pathol. 1993, 23, 163–170.
- Roeser, J., Jr. The importance of seed source and the possibilities of forest tree breeding. J. For. 1926, 24, 38–51.
- Bates, C.G. Better seeds, better trees. J. For. 1927, 25, 130–144.
- Hawksworth, F.G.; Edminster, C.B. Carlos Bates’ Dwarf Mistletoe Resistant Ponderosa Pines: A Postscript after Half a Century; USDA Forest Service, Rocky Mountain Forest and Range Experiment Station: Berkeley, CA, USA, 1981; Volume 412, p. 4.
- Roth, L.F. Dwarf mistletoe damage to small ponderosa pines. For. Sci. 1971, 17, 373–380.
- Roth, L.F. Juvenile Susceptibility of Ponderosa Pine. Phytopathology 1974, 64, 689–692.
- Harrington, T.; Wingfield, M. Diseases and the ecology of indigenous and exotic pines. In Ecology and Biogeography of Pinus; Richardson, D., Ed.; Cambridge University Press: Cambridge, UK, 1998; pp. 1–33.
- Okubamichael, D.Y. Host Specificity of the Hemiparasitic Mistletoe, Agelanthus natalitius. Master’s Thesis, University of KwaZulu-Natal, Pietermaritzburg, South Africa, 2009.
- Skrypnik, L.; Maslennikov, P.; Feduraev, P.; Pungin, A.; Belov, N. Ecological and Landscape Factors Affecting the Spread of European Mistletoe (Viscum album L.) in Urban Areas (A Case Study of the Kaliningrad City, Russia). Plants 2020, 9, 394.
- Zaroug, M.S.; Zahran, E.B.; Abbasher, A.A. Distribution And Host Range of Mistletoe (Tapinanthus globiferus) (A. Rich.) Van Tieghan) Along the Blue Nile Banks in Central Sudan. Int. J. Sci. Technol. Res. 2014, 3, 1–5.
- Ladley, J.J.; Kelly, D.; Robertson, A.W. Explosive flowering, nectar production, breeding systems, and pollinators of New Zealand mistletoes (Loranthaceae). N. Z. J. Bot. 1997, 35, 345–360.
- Lavorel, S.; Smith, M.S.; Reid, N. Spread of mistletoes (Amyema preissii) in fragmented Australian woodlands: A simulation study. Landsc. Ecol. 1999, 14, 147–160.
- Ehleringer, J.; Ullmann, I.; Lange, O.; Farquhar, G.; Cowan, I.; Schulze, E.-D.; Ziegler, H. Mistletoes: A hypothesis concerning morphological and chemical avoidance of herbivory. Oecologia 1986, 70, 234–237.
- Yoder, J.I. Parasitic plant responses to host plant signals: A model for subterranean plant–plant interactions. Curr. Opin. Plant Biol. 1999, 2, 65–70.
- Tomilov, A.; Tomilova, N.; Shin, D.H.; Jamison, D.; Torres, M.; Reagan, R.; McGray, H.; Horning, T.; Truong, R.; Nava, A. Chemical signalling between plants. In Chemical Ecology: From Gene to Ecosystem; Springer: Dordrecht, The Netherlands, 2006; Volume 16, pp. 55–69.
- Yan, Z. Resistance to haustorial development of two mistletoes, Amyema preissii (Miq.) Tieghem and Lysiana exocarpi (Behr.) Tieghem ssp. exocarpi (Loranthaceae), on host and nonhost species. Int. J. Plant Sci. 1993, 154, 386–394.
- Lech, P.; Żółciak, A.; Hildebrand, R. Occurrence of European Mistletoe (Viscum album L.) on Forest Trees in Poland and Its Dynamics of Spread in the Period 2008–2018. Forests 2020, 11, 83.
- Press, M.C.; Phoenix, G.K. Impacts of parasitic plants on natural communities. New Phytol. 2005, 166, 737–751.
- Nickrent, D.; Musselman, L. Introduction to parasitic flowering plants. Plant Health Instr. 2004.
- Pennings, S.C.; Callaway, R.M. Parasitic plants: Parallels and contrasts with herbivores. Oecologia 2002, 131, 479–489.
- Overton, J.M. Dispersal and infection in mistletoe metapopulations. J. Ecol. 1994, 82, 711–723.
- Amico, G.; Aizen, M.A. Mistletoe seed dispersal by a marsupial. Nature 2000, 408, 929–930.
- Lira-Noriega, A.; Toro-Núñez, O.; Oaks, J.R.; Mort, M.E. The roles of history and ecology in chloroplast phylogeographic patterns of the bird-dispersed plant parasite Phoradendron californicum (Viscaceae) in the Sonoran Desert. Am. J. Bot. 2015, 102, 149–164.
- Pérez-Crespo, M.; Ornelas, J.; Martén-Rodríguez, S.; González-Rodríguez, A.; Lara, C. Reproductive biology and nectar production of the Mexican endemic P. sittacanthus auriculatus (Loranthaceae), a hummingbird-pollinated mistletoe. Plant Biol. 2016, 18, 73–83.
- Burgess, V.J.; Kelly, D.; Robertson, A.W.; Ladley, J.J. Positive effects of forest edges on plant reproduction: Literature review and a case study of bee visitation to flowers of Peraxilla tetrapetala (Loranthaceae). N. Z. J. Ecol. 2006, 30, 179–190.
- Kelly, D.; Ladley, J.J.; Robertson, A.W.; Crowfoot, L. Flower predation by Zelleria maculata (Lepidoptera) on Peraxilla mistletoes: Effects of latitude and fragmentation, and impact on fruit set. N. Z. J. Ecol. 2008, 32, 186–196.
- MacRaild, L.M.; Radford, J.Q.; Bennett, A.F. Non-linear effects of landscape properties on mistletoe parasitism in fragmented agricultural landscapes. Landsc. Ecol. 2010, 25, 395–406.
- Bowen, M.E.; McAlpine, C.A.; House, A.P.; Smith, G.C. Agricultural landscape modification increases the abundance of an important food resource: Mistletoes, birds and brigalow. Biol. Conserv. 2009, 142, 122–133.
- Reid, N.; Stafford Smith, M.; Yan, Z. Ecology and population biology of mistletoes. In Forest Canopies; Lowman, M.D., Nadkarn, N.M., Eds.; Elsevier: Amsterdam, The Netherlands, 1995; pp. 285–310.
- Norton, D.A.; Ladley, J.J. Establishment and early growth of Alepis flavida in relation to Nothofagus solandri branch size. N. Z. J. Bot. 1998, 36, 213–217.
- Aukema, J.E.; Del Rio, C.M. Variation in mistletoe seed deposition: Effects of intra-and interspecific host characteristics. Ecography 2002, 25, 139–144.
- Baena-Díaz, F.; Ramírez-Barahona, S.; Ornelas, J.F. Hybridization and differential introgression associated with environmental shifts in a mistletoe species complex. Sci. Rep. 2018, 8, 5591.
- Azpeitia, F.; Lara, C. Reproductive biology and pollination of the parasitic plant Psittacanthus calyculatus (Loranthaceae) in central México1. J. Torrey Bot. Soc. 2006, 133, 429–438.
- Ramírez, M.M.; Ornelas, J.F. Polinización y producción de néctar de Psittacanthus schiedeanus (Loranthaceae) en el centro de Veracruz, México. Boletín De La Soc. Botánica De México 2010, 87, 61–67.
- Guerra, T.; Galetto, L.; Silva, W. Nectar secretion dynamic links pollinator behavior to consequences for plant reproductive success in the ornithophilous mistletoe Psittacanthus robustus. Plant Biol. 2014, 16, 956–966.
- Belchior, M.M.; Camarota, F.; Antiqueira, P.A.P.; Neves, F.S. A neotropical mistletoe influences herbivory of its host plant by driving changes in the associated insect community. Sci. Nat. 2022, 109, 27.
- Ollerton, J.; Stott, A.; Allnutt, E.; Shove, S.; Taylor, C.; Lamborn, E. Pollination niche overlap between a parasitic plant and its host. Oecologia 2007, 151, 473–485.
- Kuijt, J. Monograph of Psittacanthus (Loranthaceae). Syst. Bot. Monogr. 2009, 86, 1–362.
- Amico, G.C.; Vidal-Russell, R.; Garcia, M.A.; Nickrent, D.L. Evolutionary history of the South American mistletoe Tripodanthus (Loranthaceae) using nuclear and plastid markers. Syst. Bot. 2012, 37, 218–225.
- Ornelas, J.F.; Gándara, E.; Vásquez-Aguilar, A.A.; Ramírez-Barahona, S.; Ortiz-Rodriguez, A.E.; González, C.; Saules, M.T.M.; Ruiz-Sanchez, E. A mistletoe tale: Postglacial invasion of Psittacanthus schiedeanus (Loranthaceae) to Mesoamerican cloud forests revealed by molecular data and species distribution modeling. BMC Evol. Biol. 2016, 16, 78.
- Reid, N. Dispersal of misteltoes by honeyeaters and flowerpeckers: Components of seed dispersal quality. Ecology 1989, 70, 137–145.
- Godschalk, S. Feeding behaviour of avian dispersers of mistletoe fruit in the Loskop Dam Nature Reserve, South Africa. Afr. Zool. 1985, 20, 136–146.
- Van Ommeren, R.J.; Whitham, T.G. Changes in interactions between juniper and mistletoe mediated by shared avian frugivores: Parasitism to potential mutualism. Oecologia 2002, 130, 281–288.
- García, D.; Rodríguez-Cabal, M.A.; Amico, G.C. Seed dispersal by a frugivorous marsupial shapes the spatial scale of a mistletoe population. J. Ecol. 2009, 97, 217–229.
- Rodriguez-Cabal, M.A.; Branch, L.C. Influence of habitat factors on the distribution and abundance of a marsupial seed disperser. J. Mammal. 2011, 92, 1245–1252.
- Morales, J.M.; Rivarola, M.D.; Amico, G.; Carlo, T.A. Neighborhood effects on seed dispersal by frugivores: Testing theory with a mistletoe–marsupial system in Patagonia. Ecology 2012, 93, 741–748.
- Arriaga, L.; Franco, M.; Sarukhan, J. Identification of natural groups of trees in uneven-aged forests using multivariate methods. J. Ecol. 1988, 76, 1092–1100.
- Donohue, K. The spatial demography of mistletoe parasitism on a Yemeni Acacia. Int. J. Plant Sci. 1995, 156, 816–823.
- Gairola, S.; Bhatt, A.; Govender, Y.; Baijnath, H.; Procheş, Ş.; Ramdhani, S. Incidence and intensity of tree infestation by the mistletoe Erianthemum dregei (Eckl. & Zeyh.) V. Tieghem in Durban, South Africa. Urban For. Urban Green. 2013, 12, 315–322.
- Bilgili, E.; Coskuner, K.A.; Baysal, I.; Ozturk, M.; Usta, Y.; Eroglu, M.; Norton, D. The distribution of pine mistletoe (Viscum album ssp. austriacum) in Scots pine (Pinus sylvestris) forests: From stand to tree level. Scand. J. For. Res. 2020, 35, 20–28.
- Teixeira-Costa, L.; Coelho, F.M.; Ceccantini, G.C.T. Comparative phenology of mistletoes shows effect of different host species and temporal niche partitioning. Botany 2017, 95, 271–282.
- Yule, K.M.; Bronstein, J.L. Reproductive ecology of a parasitic plant differs by host species: Vector interactions and the maintenance of host races. Oecologia 2018, 186, 471–482.
- Cuadra-Valdés, J.; Vizentin-Bugoni, J.; Fontúrbel, F.E. An exotic magnet plant alters pollinator abundance and behavior: A field test with a native mistletoe. Biol. Invasions 2021, 23, 2515–2525.
- Cubero, J.; Hernández, L. Breeding faba bean (Vicia faba L.) for resistance to Orobanche crenata Forsk. Options Méditerranéennes 1991, 10, 51–57.
- Ringnes, D.; Stover, P.; Scharpf, R. Dwarf mistletoe resistance in ponderosa pine: Selection and testing protocols. In Proceedings of the Congresos y Jornadas-Junta de Andalucia (Espana), Cordoba, Spain, 16–18 April 1996; pp. 690–696.
- Haussmann, B.; Hess, D.; Reddy, B.; Mukuru, S.; Seetharama, N.; Kayentao, M.; Welz, H.; Geiger, H. QTL for Striga resistance in sorghum populations derived from IS 9830 and N 13. In Proceedings of the Breeding for Striga Resistance in Cereals: Proceedings of a Workshop, IITA, Ibadan, Nigeria, 18–20 August 1999; pp. 18–20.
- Mohamed, A.; Rich, P.; Housley, T.; Ejeta, G. In vitro techniques for studying mechanisms of Striga resistance in sorghum. In Proceedings of the 7th International Parasitic Weed Symposium, Nantes, France, 3–8 June 2001; pp. 96–100.
- Omanya, G.O.; Haussmann, B.I.G.; Hess, D.E.; Reddy, B.V.S.; Kayentao, M.; Welz, H.G.; Geiger, H.H. Utility of indirect and direct selection traits for improving Striga resistance in two sorghum recombinant inbred populations. Field Crops Res. 2004, 89, 237–252.
- Zhang, X.; Liu, B.; Guo, Q.; Song, L.; Chen, L.; Wang, C. Construction of a haustorium development associated SSH library in Thesium chinense and analysis of specific ESTs included by Imperata cylindrica. Biochem. Syst. Ecol. 2016, 64, 46–52.
- Ranjan, A.; Ichihashi, Y.; Farhi, M.; Zumstein, K.; Townsley, B.; David-Schwartz, R.; Sinha, N.R. De novo assembly and characterization of the transcriptome of the parasitic weed dodder identifies genes associated with plant parasitism. Plant Physiol. 2014, 166, 1186–1199.
- Ichihashi, Y.; Kusano, M.; Kobayashi, M.; Suetsugu, K.; Yoshida, S.; Wakatake, T.; Kumaishi, K.; Shibata, A.; Saito, K.; Shirasu, K.J.P.; et al. Transcriptomic and metabolomic reprogramming from roots to haustoria in the parasitic plant, Thesium chinense. Plant Cell Physiol. 2018, 59, 729–738.
- Wang, Y.; Li, X.; Zhou, W.; Li, T.; Tian, C.J.B.g. De novo assembly and transcriptome characterization of spruce dwarf mistletoe Arceuthobium sichuanense uncovers gene expression profiling associated with plant development. BMC Genom. 2016, 17, 1–14.
- Abdul Wahid, H. Identification of Dwarf Mistletoe Resistant Genes in Ziarat Junipers (Juniperus excelsa M. Bieb). Ph.D. Thesis, University of Baluchistan, Quetta, Pakistan, 2019.
- Abdul Wahid, H.; Barozai, M.Y.K.; Din, M. Identification and characterization of dwarf mistletoe responding genes in Ziarat juniper tree (Juniperus excelsa M. Bieb) through suppression subtractive hybridization and deep sequencing. Trees 2019, 33, 1027–1039.
- Kuang, J.; Wang, Y.; Mao, K.; Milne, R.; Wang, M.; Miao, N. Transcriptome Profiling of a Common Mistletoe Species Parasitizing Four Typical Host Species in Urban Southwest China. Genes 2022, 13, 1173.
- Dan, M.W.; Filiz, G. Plant Genome Editing and its Applications in Cereals. In Genetic Engineering; Farrukh, J., Ed.; IntechOpen: Rijeka, Croatia, 2016; p. 4.
- Mandal, K.; Boro, P.; Chattopadhyay, S. Micro-RNA based gene regulation: A potential way for crop improvements. Plant Gene 2021, 27, 100312.
- Yu, H.; Li, J. Breeding future crops to feed the world through de novo domestication. Nat. Commun. 2022, 13, 1171.
- Van Esse, H.P.; Reuber, T.L.; van der Does, D. Genetic modification to improve disease resistance in crops. New Phytol. 2020, 225, 70–86.
- Zaidi, S.S.-e.-A.; Mahas, A.; Vanderschuren, H.; Mahfouz, M.M. Engineering crops of the future: CRISPR approaches to develop climate-resilient and disease-resistant plants. Genome Biol. 2020, 21, 289.
- Clarke, C.R.; Timko, M.P.; Yoder, J.I.; Axtell, M.J.; Westwood, J.H. Molecular Dialog Between Parasitic Plants and Their Hosts. Annu. Rev. Phytopathol. 2019, 57, 279–299.
- Westwood, J.H.; de Pamphilis, C.W.; Das, M.; Fernández-Aparicio, M.; Honaas, L.A.; Timko, M.P.; Wafula, E.K.; Wickett, N.J.; Yoder, J.I. The Parasitic Plant Genome Project: New Tools for Understanding the Biology of Orobanche and Striga. Weed Sci. 2017, 60, 295–306.
- Neale, D.B.; Kremer, A. Forest tree genomics: Growing resources and applications. Nat. Rev. Genet. 2011, 12, 111.
- Muleo, R.; Morgante, M.; Velasco, R.; Cavallini, A.; Perrotta, G.; Baldoni, L. Olive tree genomic. In Olive Germplasm–The Olive Cultivation, Table Olive and Olive Oil Industry in Italy, 1st ed.; Muzzalupo, I., Ed.; Intechopen: Rijeka, Croatia, 2012; pp. 133–148.
- Diningrat, D.; Widiyanto, S.; Pancoro, A.; Shim, D.; Panchangam, B.; Zembower, N.; Carlson, J.E. Transcriptome of teak (Tectona grandis, Lf) in vegetative to generative stages development. J. Plant Sci. 2015, 10, 1.
- Schnell, R.J.; Priyadarshan, P. Genomics of Tree Crops; Springer Science & Business Media; Springer: New York, NY, USA, 2012.
- Gmitter, F.G.; Chen, C.; Machado, M.A.; De Souza, A.A.; Ollitrault, P.; Froehlicher, Y.; Shimizu, T. Citrus genomics. Tree Genet. Genomes 2012, 8, 611–626.
- Arias, R.S.; Borrone, J.W.; Tondo, C.L.; Kuhn, D.N.; Irish, B.M.; Schnell, R.J. Genomics of tropical fruit tree crops. In Genomics of Tree Crops; Springer: Berlin/Heidelberg, Germany, 2012; pp. 209–239.
- Deeks, S.J.; Shamoun, S.F.; Punja, Z.K. In vitro germination and development of western hemlock dwarf mistletoe. Plant Cell Tissue Organ Cult. 2001, 66, 97–105.
- Marler, M.; Pedersen, D.; Mitchell-Olds, T.; Callaway, R. A polymerase chain reaction method for detecting dwarf mistletoe infection in Douglas-fir and western larch. Can. J. For. Res. 1999, 29, 1317–1321.
- Petri, C.; Burgos, L. Transformation of fruit trees. Useful breeding tool or continued future prospect? Transgenic Res. 2005, 14, 15–26.
- Atsatt, P.R. On the evolution of leaf resemblance between mistletoes and their hosts. In Proceedings of the 2nd Symposium on Parasitic Weeds, Raleigh, NC, USA, 16–19 July 1979.
- Lev-Yadun, S. Does chemical aposematic (warning) signaling occur between host plants and their potential parasitic plants? Plant Signal. Behav. 2013, 8, e24907.
- Yoshida, S.; Cui, S.; Ichihashi, Y.; Shirasu, K. The Haustorium, a Specialized Invasive Organ in Parasitic Plants. Annu. Rev. Plant Biol. 2016, 67, 643–667.
- Chang, M.; Lynn, D.G. The haustorium and the chemistry of host recognition in parasitic angiosperms. J. Chem. Ecol. 1986, 12, 561–579.
- Sallé, G.L. Germination and Establishment of Viscum album. In The Biology of Mistletoes; Calder, D., Bernhardt, P., Eds.; Academic Press: London, UK, 1983.
- Bandaranayake, P.C.G.; Yoder, J.I. Haustorium Initiation and Early Development. In Parasitic Orobanchaceae: Parasitic Mechanisms and Control Strategies; Joel, D.M., Gressel, J., Musselman, L.J., Eds.; Springer: Berlin Heidelberg, Germany, 2013; pp. 61–74.
- Saucet, S.B.; Shirasu, K. Molecular Parasitic Plant-Host Interactions. PLoS Pathog. 2016, 12, e1005978.
- Kokla, A.; Melnyk, C.W. Developing a thief: Haustoria formation in parasitic plants. Dev. Biol. 2018, 442, 53–59.
- Keyes, W.J.; Palmer, A.G.; Erbil, W.K.; Taylor, J.V.; Apkarian, R.P.; Weeks, E.R.; Lynn, D.G. Semagenesis and the parasitic angiosperm Striga asiatica. Plant J. Cell Mol. Biol. 2007, 51, 707–716.
- Klutsch, J.G.; Erbilgin, N. Dwarf mistletoe infection in jack pine alters growth-defense relationships. Tree Physiol. 2018, 38, 1538–1547.
- Smith, J.L.; De Moraes, C.M.; Mescher, M.C. Jasmonate- and salicylate-mediated plant defense responses to insect herbivores, pathogens and parasitic plants. Pest Manag. Sci. 2009, 65, 497–503.
- Runyon, J.B.; Mescher, M.C.; De Moraes, C.M. Plant defenses against parasitic plants show similarities to those induced by herbivores and pathogens. Plant Signal. Behavior. 2010, 5, 929–931.
- Cuevas-Reyes, P.; Pérez-López, G.; Maldonado-López, Y.; González-Rodríguez, A. Effects of herbivory and mistletoe infection by Psittacanthus calyculatus on nutritional quality and chemical defense of Quercus deserticola along Mexican forest fragments. Plant Ecol. 2017, 218, 687–697.
- Teixeira-Costa, L.; Ceccantini, G. Embolism increase and anatomical modifications caused by a parasitic plant: Phoradendron crassifolium (Santalaceae) on Tapirira guianensis (Anacardiaceae). IAWA J. 2015, 36, 138–151.
- Hu, B.; Sakakibara, H.; Takebayashi, Y.; Peters, F.S.; Schumacher, J.; Eiblmeier, M.; Arab, L.; Kreuzwieser, J.; Polle, A.; Rennenberg, H. Mistletoe infestation mediates alteration of the phytohormone profile and anti-oxidative metabolism in bark and wood of its host Pinus sylvestris. Tree Physiol. 2017, 37, 676–691.
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320.
- Sallé, G.L.; Armillotta, A.; Frochot, H. Mechanisms of resistance of four cultivars of poplar against Viscum album L. In Proceedings of the 3rd International Symposium of Parasitic Weeds, Aleppo, Syria, 7–10 May 1984; Parker, C., Musselman, L.J., Polhill, R.M., Wilson, A.K., Eds.;
- Lázaro-González, A.; Hódar, J.A.; Zamora, R. Mistletoe Versus Host Pine: Does Increased Parasite Load Alter the Host Chemical Profile? J. Chem. Ecol. 2019, 45, 95–105.
- Ferrenberg, S. Dwarf Mistletoe Infection Interacts with Tree Growth Rate to Produce Opposing Direct and Indirect Effects on Resin Duct Defenses in Lodgepole Pine. Forests 2020, 11, 222.
- Bhat, K.A.; Akhtar, S.; Dar, N.A.; Bhat, M.I.; Bhat, F.A.; Rizwan, R.; Horielov, O.; Krasylenko, Y. Mistletoe Eradicator—A Novel Tool for Simultaneous Mechanical and Chemical Control of Mistletoe. J. Vis. Exp. 2022, 181, e63455.
- Schrader-Patton, C.; Grulke, N.; Bienz, C. Assessment of Ponderosa Pine Vigor Using Four-Band Aerial Imagery in South Central Oregon: Crown Objects to Landscapes. Forests 2021, 12, 612.
- Barbedo, J. A Review on the Use of Unmanned Aerial Vehicles and Imaging Sensors for Monitoring and Assessing Plant Stresses. Drones 2019, 3, 40.
- Duarte, A.; Borralho, N.; Cabral, P.; Caetano, M. Recent Advances in Forest Insect Pests and Diseases Monitoring Using UAV-Based Data: A Systematic Review. Forests 2022, 13, 911.
- Thapa, S. Detection and Mapping of Incidence of Viscum album in Pinus sylvestris Forest of Southern French Alpe Using Satellite and Airborne Optical Imagery. Master’s Thesis, University of Twente, Enskode, The Netherlands, 2013.
- Barbosa, J.; Sebastián-González, E.; Asner, G.; Knapp, D.; Anderson, C.; Martin, R.; Dirzo, R. Hemiparasite-host plant interactions in a fragmented landscape assessed via imaging spectroscopy and LiDAR. Ecol. Appl. 2016, 26, 55–66.
- Lawley, V.; Lewis, M.; Clarke, K.; Ostendorf, B. Site-based and remote sensing methods for monitoring indicators of vegetation condition: An Australian review. Ecol. Indic. 2016, 60, 1273–1283.
- Barlow, B.A.; Wiens, D. Host-Parasite Resemblance in Australian Mistletoes: The Case for Cryptic Mimicry. Evolution 1977, 31, 69–84.
- Windmuller-Campione, M.A.; Moser, R.L. Remote and Seasonal Field Detection of Eastern Spruce Dwarf Mistletoe in Northern Minnesota; Minnesota Forestry Research Notes; Department of Forest Resources, University of Minnesota: St. Paul, MN, USA, 2022; Volume 315.
- Mejia-Zuluaga, P.A.; Dozal, L.; Valdiviezo-N., J.C. Genetic Programming Approach for the Detection of Mistletoe Based on UAV Multispectral Imagery in the Conservation Area of Mexico City. Remote Sens. 2022, 14, 801.
- Pernar, R.; Bajic, M.; Ancic, M.; Seletkovic, A.; Idzojtic, M. Detection of mistletoe in digital colour infrared images of infested fir trees. Period. Biol. 2007, 109, 67–75.
- Sabrina, F.; Sohail, S.; Thakur, S.; Azad, S.; Wasimi, S. Use of deep learning approach on UAV imagery to detect mistletoe infestation. In Proceedings of the 2020 IEEE Region 10 Symposium (TENSYMP), Dhaka, Bangladesh, 5–7 June 2020; pp. 556–559.
- Ančić, M.; Pernar, R.; Bajić, M.; Seletković, A.; Kolić, J. Detecting mistletoe infestation on Silver fir using hyperspectral images. Iforest-Biogeosci. For. 2014, 7, 85.
- Maes, W.H.; Huete, A.R.; Avino, M.; Boer, M.M.; Dehaan, R.; Pendall, E.; Griebel, A.; Steppe, K. Can UAV-based infrared thermography be used to study plant-parasite interactions between mistletoe and eucalypt trees? Remote Sens. 2018, 10, 2062.
- Xiong, D.; Huang, H.; Wang, Z.; Li, Z.; Tian, C. Assessment of dwarf mistletoe (Arceuthobium sichuanense) infection in spruce trees by using hyperspectral data. For. Pathol. 2021, 51, e12669.
- Miraki, M.; Sohrabi, H.; Fatehi, P.; Kneubuehler, M. Detection of mistletoe infected trees using UAV high spatial resolution images. J. Plant Dis. Prot. 2021, 128, 1679–1689.
- León-Bañuelos, L.A.; Endara-Agramont, A.R.; Gómez-Demetrio, W.; Martínez-García, C.G.; Gabino Nava-Bernal, E. Identification of Arceuthobium globosum using unmanned aerial vehicle images in a high mountain forest of central Mexico. J. For. Res. 2020, 31, 1759–1771.