Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Immunology
Abundant immunological assays currently exist for detecting pathogens and identifying infected individuals, making detection of diseases at early stages integral to preventing their spread, together with the consequent emergence of global health crises. Lateral flow immunoassay (LFIA) is a test characterized by simplicity, low cost, and quick results. Furthermore, LFIA testing does not need well-trained individuals or laboratory settings.
- lateral flow immunoassay
- viral infections
- diagnostic tool
1. Introduction
Infectious diseases, particularly viral infections, can cause asymptomatic to life-threatening illnesses and negatively impact the global medical system [1]. Viral infections can spread through communities via blood, blood products, sex, mosquitos, food, water, and aerosol. Viruses exist in various sizes ranging from 20 to 900 nm and typically consist of genetic materials that could be single or double-stranded deoxyribonucleic acid (DNA) or ribonucleic acid (RNA) enclosed by proteins, glycoproteins or lipid coating [2]. To survive, viruses require living cells suitable for hosting the virus replication process. In response to any viral infection, the immune system generates specific antibodies to neutralize the virus and the infected cells. However, the immune responses to viruses occasionally are not successful, due to rapid viral replication and spread within the host.
Over the years, humankind has endured several virus-mediated pandemics—from 1889 to 1890 with the Asiatic flu that destroyed around one million lives [3,4], followed by the Spanish flu (1918 to 1920), and then by the Asian flu from 1957 to 1968 [5,6]. Human immunodeficiency virus (HIV) has also been infecting people since 1981 and has caused the death of more than 33 million individuals [7]. Today, the newly emerged virus, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has resulted in a global outbreak of coronavirus disease (COVID-19) for more than two years, due to viral evolution. During the COVID-19 pandemic, many lives have been lost, partially due to inadequate testing, particularly in countries with insufficient resources. As a result, many undetected COVID-19-infected symptomatic or asymptomatic individuals were released to the community and were subsequently able to spread the infection silently in society [8]. At the beginning of the pandemic, real-time reverse transcriptase polymerase chain reaction (RT-PCR) testing was the standard method of detecting COVID-19-infected subjects; however, this technique has many limitations, including the costly testing procedure, the need for highly skilled staff, and the need for fully-equipped lab settings. Therefore, developing a detection method, such as lateral flow immunoassays (LFIA), that can overcome all these limitations is essential. LFIA is a point-of-care test that detects viral antigens and the humoral immune responses to viral infections. Although LFIA can distinctively be performed without lab or trained personnel, yielding relatively rapid results, antigen-detecting tests (AT) such as LFIAs are typically less sensitive than molecular methods [9,10,11].
2. LFIA
Unlike many immunological assays, the LFIA is a rapid point-of-care test that provides results in a short time without the need for previous training or laboratory settings. These advantages have made LFIA devices an attractive tool for numerous applications beyond infectious agents’ detection, extending its usefulness to studying environmental sciences, drugs, and food, and several clinical analyses [12,13,14,15]. LFIA is a simple immunoassay that depends mainly on the accumulation of antibodies or antigens conjugated to reporter molecules on certain designated areas, known as the test or the control areas, depositing capture molecules enabling the detection of analyte-conjugate complexes on the membrane of the strip.
3. Components of LFIA Devices
As shown in Figure 1, LIFA devices are generally composed of several essential components, including a backing card, a sample pad, a conjugate pad, a membrane, and an absorbent pad, all of which are organized in a specific manner to ensure the capillary flow of the reactants across the membrane. Described in Figure 1, below, are the strip components mentioned above.
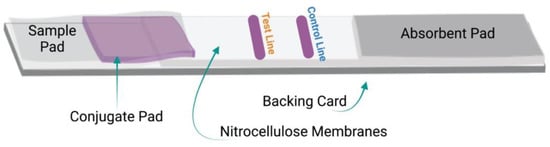
Figure 1. Components of LFIA devices: Schematic of the general components and design of the LFIA strips.
3.1. Backing Card
The various components of the LFIA strips are fixed onto a backing card to provide the whole system with rigidity and ease of handling. Typically manufactured from a plastic polymer [16,17,18], the card is divided into adhesive layers to adhere to the four major components of the LFIA strips.
3.2. Sample Pad
The sample pad is found on the first part of the strip, where the sample is applied. Two main types of materials are suitable to be utilized as a sample pad: cellulose [19] and glass fibre [20]. However, the material used for this part of the strip should undergo testing to ensure that the applied sample is optimally flowing before reaching the next component. Notably, the sample’s medium plays a vital role in the selection process of the sample pad material, and both should undergo testing to ensure optimal results. For example, for saliva and blood, the most commonly used samples to detect infectious agents, different types of sample pads must be applied to the strip. The composition of the saliva frequently varies based on the individual, time of collection, and the food and drinks consumed prior to the collection of the sample. Therefore, it is recommended to pre-treat the sample pad with an optimizing buffer to normalize the sample and maintain the system’s pH to avoid non-specific interaction [21]. As for the blood, an external filter or integrated special filter sample pad membrane are suggested as essential for preventing the flow of unwanted molecules [22,23,24]. Generally, pre-treating the sample pad improves the sample flow rate and assay reproductivity [25].
3.3. Conjugate Pad
The conjugate pad is where antibodies or antigens conjugated to reporter molecules are placed. Glass fibre is the most commonly used material for this part. However, other components are also available, including polyester and cellulose [21]. The selected material will specify the amount of conjugate to be absorbed and the speed of the conjugate’s release into the system. Some LFIA devices omit this component; instead, the conjugate is added directly to the sample [26,27].
3.4. Nitrocellulose Membrane
Typically, nitrocellulose membranes (NCM) are used to develop LFIA strips; however, in some instances, customized cellulose membranes have been utilized [28]. The NCM is the most critical component of the strip, the component in which all reactions occur, and in which the results readout appears. The NCM contains two major areas: the test and the control lines. NCMs are available with various pore sizes to control the flow speed rate of the reactants throughout the system. The selection of the membrane pore size mainly depends on the analyte size and the sample type [29]. Most commercially available NCMs are defined by their capillary flow time. The capillary flow time is the time needed for the sample anterior to travel over the membrane, typically a distance of 4 cm, and is usually defined as s/4 cm. Generally, pore sizes range from 1 to 20 µm, and the conversion into capillary flow time is approximated. For instance, 8 µm and 6 µm would equal 135 s and 180 s, respectively [21,29]. The slow flow speed is associated with higher capillary flow time [21]. As shown in Table 1, NCM with a large pore size provides lower capillary flow time, while NCM with a small pore size is associated with a high capillary flow time and slower membrane [29]. Therefore, slower NCM would increase the reaction time between the components of the NCM and the flowing conjugates as well as the analytes, thus enhancing the test sensitivity, but might increase the risk of non-specific binding [21,30]. Therefore, selecting the proper pore size is crucial for obtaining sensitive and specific LFIA strips [21]. Additionally, the sample’s viscosity is an essential factor to consider upon selecting the pore size of the membrane, because viscous samples, such as saliva, run slower than do non-viscous specimens (Table 1). The membrane’s thickness, which can be measured by gauges, is an another important factor to consider; considered together with porosity, it allows the strip developer to predict the amount of liquid needed to fill the pores of the membrane [31]. Moreover, the membrane’s thickness must be compatible with the thickness of the other components in the strip and the housing cassette to evade over-compression upon assembling the device [31]. Notably, all the commercially available NCMs in the market contains surfactant to create hydrophilic membrane and assist in the protein’s binding to the membrane.
Table 1. Relationship between the sample type, the NCM pore size, and the NCM flow speed.
| Sample Type | Recommended Pore Size | Flow Speed | Examples of NCM Capillary Flow Time |
|---|---|---|---|
| Viscous, such as saliva | Large | Fast | 75 s and 80 s |
| Medium | Medium | 120 S and 135 s | |
| Non-viscous, such as urine | Small | Slow | 170 s and 180 s |
| Medium | Medium | 120 S and 135 s |
3.5. Adsorbent Pad
Adsorbent pads are cotton liners located at the second end of the strip. These pads adsorb the remaining reagents, clearing up the strip background by maintaining the capillary flow across the membrane.
4. LFIA Preparation
Several commercial LFIA devices can be used to detect a wide variety of infectious agents in patient samples, together with the humoral responses to these agents. Nonetheless, as shown in Figure 2, the preparation process is generally the same, from preparing and assembling the major components of the strip to cutting and formulating the cassette.
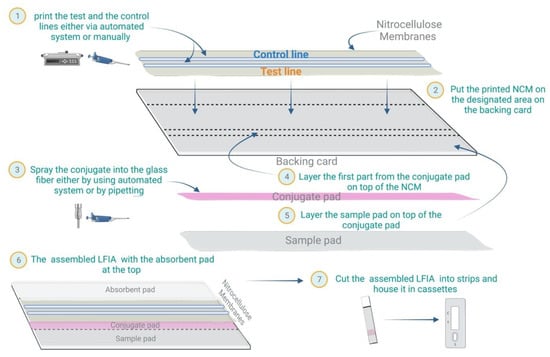
Figure 2. LFIA preparation: Schematic of the general steps for preparing LFIA devices.
4.1. NCM Preparation
As mentioned earlier, the NCM contains the test and the control lines that must be stripped into the membrane, which requires the consideration of several essential factors. These factors include the concentration of the reagents, the dispensing rate, and the stripping buffer. To optimize results, each reagent’s concentration requires adjustment according to the specific assay of interest, ranging from 0.5 to 2 mg/mL. The test and the control lines must be dispensed into the membrane using the appropriate dispenser, which might be a contact or non-contact dispenser. The dispensing rate depends on the membrane’s pore size; for instance, a membrane with large pores needs a decreased dispensing rate to achieve a similar line width to that of membranes with smaller pores. Based on experience, the dispensing rate is usually between 0.5 to 1 µl/cm to obtain a line width of 1mm. Notably, the width of the lines correlates with the signal intensity and assay sensitivity [31,32]. Phosphate buffered saline (PBS) is suitable for most of the proteins; however, some are sensitive to the pH and salt concentration of the stripping buffer. Therefore, the selected buffer should be re-evaluated in the presence of non-specific binding or upon obtaining weak signals.
4.2. Conjugate Pad Preparation
The conjugate solution can be applied to the conjugate pad either by using an air jet dispenser—the most recommended method—or by immersing the glass fibre in the conjugate solution. Since the immersion process is usually performed manually, it would require further optimization, and it is inevitably associated with inconsistencies concerning the conjugate solution’s volume upon preparing different batches of conjugate pads, unlike the automated air-jet method [20,21]. Generally, the conjugate pad can be pre-treated with a buffer that would adjust the system’s pH based on the sample type. To avoid aggregation of the conjugates, the buffer should not hold high salt concentrations. Moreover, some amount of detergents, proteins and polymers can be added to the buffer to help release the conjugate. Also, adding blocking reagents to the buffer can eliminate the need to block the NC membrane [33,34,35]. Notably, selecting the proper conjugate pad pre-treating buffer requires testing several buffers’ components and ingredients. After adding the pre-treating buffer to the conjugate pad, the pad has to be dried at 37 °C for at least 3 h before applying the conjugate solution. Moreover, the conjugate solution typically needs to be in a sugar-containing buffer to maintain long-term stability after drying the pad and interacting with the specimen [21,29,36].
4.3. Sample Pad Preparation
Sample pad treatment depends on the type of the sample intended and is usually applied to maintain the variability associated with the sample’s pH, viscosity, and salt concentration. Treating the sample pad with an optimized buffer acts as a blocking agent that can normalize the sample’s pH and salt concentration to enhance the test’s consistency and performance. Moreover, it improves the specimen capillary flow over the strip. Particularly when using saliva as a sample, the sample pad treatment usually contains salts and surfactants that break down mucins and proteins, thus decreasing the sample’s viscosity and improving flow through the strip. However, buffers containing salts and surfactants in LFIA devices designated for whole-blood samples are strongly not recommended because they may haemolyze the red blood cells within the specimen, causing the passage of unwanted lysed cells to the strip.
4.4. Assembling the LFIA Components
LFIA components can be assembled by using a fully automated system to produce large numbers of strips. For small-scale production, laminating the components can be done manually or by using lamination machines to place and hold the materials on top of the backing card.
4.5. Cutting the Assembled LFIA Strips
Following the assembly of the LFIA components, the backing cards containing all the materials are cut into strips. The cutting sizes of the backing cards are based on the design of the specific test requirements, usually ranging between 3 to 6 mm in width. Automated cutters such as a guillotine are preferred, to maintain manufacturing accuracy and reproducibility. Notably, thin strips are cost-effective but could lead to less accuracy due to the edge effect. For instance, if the strip width is 3 mm, then 0.5 mm of the strip edge is exposed to unusual flow, representing 33% of the strip, but if the strip is 6 mm, then only 16% of the strip width is affected [32].
4.6. Assembling of the Cassette
Typically, the cassette is designed after optimizing the entire system to fit the length, width, and thickness needed for all the components. LFIA strips must be housed in a solid, well-designed cassette that can be easily handled, which is also reproducible and reliable. This part of the LFIA device is a crucial component that is usually overlooked, one which applies pressure on specific points of the strip to maintain optimal flow control while conserving the flow rate. Moreover, the cassette design must be optimized to avoid strip flooding.
This entry is adapted from the peer-reviewed paper 10.3390/mi13111901
This entry is offline, you can click here to edit this entry!
