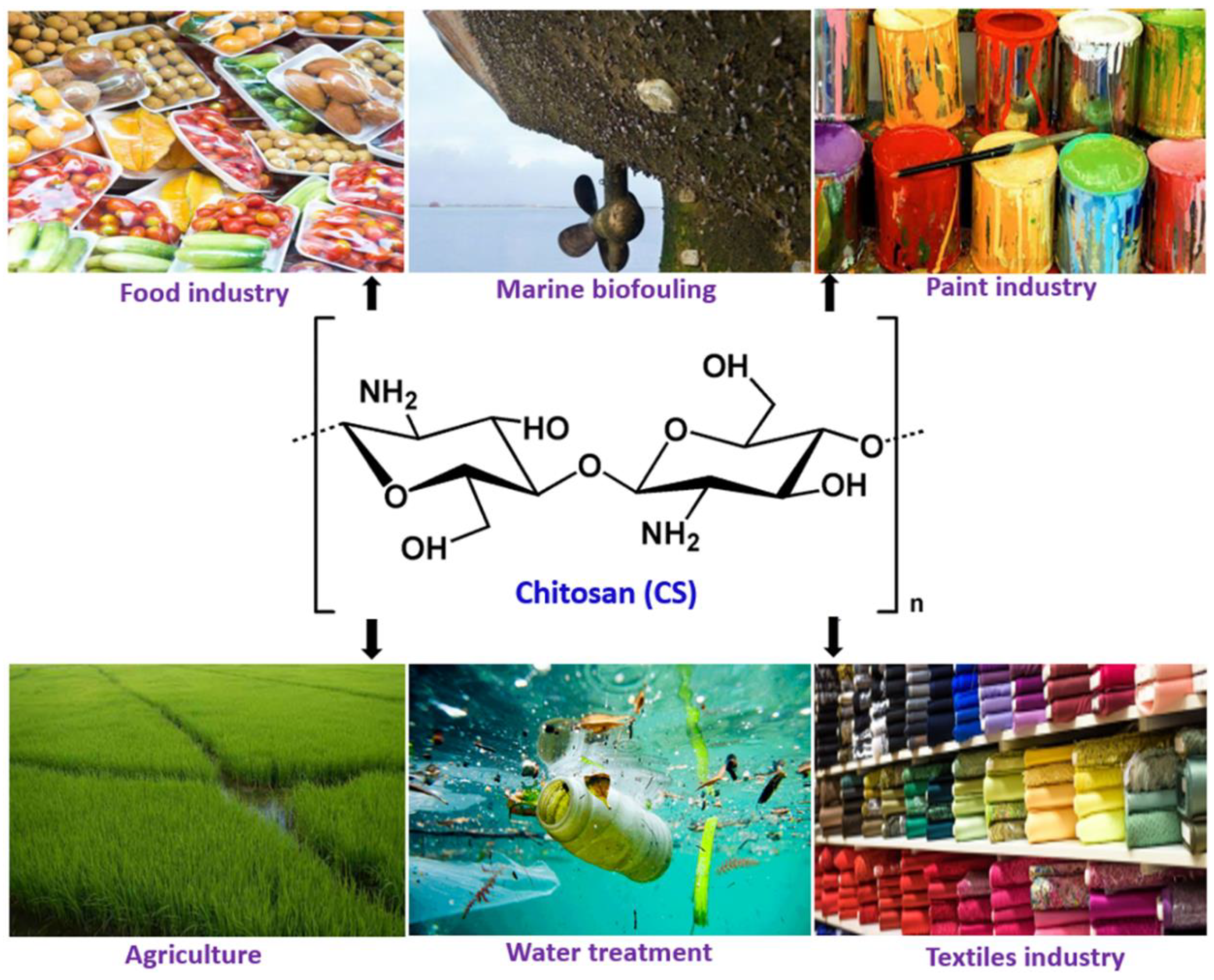
Millions of tons of crustaceans are produced every year and consumed as protein-rich seafood but the shells and other non-edible parts constituting about half the body mass are wasted. The crustacean shells are a prominent source of polysaccharide (chitin) and protein. Chitosan, a de-acetylated form of chitin obtained from the crustacean waste are used for a variety of medical applications. In recent times, it has also found use in food and paint industries including marine antifouling coatings, due to its characteristic properties, like solubility in weak acids, film-forming ability, pH-sensitivity, antifouling properties, biodegradability, and biocompatibility. Chitosan composite coatings in food, paint and water treatment solutions have been developed. In food industries, chitosan-based composite films and coatings are applied for prolonging the post-harvest life of fruits and vegetables, while anti-corrosion and self-healing properties are mainly explored for antifouling applications in paints and metal ion chelation and antifouling properties are useful for water treatment.
- Crustacean waste
- Chitosan
- Nanocomposite
- Films or Coatings
- Antimicrobial activity
- Anti-corrosion
- Antifouling
- Food preservation
- Fruits and vegetable
- Water Treatment
1. Introduction
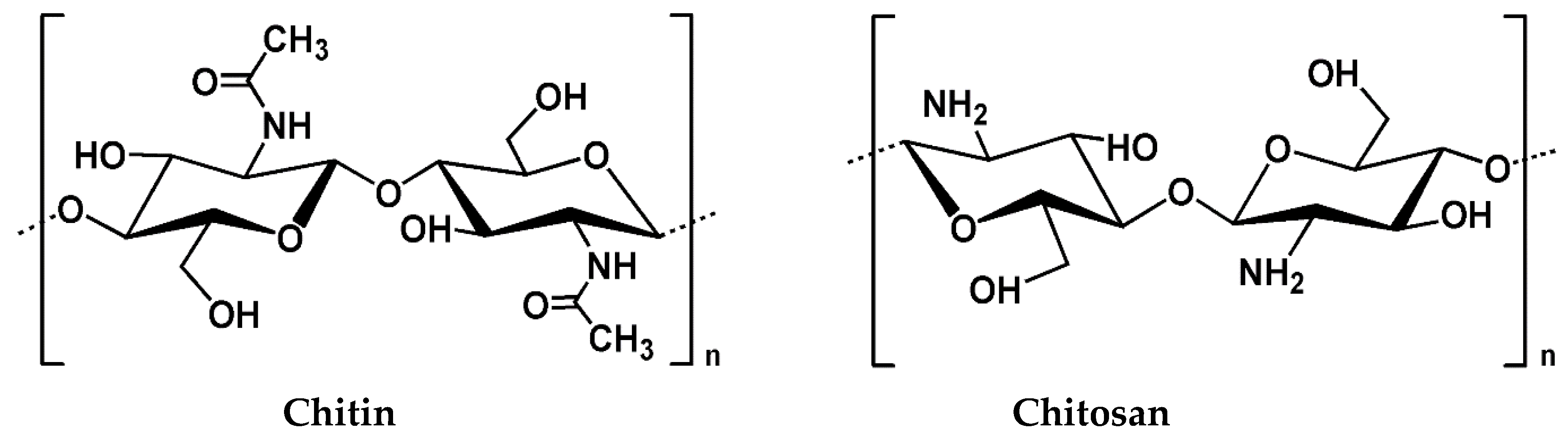
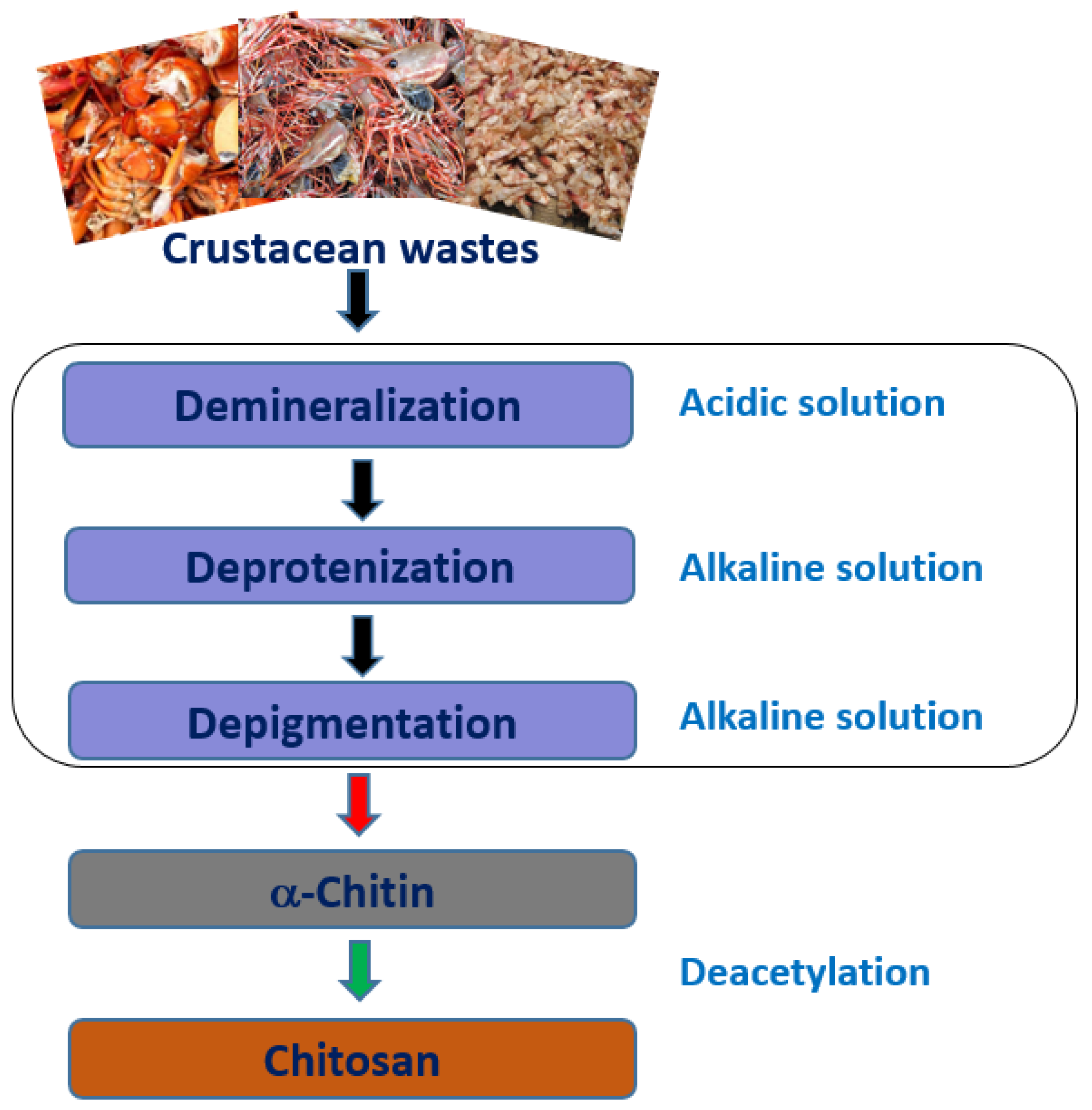
Millions of tons of crustaceans such as crabs, shrimps, lobsters, and krill are consumed as protein-rich seafood worldwide every year. The shells of the crustaceans and other non-edible parts which are about half the body mass, a prominent source of chitin and protein, are generally discarded as waste. Although chitin is the second largest natural polysaccharide on earth, after cellulose, it is not widely used for fabrication of products or as a food commodity due to its insolubility in commonly used solvents [1,2]. Chitosan (CH) can be obtained commercially from crustacean wastes and the cell walls of some fungi by the deacetylation of chitin [3,4,5]. The United States Food and Drug Administration (USFDA) has recommended chitosan a GRAS (Generally Recognized as Safe) material, which is increasingly attracting attention for potential applications in food, agriculture, and biomedicine.
Chitosan is a biopolymer and “hydrocolloid”. Although most hydrocolloids are neutral or negatively charged at acidic pH, chitosan is charged positively due to the presence of highly reactive amino groups. Chitosan is not extensively available in nature and is usually derived from chitin by the partial deacetylation in alkaline solutions at elevated temperatures, which is a linear polysaccharide composed of N-acetyl, D-glucosamine, and D-glucosamine units [6]. Chitosan has been used in food and paint applications owing to its superior characteristic properties, such as degradability, solubility in weak acids, pH-sensitivity, film-forming property, biocompatibility, non-antigenic properties, absence of toxicity, and low-cost [7,8,9]. Moreover, because of its natural origin and multiple possible applications, like preparation of biodegradable films, blends, coatings, composites, nanocomposites, etc., it has attracted the attention of both the scientific community and industries, particularly involved in food and paints applications (Figure 1).[1]
2. Chitosan and its Properties
2.1. Source and Extraction
2.2. Physico-Chemical Properties of Chitosan
2.2.1. Degree of Deacetylation (DD)
2.2.2. Molecular Weight (MW)
2.2.3. Solubility
2.3. Antimicrobial Properties
2.4. Self-Healing Properties
The ability of a material to heal or repair damages automatically or with some external stimulation independently is called self-healing. Numerous polymeric materials with self-healing capabilities have been fabricated in recent years [62,63,64,65]. Self-healing materials, being capable of forming reversible bonds or reactions in the networks, enhance the durability of the materials. An example of chitosan-based self-healing materials that have attracted attention are anticorrosion self-healing paints [66,67,68,69]. Despite significant achievements in the development of chitosan-based self-healing materials, several challenges still need to be addressed for wider applications. An appropriate balance between mechanical strength, self-healing capacities, and mechanical robustness is required for the fabrication of newly developed, chitosan-based, self-healing materials.
Applications of self-healing coatings include automotive refinish on the backside of smartphones to stop the development of corrosion in scratches [70], etc. Due to excellent film-forming properties, superior adhesion to metallic surfaces, and self-healing abilities, chitosan-based self-healing coatings have been effective in protecting metal surfaces and metallic pieces [71,72]. Two main approaches have been pursued for corrosion protection in self-healing coatings: (i) the fixing of defects by adding polymerizing agents in polymeric coating matrix and (ii) by using corrosion inhibitors that can protect corroding areas [73]. In anti-corrosion paints, self-healing refers to both dynamic care of the substrate and structural repair of the coatings, offering superior protecting ability and increased longevity of the coating compared to other protective coatings [71]. Chitosan- and cerium (Ce)-based self-healing coatings have been reported to protect aluminium alloy 2024 from corrosion [68,74]. 2-Mercaptobenzothiazole (MBT) has been used as an effective corrosion inhibitor in chitosan-based coating for aluminium alloys 2024. The study revealed that MBT has strong inhibiting ability and even after one week in a full immersion condition no corrosion attack was reported [71]. In the study, the surface properties of the chitosan coatings were also improved by chemical grafting using poly (ethylene-alt-maleic anhydride) (PEMA) and poly (maleic anhydride-alt-1-octadecene) (PMAO) to increase its hydrophobicity, which is important for corrosion protection in atmospheric conditions. Due to good wettability and adhesion properties it was argued that chitosan provides corrosion protection, simultaneously working as a reservoir for the corrosion inhibitor which prevents the formation of pittings on aluminium alloy. The grafting of chitosan at the coating/solution interface with PEMA and PMAO provided an adequate hydrophobic effect, especially in the case of chitosan loaded with MBT, leading to the delay of ingress of electrolyte towards the metal interface. The combination of active corrosion protection due to MBT and the surface hydrophobicity conferred by grafting could be the reason behind the efficient protection from corrosion to aluminium alloy 2024 [71].
3. Chitosan-Based Nanocomposites
Previously, many studies have reported chemical modification of chitosan either by coupling with small molecules or grafting with polymers, for changing/improvement or better use of the intrinsic properties of chitosan. Chitosan has been grafted with poly-lactide to form polymeric amphiphilic micelles [75] or polyethyleneimine (PEI) to form a branched PEI-g-chitosan with lowered cytotoxicity but higher gene transfection efficiency compared to PEI [76]. More recently, chitosan-based nanocomposites have emerged, where both polymer and nanoparticles contribute to the improvement or enhancement of specific properties. Dispersed nanomaterials contained in chitosan polymer matrix not only improves the physical, mechanical, and thermal stability of chitosan but also endows the composite with its intrinsic properties, such as high surface area or extraordinary physicochemical properties. Chitosan nanocomposites formed between chitosan and metal/metal oxide, carbon, polymer, or clay materials via physical or chemical interaction and their applications in food, paints, and environmental fields are summarized in Table 1.
3.1. Chitosan-Metal/Metal Oxide
3.2. Chitosan-Carbon Materials
3.3. Chitosan-Polymer Mixture or Copolymer
3.4. Chitosan-Clay Composites
Clay is finely grained soil mainly composed of metal oxides or hydroxides with traces of organic matters. Owing to its small particle size (ca. 1 μm) and excellent colloidal properties, clay has been widely used in different applications, such as for water purification, as an odor absorbent, and as a lubricant in construction industries. Because of the structural characteristics, clay nanotubes and platelets can be used to load active agent to improve the passive barrier performances of anticorrosive coating [96]. Due to the electrostatic interaction between chitosan and clay, the composites are normally combined through adsorption, gelation, or intercalation. In chitosan-clay nanocomposites, clay exhibits its characteristic properties, such as absorption of specific species [97,98] or hemostatic properties [99], while chitosan can provide a higher loading or the cross-linked chitosan network as a support or scaffold for clay (Table 1).
Table 1. Examples of chitosan-based nanocomposites and their applications.
|
Chitosan Molecular Weight/Viscosity |
Type of Nanomaterials in Composite |
Name of Nanomaterial/Polymer/Clay |
Preparation Method of Chitosan Nanocomposite |
Form of Chitosan Nanocomposites |
Specific Application |
Key/Enhanced Properties |
Application Field |
Reference |
|---|---|---|---|---|---|---|---|---|
|
100 kDa |
Metal |
Ag nanoparticles |
In situ reduction on chitosan |
Thin film coating on bandage |
Antibacterial activity against E. coliand S. aureus |
Inactivation bacterial metabolism |
Antimicrobial |
[100] |
|
Medium molecular weight |
Metal |
Ag nanoparticles |
In situ reduction on chitosan |
Ag nanoparticles anchored on chitosan particles |
Sensing of ammonia in solution |
Sensitive in optical absorption intensity and wavelength |
Environment |
[101] |
|
Medium molecular weight |
Metal oxide |
ZnO nanoparticles |
Blending |
Thin film coating |
Antifouling prevention |
Anti-diatom activity and antibacterial activity against the marine bacterium |
Anti-biofouling |
|
|
Low viscosity |
Metal oxide |
SiO2 nanoparticles |
In situ Stöber method grown on chitosan |
Slurry packed in liquid chromatography (LC) column |
Adsorption of rare-earth elements |
High adsorption efficiency, selectivity, and reusability |
Environmental |
[87] |
|
190–310 kDa |
Carbon |
Graphene oxide |
Cross-linking |
Thin film |
Antimicrobial against E. coliand B. subtilis |
Improved mechanical and antimicrobial properties |
Antimicrobial |
[88] |
|
300 kDa |
Carbon |
Graphene oxide |
Cross-linking |
Hydrogel |
Removal of dyes and metal ions from water |
Tunable surface charge; efficient removal of pollutants |
Environmental |
[89] |
|
N/A |
Polymer |
low density poly-ethylene (LDPE) film |
Grafting |
Coating |
Significant changes in surface wettability |
Improved anti-thrombogenic properties |
Antifouling |
[92] |
|
N/A |
Clay |
Halloysite clay nanotubes |
Electrostatical adsorption |
Coating |
Anticorrosive protective |
Improved passive barrier protective and self-healing |
Environmental |
[96] |
|
50–190 kDa |
Clay |
Bentonite and sepiolite |
Blend |
Thin film |
Winemaking application |
Enhanced immobilization of protease but negatively affected catalytic properties |
Antimicrobial |
[97] |
|
Medium molecular weight |
Clay |
Bentonite |
Gelation and lyophilization |
Bead |
Carbon dioxide adsorption |
High adsorption capacity under moderate condition |
Environmental |
[98] |
4. Applications of Chitosan-Based Nanocomposites
4.1. Water Purification
Synthetic dyes are used increasingly for industrial applications, particularly in the textile industry, which leads to severe water pollution because of the discharge of unutilized dyes into water bodies. Over 10,000 different colorants (dyes and pigments) are used in textile industries and over 7 × 105 tons of synthetic dyes are annually produced worldwide [102,103]. Most of the synthetic dyestuffs are discharged into the ecosystem without appropriate treatments, thus triggering global environmental problems [103]. Removal of dyes from water bodies is challenging because of their inert nature and existence in low concentrations. Chitosan has received attention in water purification as it is an inexpensive biopolymer and also due to the presence of a large number of reactive amino (-NH2) and hydroxyl (-OH) groups. Adsorption of acid dyes on chitosan and modified chitosan materials occurs because of the electrostatic interaction between negatively charged dye ions and the protonated amino groups [104]. Shen et al. demonstrated that the removal of dyes from alkaline effluent involves chelating interactions rather than electrostatic interactions [105].
The removal of dye from wastewater using nanocomposites of chitosan has been achieved using several processes, such as physical adsorption, ion-exchange, hydrogen bonds, hydrophobic attractions, and chemical bonding. Chitosan-based composite fibers (MNPs/ZnPc-CS) and pellets of zinc photocatalysts (ZnPc)-supported metallic and bimetallic nanoparticles have been synthesized by Ali et al. for metal ions uptake [106]. The MNPs/ZnPc-CS fibers were used as dip-catalysts for the reduction of nitrophenols and azo dyes like methyl orange (MO) and congo red (CR). The results showed that the developed composites exhibited excellent catalytic efficiency and recyclability in the reduction of these dyes.
4.2. Antifouling Paints and Coatings
Marine biofouling is the undesirable growth of organisms on submerged surfaces in ponds, rivers, estuaries, and oceans [108]. Any submerged, clean, and unprotected substrate are quickly colonized by microorganisms (bacteria, diatoms, and unicellular eukaryotes) and later by larvae of invertebrates and spores of algae [109,110]. Biofouling has huge economic impacts in maritime industries [111]. Biofouling significantly increases vessel drag, fuel consumption, clogs membranes and pipes, and also interfere with the stability of sensors attached to the vessels. Worldwide, countries spend billions of US dollars in order to manage and prevent this problem [112]. Currently, antifouling paints utilize toxic biocides that pollute the environment [111]. Thus, new low-cost and non-toxic antifouling paints are urgently needed. Chitosan has been proposed as a promising antifouling agent due to its antimicrobial properties [113]. Plastic substrates coated with 2.5% chitosan significantly reduced the settlement of bryozoan Bugula neritina compared to untreated substrates. Chitosan mixed with a non-toxic paint base applied to plastic substrates were found to protect from bacterial fouling over one week of laboratory experiments [114]. Chitosan incorporated into a silicon-polyurethane marine paint was exposed to biofouling for over two months in cold seawater [113]. Experiments demonstrated a short-term antibacterial action of chitosan, while no anti-algal action was observed. In contrast, chitosan paints significantly reduced densities of micro-fouling on the surface of a sea glider exposed to periodical oscillations of environmental conditions in the Sea of Oman. Moreover, it was found that the structure of microbial communities formed on chitosan paints was similar to unprotected surfaces and was totally different from what is found on surfaces coated with copper-based antifouling paints, suggesting that chitosan-based coatings have a minimal negative impact on marine communities [115].
Few researchers have combined chitosan with nanoparticles in order to increase antifouling properties and stability of coatings. Chitosan-ZnO hybrid coatings were found to inhibit bacterial and diatom fouling more efficiently than chitosan coatings alone [86]. Nanocomposite films of chitosan mixed with silver nanoparticles were found to be effective against E. coli and Bacillus bacteria and thus it was proposed that these “green” films can be used as packaging materials [116]. Nitric oxide (NO)-releasing chitosan coatings were found to exhibit dose-dependent behavior for the degradation of biofilms of Pseudomonas aeruginosa [117]. The effect of NO-chitosan coating was better than chitosan alone, suggesting synergistic action of chitosan and NO. Chitosan functionalized with polyelectrolyte brushes were tested against bacteria in laboratory experiments and against biofouling in static field experiments in the Mediterranean sea, showing promising antifouling activities [118].
Chitosan membranes show strong antibacterial properties [119] and the growth of gram-positive bacteria was found to reduce more significantly than gram-negative bacteria. Cellulose membranes with smaller pore sizes, when modified with higher molecular weight chitosan, were found to have improved antibacterial activity against gram-positive Staphylococcus aureus and gram-negative E. coli bacteria [120]. Forward osmosis (FO) membrane coated with chitosan and graphene oxide nanosheets on a porous support layer, was found to be effective in fouling rejection of alginate in laboratory experiments [121]. Silicone and glass surfaces coated with chitosan nanocoatings were able to prevent the adsorption of proteins and biofilm formation by S. aureus wherein the antifouling effect was found to depend on the coating thickness [122]. Chitosan-modified polyacrylonitrile (PAN) hollow fiber membranes were reported to have better antibacterial properties against E. coli and Bacillus subtilis compared to unmodified PAN membranes [123]. Composite polyethersulfone (PES) nanofiltration membranes synthesized by blending O-carboxymethyl chitosan (O-CMC) and iron oxide (Fe3O4) nanoparticles were found to have considerably higher water flux, permeation, and fouling resistance compared to PES membranes [124]. PES membranes prepared using O-CMC and silver nanoparticles were found to prevent protein fouling as well as a good antibacterial property towards E. coli and S. aureus [125].
4.3. Shelf-Life Extension of Fruits and Vegetables
During postharvest transportation and storage, fresh produce (fruits and vegetables) undergo quality deteriorations due to the various physiology reactions and processes, such as postharvest respiration, ripening, ethylene production, and senescence. These physiological changes are directly influenced by the surrounding environment, i.e., temperature, oxygen, humidity, and light, that lead to loss of water, texture, color, and nutrients of the produce [126,127]. Microbiological spoilage leads to postharvest losses of about 15% to 50% of fruits and vegetables produced worldwide [128]. In developing countries, the percentages of product losses are quite high due to a lack of appropriate technologies for postharvest storage of fruits and vegetables. The physiological, biochemical, and environmental changes promote the growth of postharvest pathogens that are the major causes of loss/damages in the supply chain. However, controlling postharvest destruction of fresh produce involves the application of synthetic antimicrobials, fungicides, and insecticides. Consumer concerns about the adverse effect of synthetic chemical residues on human health and environment and the chances of the development of pathogen resistance have led global scientific research towards the development of alternative strategies for the preservation of fresh produce [129].
The application of a natural polymer (biopolymer)-based films and coatings on food surfaces have recently gained interest for the shelf-life extension of fresh produce due to their similar functions to those of conventional protection or synthetic packaging. Out of several natural polymers, the use of chitosan-based treatment at the postharvest stages has been considered to be a suitable alternative treatment to replace the use of synthetic chemicals [130]. Chitosan-based films and coatings have been reported to effectively extend quality and storability of food products in general, and of fresh agricultural produce in particular, due to its excellent natural antioxidant, antibacterial, and antifungal activities [131,132,133,134].
4.3.1. Packaging Films
One of the main research areas in food industries has focused on developing new packaging techniques capable of improving post-harvest life of fresh foods based on their interaction with packaging. Such techniques called "active packaging" is defined as the incorporation of an active system/materials into packaging film or a container to maintain the quality or extending the shelf-life of food products. In particular, antimicrobial packaging is one of the most innovative and promising active packaging systems developed over the last decade, for inhibiting microbial growth and action, leading to the maintenance of food quality with improved shelf-life [132,135,136].
Biopolymer-based packaging films have received considerable interest as an alternative packaging material to plastics. Bioplastics are usually degradable under appropriate conditions of moisture, temperature, and oxygen availability and do not produce any toxic residues. Major problems associated with biodegradable polymers are three-fold (3P): performance, processing, and price. Performance and processing related problems are universal to almost all biodegradable polymers irrespective of their origin [137]. In particular, brittleness, low heat-distortion temperature, high gas-vapor barrier properties, and poor resistance to harsh processing operations limit extensive applications of biopolymers. Application of nanotechnology forming nanocomposites has opened new options for improving properties of biopolymers. Through the incorporation of nanoscale materials as a filler into biopolymer matrices markedly improve mechanical, thermal, barrier, and other physio-chemical properties, compared to base polymers and conventional (microscale) composites [138]. Nano-sized fillers can be either inorganic or organic materials, such as clay (e.g., montmorillonite, attapulgite), natural antimicrobial agents (e.g., nisin), metal (e.g., silver, gold), and metal oxides (e.g., zinc oxide (ZnO), titanium dioxide (TiO2)) leading to antimicrobial activity, thermal stability and improved mechanical properties of biopolymer films [131,133,137,139,140,141]. Chitosan-ZnO hybrid coatings on polyethylene films have been reported to reduce the growth of pathogenic bacteria and fungi [85], as well as increased the shelf life of okra (Abelmoschus esculentus) vegetables [142]. Zhang et al. developed a chitosan-TiO2 composite film that was effective against E. coli, S. aureus, Candida albicans, and Aspergillus niger with 100% sterilization achieved within 12 h [131]. The composite film could successfully protect red grapes from microbial infection thus enhancing their shelf-life (Figure 5).
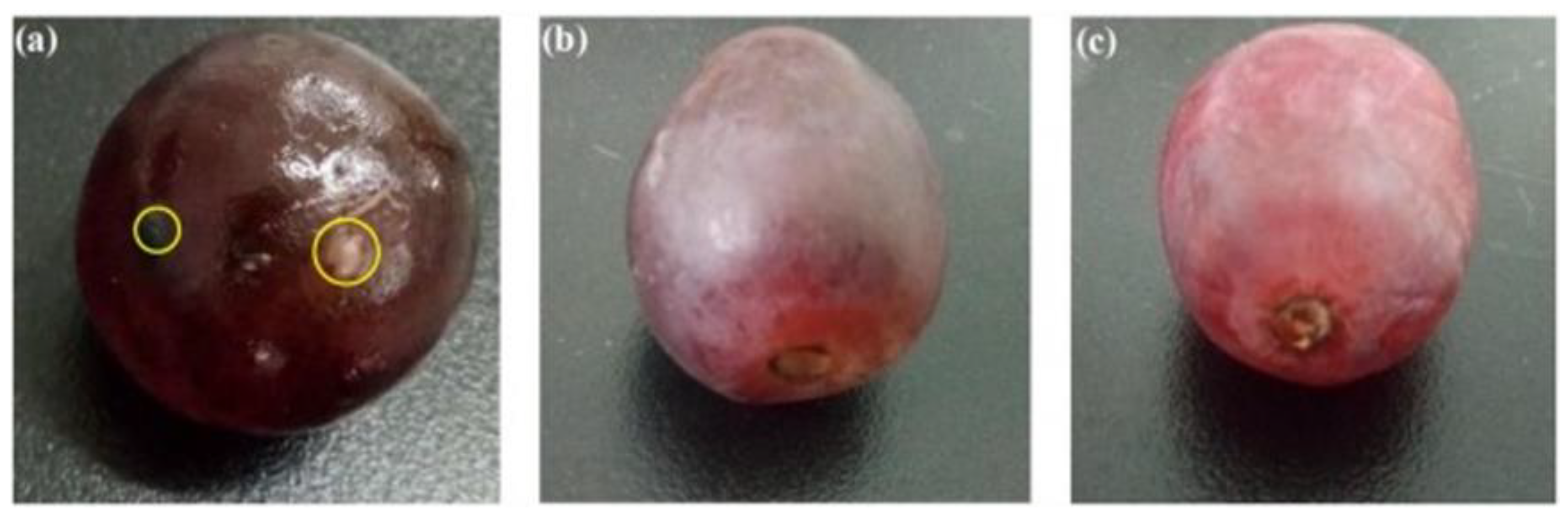
Figure 5. Preservation of red grapes wrapped with (a) polyethylene film, (b) pure chitosan film, and (c) chitosan-TiO2 film, stored at 37 °C for six days. (Reproduced with permission from [131], Copyright © 2017, Elsevier).
4.3.2. Coatings of Fruits and Vegetables
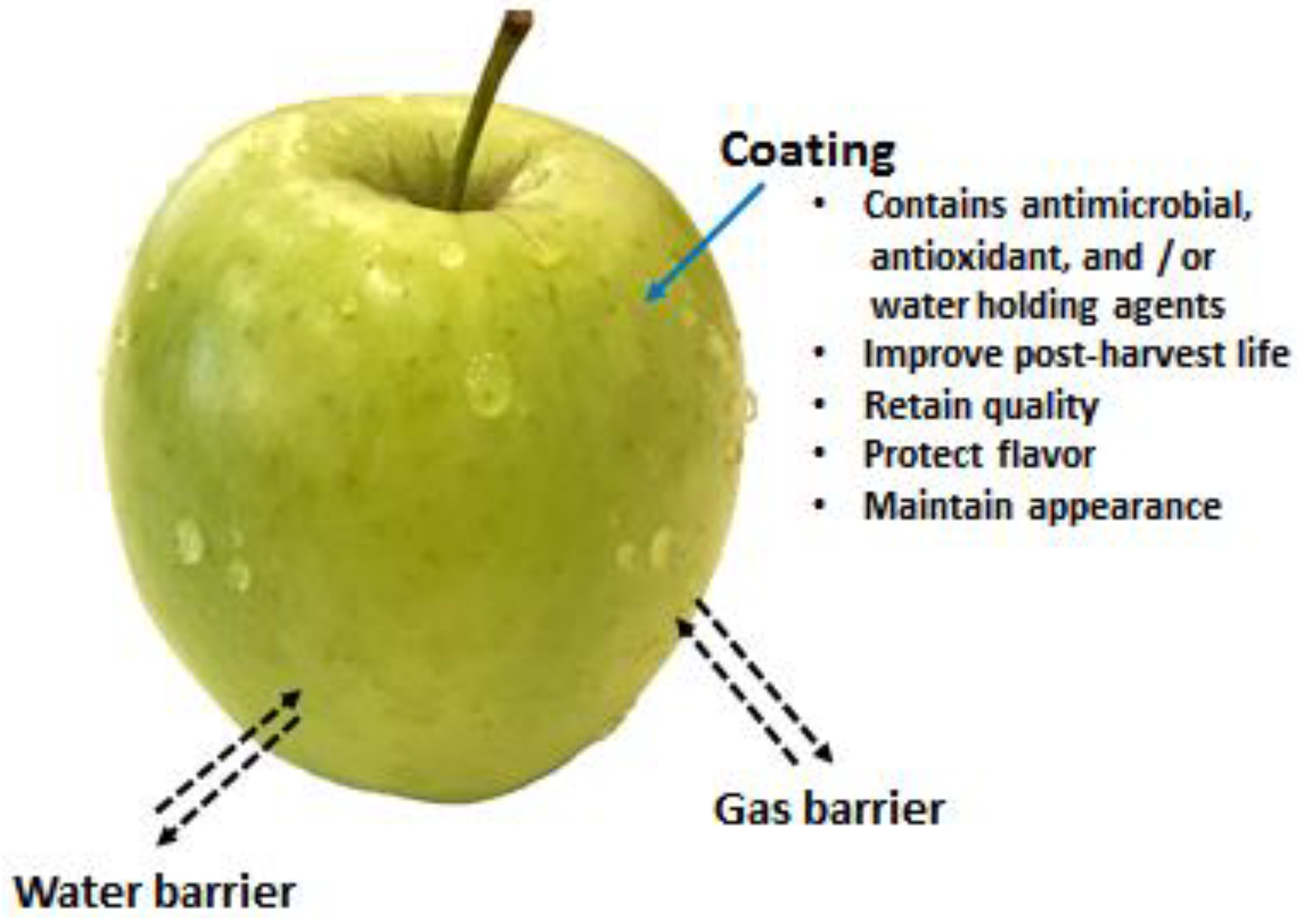
Coatings provide a thin layer of materials on food surfaces to maintain or control the ingress of gases, moisture, and solutes from the environment. Additionally, coatings can also act as a carrier for functional antimicrobial and antioxidant substances to additionally enhance its functionality for ensuring food quality and food safety of fresh produce (Figure 6). Coatings on the surface of fruits and vegetables could provide the following functions:
-
Offer barrier properties against moisture and oxygen
-
Help to deliver antimicrobial activity to inhibit or delay the microbial growth
-
Deliver antioxidant effects that help to reduce the oxidation process, loss of color, vitamins, etc.
-
Help to maintain the loss of volatile components and stop acquiring foreign odors
References
- Santosh Kumar; Fei Ye; Sergey Dobretsov; Joydeep Dutta; Chitosan Nanocomposite Coatings for Food, Paints, and Water Treatment Applications. Applied Sciences 2019, 9, 2409, 10.3390/app9122409.
- Tsigos, I.; Martinou, A.; Kafetzopoulos, D.; Bouriotis, V. Chitin deacetylases: New, versatile tools in biotechnology. Trends Biotechnol. 2000, 18, 305–312.
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Technol. 2006, 97, 1061–1085.
- Rinaudo, M. Chitin and chitosan: Properties and applications. Polym. Sci. 2006, 31, 603–632.
- Dhillon, G.S.; Kaur, S.; Brar, S.K.; Verma, M. Green synthesis approach: Extraction of chitosan from fungus mycelia. Rev. Biotechnol. 2012, 33, 379–403.
- Benhabiles, M.S.; Salah, R.; Lounici, H.; Drouiche, N.; Goosen, M.F.A.; Mameri, N. Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll. 2012, 29, 48–56.
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of Spectroscopic Methods for Structural Analysis of Chitin and Chitosan. Drugs 2010, 8, 1567–1636.
- Bonilla, J.; Fortunati, E.; Atarés, L.; Chiralt, A.; Kenny, J.M. Physical, structural and antimicrobial properties of poly vinyl alcohol–chitosan biodegradable films. Food Hydrocoll. 2014, 35, 463–470.
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Polym. J. 2013, 49, 780–792.
- Kumari, S.; Rath, P.K. Extraction and Characterization of Chitin and Chitosan from (Labeo rohit) Fish Scales. Procedia Mater. Sci. 2014, 6, 482–489.
- Doiphode, N.; Joshi, C.; Ghormade, V.; Deshpande, M.V. Biotechnological Applications of Dimorphic Yeasts. In Yeast Biotechnology: Diversity and Applications; Springer: Dordrecht, The Netherlands, 2009; pp. 635–650.
- Amorim, R.V.S.; Ledingham, W.M.; Kennedy, J.F.; Campos-Takaki, G.M. Chitosan from Syncephalastrum racemosum Using Sugar Cane Substrates as Inexpensive Carbon Sources. Food Biotechnol. 2006, 20, 43–53.
- Tharanathan, R.N.; Kittur, F.S. Chitin—The Undisputed Biomolecule of Great Potential. Rev. Food Sci. Nutr. 2003, 43, 61–87.
- Nouri, M.; Khodaiyan, F.; Razavi, S.H.; Mousavi, M. Improvement of chitosan production from Persian Gulf shrimp waste by response surface methodology. Food Hydrocoll. 2016, 59, 50–58.
- Teli, M.D.; Sheikh, J. Extraction of chitosan from shrimp shells waste and application in antibacterial finishing of bamboo rayon. J. Biol. Macromol. 2012, 50, 1195–1200.
- Sedaghat, F.; Yousefzadi, M.; Toiserkani, H.; Najafipour, S. Bioconversion of shrimp waste Penaeus merguiensis using lactic acid fermentation: An alternative procedure for chemical extraction of chitin and chitosan. J. Biol. Macromol. 2017, 104, 883–888.
- Younes, I.; Ghorbel-Bellaaj, O.; Nasri, R.; Chaabouni, M.; Rinaudo, M.; Nasri, M. Chitin and chitosan preparation from shrimp shells using optimized enzymatic deproteinization. Process Biochem. 2012, 47, 2032–2039.
- Mohammed, M.H.; Williams, P.A.; Tverezovskaya, O. Extraction of chitin from prawn shells and conversion to low molecular mass chitosan. Food Hydrocoll. 2013, 31, 166–171.
- Kumari, S.; Kumar Annamareddy, S.H.; Abanti, S.; Kumar Rath, P. Physicochemical properties and characterization of chitosan synthesized from fish scales, crab and shrimp shells. J. Biol. Macromol. 2017, 104, 1697–1705.
- Abdelmalek, B.E.; Sila, A.; Haddar, A.; Bougatef, A.; Ayadi, M.A. β-Chitin and chitosan from squid gladius: Biological activities of chitosan and its application as clarifying agent for apple juice. J. Biol. Macromol. 2017, 104, 953–962.
- Marei, N.H.; El-Samie, E.A.; Salah, T.; Saad, G.R.; Elwahy, A.H.M. Isolation and characterization of chitosan from different local insects in Egypt. J. Biol. Macromol. 2016, 82, 871–877.
- Nemtsev, S.V.; Zueva, O.Y.; Khismatullin, M.R.; Albulov, A.I.; Varlamov, V.P. Isolation of Chitin and Chitosan from Honeybees. Biochem. Microbiol. 2004, 40, 39–43.
- Abdel-Gawad, K.M.; Hifney, A.F.; Fawzy, M.A.; Gomaa, M. Technology optimization of chitosan production from Aspergillus niger biomass and its functional activities. Food Hydrocoll. 2017, 63, 593–601.
- Paulino, A.T.; Simionato, J.I.; Garcia, J.C.; Nozaki, J. Characterization of chitosan and chitin produced from silkworm crysalides. Polym. 2006, 64, 98–103.
- Baron, R.D.; Pérez, L.L.; Salcedo, J.M.; Córdoba, L.P.; Sobral, P.J.d.A. Production and characterization of films based on blends of chitosan from blue crab (Callinectes sapidus) waste and pectin from Orange (Citrus sinensis Osbeck) peel. J. Biol. Macromol. 2017, 98, 676–683.
- Nguyen, T.T.; Barber, A.R.; Corbin, K.; Zhang, W. Lobster processing by-products as valuable bioresource of marine functional ingredients, nutraceuticals, and pharmaceuticals. Bioprocess. 2017, 4, 27.
- Srinivasan, H.; Kanayairam, V.; Ravichandran, R. Chitin and chitosan preparation from shrimp shells Penaeus monodon and its human ovarian cancer cell line, PA-1. J. Biol. Macromol. 2018, 107, 662–667.
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. J. Biol. Macromol. 2018, 120, 1181–1189.
- Yuan, Y.; Chesnutt, B.M.; Haggard, W.O.; Bumgardner, J.D. Deacetylation of Chitosan: Material Characterization and in vitro Evaluation via Albumin Adsorption and Pre-Osteoblastic Cell Cultures. Materials 2011, 4, 1399–1416.
- Jiang, X.; Chen, L.; Zhong, W. A new linear potentiometric titration method for the determination of deacetylation degree of chitosan. Polym. 2003, 54, 457–463.
- Dos Santos, Z.M.; Caroni, A.L.P.F.; Pereira, M.R.; da Silva, D.R.; Fonseca, J.L.C. Determination of deacetylation degree of chitosan: A comparison between conductometric titration and CHN elemental analysis. Res. 2009, 344, 2591–2595.
- Kasaai, M.R. Determination of the degree of N-acetylation for chitin and chitosan by various NMR spectroscopy techniques: A review. Polym. 2010, 79, 801–810.
- Desbrières, J.; Martinez, C.; Rinaudo, M. Hydrophobic derivatives of chitosan: Characterization and rheological behaviour. J. Biol. Macromol. 1996, 19, 21–28.
- Kasaai, M. A review of several reported procedures to determine the degree of N-acetylation for chitin and chitosan using infrared spectroscopy. Polym. 2008, 71, 497–508.
- Wu, T.; Zivanovic, S. Determination of the degree of acetylation (DA) of chitin and chitosan by an improved first derivative UV method. Polym. 2008, 73, 248–253.
- Wu, C.; Kao, C.Y.; Tseng, S.-Y.; Chen, K.C.; Chen, S.-F. Determination of the degree of deacetylation of chitosan by capillary zone electrophoresis. Polym. 2014, 111, 236–244.
- Zając, A.; Hanuza, J.; Wandas, M.; Dymińska, L. Determination of N-acetylation degree in chitosan using Raman spectroscopy. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 114–120.
- Li, X.; Xia, W. Effects of concentration, degree of deacetylation and molecular weight on emulsifying properties of chitosan. J. Biol. Macromol. 2011, 48, 768–772.
- Zhuang, C.; Zhong, Y.; Zhao, Y. Effect of deacetylation degree on properties of Chitosan films using electrostatic spraying technique. Food Control 2019, 97, 25–31.
- Paul, T.; Halder, S.K.; Das, A.; Ghosh, K.; Mandal, A.; Payra, P.; Barman, P.; Das Mohapatra, P.K.; Pati, B.R.; Mondal, K.C. Production of chitin and bioactive materials from Black tiger shrimp (Penaeus monodon) shell waste by the treatment of bacterial protease cocktail. 3 Biotech 2015, 5, 483–493.
- Peniche, C.; Peniche, H.; Pérez, J. Chitosan based self-assembled nanoparticles in drug delivery. Polymers 2018, 10, 235.
- Jongsri, P.; Wangsomboondee, T.; Rojsitthisak, P.; Seraypheap, K. Effect of molecular weights of chitosan coating on postharvest quality and physicochemical characteristics of mango fruit. LWT 2016, 73, 28–36.
- Zhong, Y.; Zhuang, C.; Gu, W.; Zhao, Y. Effect of molecular weight on the properties of chitosan films prepared using electrostatic spraying technique. Polym. 2019, 212, 197–205.
- Kim, K.W.; Min, B.J.; Kim, Y.-T.; Kimmel, R.M.; Cooksey, K.; Park, S.I. Antimicrobial activity against foodborne pathogens of chitosan biopolymer films of different molecular weights. LWT -Food Sci. Technol. 2011, 44, 565–569.
- Zheng, L.-Y.; Zhu, J.-F. Study on antimicrobial activity of chitosan with different molecular weights. Polym. 2003, 54, 527–530.
- No, H.K.; Park, N.Y.; Lee, S.H.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. J. Food Microbiol. 2002, 74, 65–72.
- Shelma, R.; Sharma, C.P. Acyl modified chitosan derivatives for oral delivery of insulin and curcumin. Mater. Sci. Mater. Med. 2010, 21, 2133–2140.
- Wu, M.; Long, Z.; Xiao, H.; Dong, C. Preparation of N, N, N-trimethyl chitosan via a novel approach using dimethyl carbonate. Polym. 2017, 169, 83–91.
- Jagadish, R.S.; Divyashree, K.N.; Viswanath, P.; Srinivas, P.; Raj, B. Preparation of N-vanillyl chitosan and 4-hydroxybenzyl chitosan and their physico-mechanical, optical, barrier, and antimicrobial properties. Polym. 2012, 87, 110–116.
- Jeong, Y.-I.; Kim, D.-G.; Jang, M.-K.; Nah, J.-W. Preparation and spectroscopic characterization of methoxy poly(ethylene glycol)-grafted water-soluble chitosan. Res. 2008, 343, 282–289.
- Mohamed, N.A.; Abd El-Ghany, N.A. Preparation and antimicrobial activity of some carboxymethyl chitosan acyl thiourea derivatives. J. Biol. Macromol. 2012, 50, 1280–1285.
- Botelho da Silva, S.; Krolicka, M.; van den Broek, L.A.M.; Frissen, A.E.; Boeriu, C.G. Water-soluble chitosan derivatives and pH-responsive hydrogels by selective C-6 oxidation mediated by TEMPO-laccase redox system. Polym. 2018, 186, 299–309.
- Fu, Y.; Xiao, C.; Liu, J. Facile fabrication of quaternary water soluble chitosan-sodium alginate gel and its affinity characteristic toward multivalent metal ion. Technol. Innov. 2019, 13, 340–345.
- Alfaro, L.; Chotiko, A.; Chouljenko, A.; Janes, M.; King, J.M.; Sathivel, S. Development of water-soluble chitosan powder and its antimicrobial effect against inoculated Listeria innocua NRRL B-33016 on shrimp. Food Control 2018, 85, 453–458.
- Chouljenko, A.; Chotiko, A.; Reyes, V.; Alfaro, L.; Liu, C.; Dzandu, B.; Sathivel, S. Application of water-soluble chitosan to shrimp for quality retention. LWT 2016, 74, 571–579.
- Allan, C.R.; Hadwiger, L.A. The fungicidal effect of chitosan on fungi of varying cell wall composition. Mycol. 1979, 3, 285–287.
- Sarwar, A.; Katas, H.; Zin, N.M. Antibacterial effects of chitosan–tripolyphosphate nanoparticles: Impact of particle size molecular weight. Nanoparticle Res. 2014, 16, 2517.
- Xing, K.; Chen, X.G.; Kong, M.; Liu, C.S.; Cha, D.S.; Park, H.J. Effect of oleoyl-chitosan nanoparticles as a novel antibacterial dispersion system on viability, membrane permeability and cell morphology of Escherichia coli and Staphylococcus aureus. Polym. 2009, 76, 17–22.
- Ma, Z.; Garrido-Maestu, A.; Jeong, K.C. Application, mode of action, and in vivo activity of chitosan and its micro- and nanoparticles as antimicrobial agents: A review. Polym. 2017, 176, 257–265.
- Tamara, F.; Lin, C.; Mi, F.-L.; Ho, Y.-C. Antibacterial Effects of Chitosan/Cationic Peptide Nanoparticles. Nanomaterials 2018, 8, 88.
- Liu, H.; Du, Y.; Wang, X.; Sun, L. Chitosan kills bacteria through cell membrane damage. J. Food Microbiol. 2004, 95, 147–155.
- Li, X.; Feng, X.; Yang, S.; Fu, G.; Wang, T.; Su, Z. Chitosan kills Escherichia coli through damage to be of cell membrane mechanism. Polym. 2010, 79, 493–499.
- Ataei, S.; Khorasani, S.N.; Neisiany, R.E. Biofriendly vegetable oil healing agents used for developing self-healing coatings: A review. Org. Coat. 2019, 129, 77–95.
- Scheiner, M.; Dickens, T.J.; Okoli, O. Progress towards self-healing polymers for composite structural applications. Polymer 2016, 83, 260–282.
- Garcia, S.J. Effect of polymer architecture on the intrinsic self-healing character of polymers. Polym. J. 2014, 53, 118–125.
- Mauldin, T.C.; Kessler, M.R. Self-healing polymers and composites. Mater. Rev. 2010, 55, 317–346.
- Szabó, T.; Molnár-Nagy, L.; Bognár, J.; Nyikos, L.; Telegdi, J. Self-healing microcapsules and slow release microspheres in paints. Org. Coat. 2011, 72, 52–57.
- Hefni, H.H.H.; Azzam, E.M.; Badr, E.A.; Hussein, M.; Tawfik, S.M. Synthesis, characterization and anticorrosion potentials of chitosan-g-PEG assembled on silver nanoparticles. J. Biol. Macromol. 2016, 83, 297–305.
- Carneiro, J.; Tedim, J.; Fernandes, S.C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Gandini, A.; Ferreira, M.G.S.; Zheludkevich, M.L. Chitosan-based self-healing protective coatings doped with cerium nitrate for corrosion protection of aluminum alloy 2024. Org. Coat. 2012, 75, 8–13.
- Bao, Q.; Zhang, D.; Wan, Y. 2-Mercaptobenzothiazole doped chitosan/11-alkanethiolate acid composite coating: Dual function for copper protection. Surface Sci. 2011, 257, 10529–10534.
- Ulaeto, S.B.; Pancrecious, J.K.; Rajan, T.P.D.; Pai, B.C. Chapter 17—Smart Coatings. In Noble Metal-Metal Oxide Hybrid Nanoparticles; Mohapatra, S., Nguyen, T.A., Nguyen-Tri, P., Eds.; Woodhead Publishing, Duxford, United Kingdom: 2019; pp. 341–372.
- Carneiro, J.; Tedim, J.; Fernandes, S.C.M.; Freire, C.S.R.; Gandini, A.; Ferreira, M.G.S.; Zheludkevich, M.L. Functionalized chitosan-based coatings for active corrosion protection. Surface Coat. Technol. 2013, 226, 51–59.
- Mohandas, A.; Deepthi, S.; Biswas, R.; Jayakumar, R. Chitosan based metallic nanocomposite scaffolds as antimicrobial wound dressings. Mater. 2018, 3, 267–277.
- Ding, F.; Li, H.; Du, Y.; Shi, X. Recent advances in chitosan-based self-healing materials. Che. Intermed. 2018, 44, 4827–4840.
- Zheludkevich, M.L.; Tedim, J.; Freire, C.S.R.; Fernandes, S.C.M.; Kallip, S.; Lisenkov, A.; Gandini, A.; Ferreira, M.G.S. Self-healing protective coatings with “green” chitosan based pre-layer reservoir of corrosion inhibitor. Mater. Chem. 2011, 21, 4805.
- Wu, Y.; Zheng, Y.; Yang, W.; Wang, C.; Hu, J.Fu, S. Synthesis and characterization of a novel amphiphilic chitosan–polylactide graft copolymer. Carbohydrate Polymers 2005; 59, 165-171.
- Wong, K.; Sun, G.; Zhang, X.; Dai, H.; Liu, Y.; He, C.; Leong, K.W. PEI-g-chitosan, a Novel Gene Delivery System with Transfection Efficiency Comparable to Polyethylenimine in vitro and after Liver Administration in vivo. Bioconjugate Chem. 2006, 17, 152–158.
- Kumar, S.; Bhattacharya, W.; Singh, M.; Halder, D.; Mitra, A. Plant latex capped colloidal silver nanoparticles: A potent anti-biofilm and fungicidal formulation. Mol. Liq. 2017, 230, 705–713.
- Kumari, R.; Brahma, G.; Rajak, S.; Singh, M.; Kumar, S. Antimicrobial activity of green silver nanoparticles produced using aqueous leaf extract of Hydrocotyle rotundifolia. Pharm. Exp. Med. 2016, 16, 195–201.
- Swargiary, M.; Kumar, S. One pot phytosynthesis of gold nanoparticles using aqueous extract of elephant apple- an eco-friendly approach. Pharm. Exp. Med. 2017, 17, 285–289.
- Pang, X.; Zhitomirsky, I. Electrodeposition of hydroxyapatite–silver–chitosan nanocomposite coatings. Surface Coat. Technol. 2008, 202, 3815–3821.
- Mishra, S.K.; Ferreira, J.M.F.; Kannan, S. Mechanically stable antimicrobial chitosan–PVA–silver nanocomposite coatings deposited on titanium implants. Polym. 2015, 121, 37–48.
- Pounraj, S.; Somu, P.; Paul, S. Chitosan and graphene oxide hybrid nanocomposite film doped with silver nanoparticles efficiently prevents biofouling. Surface Sci. 2018, 452, 487–497.
- Lin, B.; Luo, Y.; Teng, Z.; Zhang, B.; Zhou, B.; Wang, Q. Development of silver/titanium dioxide/chitosan adipate nanocomposite as an antibacterial coating for fruit storage. LWT-Food Sci. Technol. 2015, 63, 1206–1213.
- Ortiz-Duarte, G.; Pérez-Cabrera, L.E.; Artés-Hernández, F.; Martínez-Hernández, G.B. Ag-chitosan nanocomposites in edible coatings affect the quality of fresh-cut melon. Postharvest Biol. Technol. 2019, 147, 174–184.
- Al-Naamani, L.; Dobretsov, S.; Dutta, J. Chitosan-zinc oxide nanoparticle composite coating for active food packaging applications. Food Sci. Emerg. Technol. 2016, 38, 231–237.
- Al-Naamani, L.; Dobretsov, S.; Dutta, J.; Burgess, J.G. Chitosan-zinc oxide nanocomposite coatings for the prevention of marine biofouling. Chemosphere 2017, 168, 408–417.
- Roosen, J.; Spooren, J.; Binnemans, K. Adsorption performance of functionalized chitosan–silica hybrid materials toward rare earths. Mater. Chem. A 2014, 2, 19415–19426.
- Grande, C.D.; Mangadlao, J.; Fan, J.; De Leon, A.; Delgado-Ospina, J.; Rojas, J.G.; Rodrigues, D.F.; Advincula, R. Chitosan Cross-Linked Graphene Oxide Nanocomposite Films with Antimicrobial Activity for Application in Food Industry. Symp. 2017, 374, 1600114.
- Yan, H.; Yang, H.; Li, A.; Cheng, R. pH-tunable surface charge of chitosan/graphene oxide composite adsorbent for efficient removal of multiple pollutants from water. Eng. J. 2016, 284, 1397–1405.
- Dong, X.; Wei, C.; Liang, J.; Liu, T.; Kong, D.; Lv, F. Thermosensitive hydrogel loaded with chitosan-carbon nanotubes for near infrared light triggered drug delivery. Colloids Surfaces B Biointerfaces 2017, 154, 253–262.
- Papadimitriou, L.; Kaliva, M.; Vamvakaki, M.; Chatzinikolaidou, M. Immunomodulatory Potential of Chitosan-graft-poly(ε-caprolactone) Copolymers toward the Polarization of Bone-Marrow-Derived Macrophages. ACS Biomater. Sci. Eng. 2017, 3, 1341–1349.
- Pandiyaraj, K.N.; Ramkumar, M.C.; Arun Kumar, A.; Padmanabhan, P.V.A.; Pichumani, M.; Bendavid, A.; Cools, P.; De Geyter, N.; Morent, R.; Kumar, V.; et al. Evaluation of surface properties of low density polyethylene (LDPE) films tailored by atmospheric pressure non-thermal plasma (APNTP) assisted co-polymerization and immobilization of chitosan for improvement of antifouling properties. Sci. Eng. C 2019, 94, 150–160.
- Trivedi, P.; Saloranta-Simell, T.; Maver, U.; Gradišnik, L.; Prabhakar, N.; Smått, J.-H.; Mohan, T.; Gericke, M.; Heinze, T.; Fardim, P. Chitosan–Cellulose Multifunctional Hydrogel Beads: Design, Characterization and Evaluation of Cytocompatibility with Breast Adenocarcinoma and Osteoblast Cells. Bioengineering 2018, 5, 3.
- Wang, Z.; Shi, Y.; Yang, X.; Xiong, Y.; Li, Y.; Chen, B.; Lai, W.-F.; Rogach, A.L. Water-Soluble Biocompatible Copolymer Hypromellose Grafted Chitosan Able to Load Exogenous Agents and Copper Nanoclusters with Aggregation-Induced Emission. Funct. Mater. 2018, 28, 1802848.
- Wang, G.; Wang, X.; Huang, L. Feasibility of chitosan-alginate (Chi-Alg) hydrogel used as scaffold for neural tissue engineering: A pilot study in vitro. Biotechnol. Equip. 2017, 31, 766–773.
- Njoku, D.I.; Cui, M.; Xiao, H.; Shang, B.; Li, Y. Understanding the anticorrosive protective mechanisms of modified epoxy coatings with improved barrier, active and self-healing functionalities: EIS and spectroscopic techniques. Rep. 2017, 7, 15597.
- Benucci, I.; Liburdi, K.; Cacciotti, I.; Lombardelli, C.; Zappino, M.; Nanni, F.; Esti, M. Chitosan/clay nanocomposite films as supports for enzyme immobilization: An innovative green approach for winemaking applications. Food Hydrocoll. 2018, 74, 124–131.
- Azharul Islam, M.; Tan, Y.L.; Atikul Islam, M.; Romić, M.; Hameed, B.H. Chitosan–bleaching earth clay composite as an efficient adsorbent for carbon dioxide adsorption: Process optimization. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 554, 9–15.
- Li, X.; Li, Y.-C.; Chen, M.; Shi, Q.; Sun, R.; Wang, X. Chitosan/rectorite nanocomposite with injectable functionality for skin hemostasis. Mater. Chem. B 2018, 6, 6544–6549.
- Susilowati, E.; MaryaniAshadi, *. REPLACE .*. Preparation of silver-chitosan nanocomposites and coating
- on bandage for antibacterial wound dressing application. AIP Conf. Proc. 2016, 1710, 030015
- El-Sherbiny, I.M.; Hefnawy, A.; Salih, E. New core–shell hyperbranched chitosan-based nanoparticles as
- optical sensor for ammonia detection. Int. J. Biol. Macromol. 2016, 86, 782–788.
- Ogugbue, C.J.; Sawidis, T. Bioremediation and Detoxification of Synthetic Wastewater Containing Triarylmethane Dyes by Aeromonas hydrophila Isolated from Industrial Effluent. Res. Int. 2011, 2011, 1–11.
- Brüschweiler, B.J. Toxicity of non-regulated aromatic amines from azo dyes in textiles: Knowns and unknowns. Lett. 2013, 221, S54.
- Wang, J.; Zhuang, S. Removal of various pollutants from water and wastewater by modified chitosan adsorbents. Rev. Environ. Sci. Technol. 2017, 47, 2331–2386.
- Shen, C.; Shen, Y.; Wen, Y.; Wang, H.; Liu, W. Fast and highly efficient removal of dyes under alkaline conditions using magnetic chitosan-Fe(III) hydrogel. Water Res. 2011, 45, 5200–5210.
- Ali, F.; Khan, S.B.; Kamal, T.; Anwar, Y.; Alamry, K.A.; Asiri, A.M. Anti-bacterial chitosan/zinc phthalocyanine fibers supported metallic and bimetallic nanoparticles for the removal of organic pollutants. Polym. 2017, 173, 676–689.
- Zhou, J.; Lü, Q.-F.; Luo, J.-J. Efficient removal of organic dyes from aqueous solution by rapid adsorption onto polypyrrole–based composites. Clean. Prod. 2017, 167, 739–748.
- Wahl, M. Marine epibiosis. I. Fouling and antifouling: Some basic aspects. Ecol. Progr. Ser. 1989, 58, 175–189.
- Qian, P.Y.; Lau, S.C.K.; Dahms, H.U.; Dobretsov, S.; Harder, T. Marine Biofilms as Mediators of Colonization by Marine Macroorganisms: Implications for Antifouling and Aquaculture. Biotechnol. 2007, 9, 399–410.
- Salta, M.; Wharton, J.A.; Blache, Y.; Stokes, K.R.; Briand, J.-F. Marine biofilms on artificial surfaces: Structure and dynamics. Microbiol. 2013, 15, 2879–2893.
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology—Past, present and future steps towards efficient and environmentally friendly antifouling coatings. Org. Coat. 2004, 50, 75–104.
- Schultz, M.P.; Bendick, J.A.; Holm, E.R.; Hertel, W.M. Economic impact of biofouling on a naval surface ship. Biofouling 2010, 27, 87–98.
- Pelletier, É.; Bonnet, C.; Lemarchand, K. Biofouling Growth in Cold Estuarine Waters and Evaluation of Some Chitosan and Copper Anti-Fouling Paints. J. Mol. Sci. 2009, 10, 3209–3223.
- Al-Naamani, L.S. Antifouling properties of chitosan coatings on plastic substrates. Agric. Mar. Sci. [JAMS] 2019, 23, 92.
- Dobretsov, S.; Abed, R.M.M.; Muthukrishnan, T.; Sathe, P.; Al-Naamani, L.; Queste, B.Y.; Piontkovski, S. Living on the edge: Biofilms developing in oscillating environmental conditions. Biofouling 2018, 34, 1064–1077.
- Thomas, V.; Yallapu, M.M.; Sreedhar, B.; Bajpai, S.K. Fabrication, Characterization of Chitosan/Nanosilver Film and Its Potential Antibacterial Application. Biomater. Sci. Polym. Ed. 2009, 20, 2129–2144.
- Reighard, K.P.; Hill, D.B.; Dixon, G.A.; Worley, B.V.; Schoenfisch, M.H. Disruption and eradication of aeruginosabiofilms using nitric oxide-releasing chitosan oligosaccharides. Biofouling 2015, 31, 775–787.
- Elshaarawy, R.F.M.; Mustafa, F.H.A.; van Geelen, L.; Abou-Taleb, A.E.A.; Tadros, H.R.Z.; Kalscheuer, R.; Janiak, C. Mining marine shell wastes for polyelectrolyte chitosan anti-biofoulants: Fabrication of high-performance economic and ecofriendly anti-biofouling coatings. Polym. 2017, 172, 352–364.
- Takahashi, T.; Imai, M.; Suzuki, I.; Sawai, J. Growth inhibitory effect on bacteria of chitosan membranes regulated with deacetylation degree. Eng. J. 2008, 40, 485–491.
- Nigmatullin, R.; Konovalova, V.; Pobigay, G. Development of antimicrobial membranes via the surface tethering of chitosan. Appl. Polym. Sci. 2009, 111, 1697–1705.
- Salehi, H.; Rastgar, M.; Shakeri, A. Anti-fouling and high water permeable forward osmosis membrane fabricated via layer by layer assembly of chitosan/graphene oxide. Surface Sci. 2017, 413, 99–108.
- Bulwan, M.; Wójcik, K.; Zapotoczny, S.; Nowakowska, M. Chitosan-Based Ultrathin Films as Antifouling, Anticoagulant and Antibacterial Protective Coatings. Biomater. Sci. Polym. Ed. 2012, 23, 1963–1980.
- Shanthana Lakshmi, D.; Jaiswar, S.; saxena, M.; Tasselli, F.; Raval, H.D. Preparation and performance of biofouling resistant PAN/chitosan hollow fiber membranes. 3 Biotech 2017, 7, 224.
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H.; Beygzadeh, M. Novel high flux antifouling nanofiltration membranes for dye removal containing carboxymethyl chitosan coated Fe3O4 nanoparticles. Desalination 2014, 349, 145–154.
- Wang, R.; Song, X.; Xiang, T.; Liu, Q.; Su, B.; Zhao, W.; Zhao, C. Mussel-inspired chitosan-polyurethane coatings for improving the antifouling and antibacterial properties of polyethersulfone membranes. Polym. 2017, 168, 310–319.
- Yousuf, B.; Qadri, O.S.; Srivastava, A.K. Recent developments in shelf-life extension of fresh-cut fruits and vegetables by application of different edible coatings: A review. LWT 2018, 89, 198–209.
- Gong, T.; Li, C.; Bian, B.; Wu, Y.; Dawuda, M.M.; Liao, W. Advances in application of small molecule compounds for extending the shelf life of perishable horticultural products: A review. Hortic. 2018, 230, 25–34.
- Swaminathan, M.S. Food Losses and Food Waste. In Combating Hunger and Achieving Food Security; Cambridge University Press: Cambridge, UK, 2015, pp. 37–46.
- Ma, L.; Zhang, M.; Bhandari, B.; Gao, Z. Recent developments in novel shelf life extension technologies of fresh-cut fruits and vegetables. Trends Food Sci. Technol. 2017, 64, 23–38.
- Yuan, G.; Chen, X.; Li, D. Chitosan films and coatings containing essential oils: The antioxidant and antimicrobial activity, and application in food systems. Food Res. Int. 2016, 89, 117–128.
- Zhang, X.; Xiao, G.; Wang, Y.; Zhao, Y.; Su, H.; Tan, T. Preparation of chitosan-TiO2 composite film with efficient antimicrobial activities under visible light for food packaging applications. Polym. 2017, 169, 101–107.
- Shankar, S.; Rhim, J.-W. Preparation of sulfur nanoparticle-incorporated antimicrobial chitosan films. Food Hydrocoll. 2018, 82, 116–123.
- Xu, D.A.N.; Qin, H.-R.; Ren, D.A.N.; Yu, Y.-L. Influence of Coating Time on the Preservation Performance of Chitosan/Montmorillonite Composite Coating on Tangerine Fruits. In The 21st IAPRI World Conference on Packaging; DEStech Publications, Inc.: Zhuhai, China, 2018.
- Cosme Silva, G.M.; Silva, W.B.; Medeiros, D.B.; Salvador, A.R.; Cordeiro, M.H.M.; da Silva, N.M.; Santana, D.B.; Mizobutsi, G.P. The chitosan affects severely the carbon metabolism in mango (Mangifera indica L. cv. Palmer) fruit during storage. Food Chem. 2017, 237, 372–378.
- Salari, M.; Sowti Khiabani, M.; Rezaei Mokarram, R.; Ghanbarzadeh, B.; Samadi Kafil, H. Development and evaluation of chitosan based active nanocomposite films containing bacterial cellulose nanocrystals and silver nanoparticles. Food Hydrocoll. 2018, 84, 414–423.
- Naskar, A.; Khan, H.; Sarkar, R.; Kumar, S.; Halder, D.; Jana, S. Anti-biofilm activity and food packaging application of room temperature solution process based polyethylene glycol capped Ag-ZnO-graphene nanocomposite. Sci. Eng. C 2018, 91, 743–753.
- Kanmani, P.; Rhim, J.-W. Physical, mechanical and antimicrobial properties of gelatin based active nanocomposite films containing AgNPs and nanoclay. Food Hydrocoll. 2014, 35, 644–652.
- Youssef, A.M.; El-Sayed, S.M. Bionanocomposites materials for food packaging applications: Concepts and future outlook. Polym. 2018, 193, 19–27.
- Kumar, S.; Shukla, A.; Baul, P.P.; Mitra, A.; Halder, D. Biodegradable hybrid nanocomposites of chitosan/gelatin and silver nanoparticles for active food packaging applications. Food Packag. Shelf Life 2018, 16, 178–184.
- Basumatary, K.; Daimary, P.; Das, S.K.; Thapa, M.; Singh, M.; Mukherjee, A.; Kumar, S. Lagerstroemia speciosa fruit-mediated synthesis of silver nanoparticles and its application as filler in agar based nanocomposite films for antimicrobial food packaging. Food Packag. Shelf Life 2018, 17, 99–106.
- Kumar, S.; Mitra, A.; Halder, D. Centella asiatica leaf mediated synthesis of silver nanocolloid and its application as filler in gelatin based antimicrobial nanocomposite film. LWT 2017, 75, 293–300.
- Al-Naamani, L.; Dutta, J.; Dobretsov, S. Nanocomposite Zinc Oxide-Chitosan Coatings on Polyethylene Films for Extending Storage Life of Okra (Abelmoschus esculentus). Nanomaterials 2018, 8, 479.
