Metabolic syndrome (MetS) prevalence continues to climb significantly worldwide in today’s ad libitum society. MetS have tremendous societal and economic ramifications, making it imperative to develop effective strategies for preventing and controlling it to alleviate this growing burden. Periodontal disease and MetS are associated with several risk factors. Studies in the past have demonstrated that obesity, cardiovascular illness, and type 2 diabetes mellitus have a negative effect on the severity of periodontal disease. Patients with metabolic syndrome have elevated serum levels of proinflammatory mediators such as tumour necrosis factor-alpha interleukin-6 and C-reactive protein. Remarkably, Intermittent fasting is underpinned by scientific evidence, claiming to be the most effective non-pharmacological, potential therapeutic alternative for combating a wide range of metabolic, inflammatory, and lifestyle-related diseases.
- intermittent fasting
- metabolic syndrome
- calorie restriction
- periodontal diseases
1. Introduction
2. Impact of IF on PD and Mets
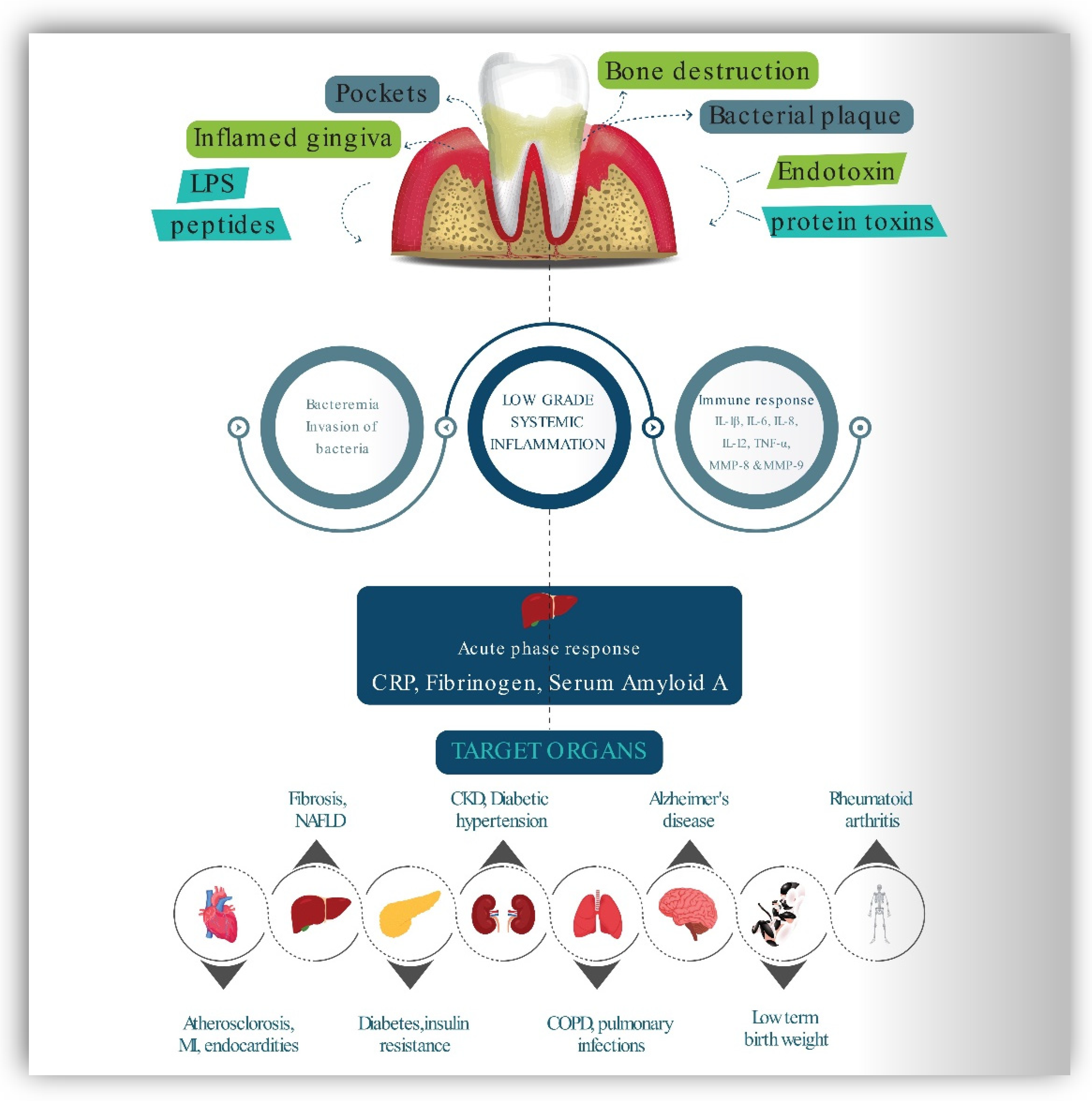
2.1. Periodontal Diseases
2.2. Activation and Regulation of Periodontal Inflammation by AMPK Pathway and Role of Sirtuins
2.3. Impact of Intermittent Fasting and Calorie Restriction on Periodontal Inflammation Diseases
2.4. Inflammation and Immune Mediation between PD and MetS
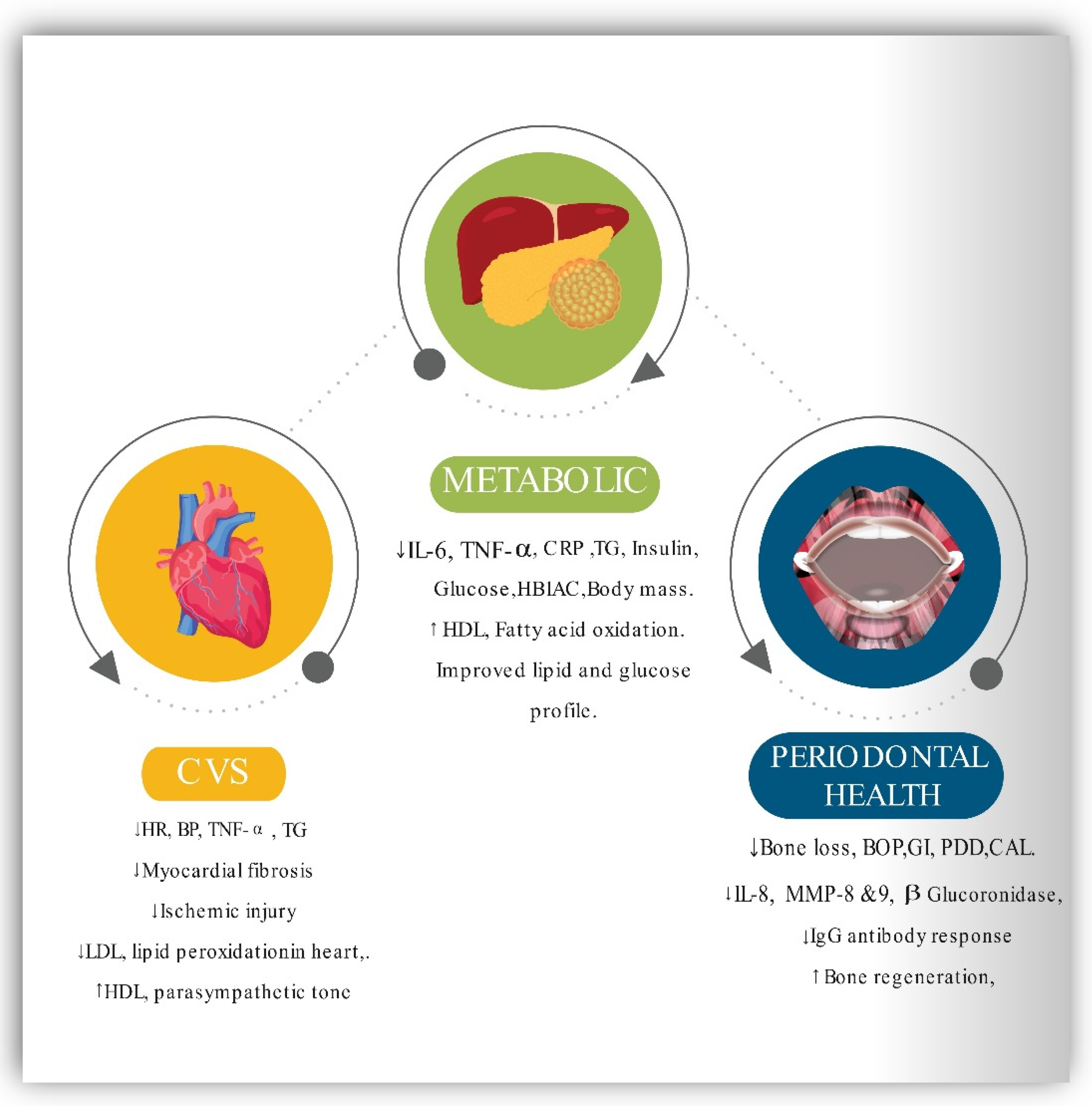
2.5. Impact of Intermittent Fasting on MetS
This entry is adapted from the peer-reviewed paper 10.3390/ijerph192114536
References
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C.; et al. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645.
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12.
- Bovolini, A.; Garcia, J.; Andrade, M.A.; Duarte, J.A. Metabolic Syndrome Pathophysiology and Predisposing Factors. Int. J. Sports Med. 2021, 42, 199–214.
- Global Nutrition Report. 2020. Available online: https://globalnutritionreport.org/reports/2020-global-nutrition-report/ (accessed on 17 July 2020).
- Bulló, M.; García-Lorda, P.; Megias, I.; Salas-Salvadó, J. Systemic Inflammation, Adipose Tissue Tumor Necrosis Factor, and Leptin Expression. Obes. Res. 2003, 11, 525–531.
- Taylor, G.W. Periodontal Treatment and Its Effects on Glycemic Control: A Review of the Evidence. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1999, 87, 311–316.
- Emrich, L.J.; Shlossman, M.; Genco, R.J. Periodontal Disease in Non-Insulin-Dependent Diabetes Mellitus. J. Periodontol. 1991, 62, 123–131.
- Pischon, N.; Heng, N.; Bernimoulin, J.-P.; Kleber, B.-M.; Willich, S.N.; Pischon, T. Obesity, Inflammation, and Periodontal Disease. J. Dent. Res. 2007, 86, 400–409.
- Baumgartner, S.; Imfeld, T.; Schicht, O.; Rath, C.; Persson, R.; Persson, G.R. The Impact of the Stone Age Diet on Gingival Conditions in the Absence of Oral Hygiene. J. Periodontol. 2009, 80, 759–768.
- El Makaky, Y.; Beltagy, T.; El Makakey, A. The Effects of an Anti-Inflammatory Diet on Gingival Health in Children (Randomized Controlled Trial). Egypt. Dent. J. 2019, 65, 1995–2002.
- Woelber, J.P.; Bremer, K.; Vach, K.; König, D.; Hellwig, E.; Ratka-Krüger, P.; Al-Ahmad, A.; Tennert, C. An Oral Health Optimized Diet Can Reduce Gingival and Periodontal Inflammation in Humans—A Randomized Controlled Pilot Study. BMC Oral Health 2017, 17, 28.
- Iacopino, A.M.; Cutler, C.W. Pathophysiological Relationships between Periodontitis and Systemic Disease: Recent Concepts Involving Serum Lipids. J. Periodontol. 2000, 71, 1375–1384.
- Kim, J.; Amar, S. Periodontal Disease and Systemic Conditions: A Bidirectional Relationship. Odontology 2006, 94, 10–21.
- Yu, Y.-H.; Chasman, D.I.; Buring, J.E.; Rose, L.; Ridker, P.M. Cardiovascular Risks Associated with Incident and Prevalent Periodontal Disease. J. Clin. Periodontol. 2015, 42, 21–28.
- Makkar, H.; Reynolds, M.A.; Wadhawan, A.; Dagdag, A.; Merchant, A.T.; Postolache, T.T. Periodontal, Metabolic, and Cardiovascular Disease: Exploring the Role of Inflammation and Mental Health. Pteridines 2018, 29, 124–163.
- Desvarieux, M.; Demmer, R.T.; Jacobs, D.R.; Rundek, T.; Boden-Albala, B.; Sacco, R.L.; Papapanou, P.N. Periodontal Bacteria and Hypertension: The Oral Infections and Vascular Disease Epidemiology Study (INVEST). J. Hypertens. 2010, 28, 1413–1421.
- Arcaro, G.; Cretti, A.; Balzano, S.; Lechi, A.; Muggeo, M.; Bonora, E.; Bonadonna, R.C. Insulin Causes Endothelial Dysfunction in Humans: Sites and Mechanisms. Circulation 2002, 105, 576–582.
- Kim, J.; Montagnani, M.; Koh, K.K.; Quon, M.J. Reciprocal Relationships between Insulin Resistance and Endothelial Dysfunction: Molecular and Pathophysiological Mechanisms. Circulation 2006, 113, 1888–1904.
- Longo, V.D.; Mattson, M.P. Fasting: Molecular Mechanisms and Clinical Applications. Cell Metab. 2014, 19, 181–192.
- Grajower, M.M.; Horne, B.D. Clinical Management of Intermittent Fasting in Patients with Diabetes Mellitus. Nutrients 2019, 11, 873.
- Varady, K.A.; Bhutani, S.; Church, E.C.; Klempel, M.C. Short-Term Modified Alternate-Day Fasting: A Novel Dietary Strategy for Weight Loss and Cardioprotection in Obese Adults. Am. J. Clin. Nutr. 2009, 90, 1138–1143.
- Templeman, I.; Thompson, D.; Gonzalez, J.; Walhin, J.-P.; Reeves, S.; Rogers, P.J.; Brunstrom, J.M.; Karagounis, L.G.; Tsintzas, K.; Betts, J.A. Intermittent Fasting, Energy Balance and Associated Health Outcomes in Adults: Study Protocol for a Randomised Controlled Trial. Trials 2018, 19, 86.
- Herzig, S.; Shaw, R.J. AMPK: Guardian of Metabolism and Mitochondrial Homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135.
- Kane, A.E.; Sinclair, D.A. Sirtuins and NAD+ in the Development and Treatment of Metabolic and Cardiovascular Diseases. Circ. Res. 2018, 123, 868–885.
- De Cabo, R.; Mattson, M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019, 381, 2541–2551.
- DOGAN, S.; ROGOZINA, O.P.; LOKSHIN, A.E.; GRANDE, J.P.; CLEARY, M.P. Effects of Chronic vs. Intermittent Calorie Restriction on Mammary Tumor Incidence and Serum Adiponectin and Leptin Levels in MMTV-TGF-α Mice at Different Ages. Oncol. Lett. 2010, 1, 167–176.
- Di Francesco, A.; Di Germanio, C.; Bernier, M.; de Cabo, R. A Time to Fast. Science 2018, 362, 770–775.
- Mattson, M.P.; Moehl, K.; Ghena, N.; Schmaedick, M.; Cheng, A. Intermittent Metabolic Switching, Neuroplasticity and Brain Health. Nat. Rev. Neurosci. 2018, 19, 63–80.
- Panda, S. Circadian Physiology of Metabolism. Science 2016, 354, 1008–1015.
- Hasturk, H.; Kantarci, A. Activation and Resolution of Periodontal Inflammation and Its Systemic Impact. Periodontol. 2000 2015, 69, 255–273.
- Sorsa, T.; Ingman, T.; Suomalainen, K.; Haapasalo, M.; Konttinen, Y.T.; Lindy, O.; Saari, H.; Uitto, V.J. Identification of Proteases from Periodontopathogenic Bacteria as Activators of Latent Human Neutrophil and Fibroblast-Type Interstitial Collagenases. Infect. Immun. 1992, 60, 4491–4495.
- Chapple, I.L.C.; Matthews, J.B. The Role of Reactive Oxygen and Antioxidant Species in Periodontal Tissue Destruction. Periodontol. 2000 2007, 43, 160–232.
- Nagpal, R.; Yamashiro, Y.; Izumi, Y. The Two-Way Association of Periodontal Infection with Systemic Disorders: An Overview. Mediat. Inflamm. 2015, 2015, 793898.
- Konkel, J.E.; O’Boyle, C.; Krishnan, S. Distal Consequences of Oral Inflammation. Front. Immunol. 2019, 10, 1403.
- Kaur, S.; White, S.; Bartold, M. Periodontal Disease as a Risk Factor for Rheumatoid Arthritis: A Systematic Review. JBI Libr. Syst. Rev. 2012, 10, 1–12.
- Pitiphat, W.; Joshipura, K.J.; Gillman, M.W.; Williams, P.L.; Douglass, C.W.; Rich-Edwards, J.W. Maternal Periodontitis and Adverse Pregnancy Outcomes. Commun. Dent. Oral Epidemiol. 2008, 36, 3–11.
- Pan, Z.; Dong, H.; Huang, N.; Fang, J. Oxidative Stress and Inflammation Regulation of Sirtuins: New Insights into Common Oral Diseases. Front. Physiol. 2022, 13, 953078.
- Chen, J.; Zhang, Y.; Gao, J.; Li, T.; Gan, X.; Yu, H. Sirtuin 3 Deficiency Exacerbates Age-Related Periodontal Disease. J. Periodontal Res. 2021, 56, 1163–1173.
- Huang, L.; Sun, H.; Song, F.; Cao, Z.; Jiang, X.; Zhang, L.; Li, Z.; Huang, C. SIRT6 Overexpression Inhibits Cementogenesis by Suppressing Glucose Transporter 1. J. Cell. Physiol. 2019, 234, 4005–4014.
- der Velden, U.V.; Kuzmanova, D.; Chapple, I.L.C. Micronutritional Approaches to Periodontal Therapy. J. Clin. Periodontol. 2011, 38, 142–158.
- Al-Zahrani, M.S.; Borawski, E.A.; Bissada, N.F. Periodontitis and Three Health-Enhancing Behaviors: Maintaining Normal Weight, Engaging in Recommended Level of Exercise, and Consuming a High-Quality Diet. J. Periodontol. 2005, 76, 1362–1366.
- Parveen, S. Impact of Calorie Restriction and Intermittent Fasting on Periodontal Health. Periodontol. 2000 2021, 87, 315–324.
- Wulansari, L.; Kaboosaya, B.; Khan, M.; Takahashi, M.; Nakata, H.; Kuroda, S.; Aoki, K.; Kasugai, S. Beneficial Effects of Fasting Regimens on Periodontal Tissues in Experimental Periodontitis Mice Model. J. Int. Dent. Med. Res. 2018, 11, 362–369.
- Branch-Mays, G.L.; Dawson, D.R.; Gunsolley, J.C.; Reynolds, M.A.; Ebersole, J.L.; Novak, K.F.; Mattison, J.A.; Ingram, D.K.; Novak, M.J. The Effects of a Calorie-Reduced Diet on Periodontal Inflammation and Disease in a Non-Human Primate Model. J. Periodontol. 2008, 79, 1184–1191.
- Ebersole, J.L.; Steffen, M.J.; Reynolds, M.A.; Branch-Mays, G.L.; Dawson, D.R.; Novak, K.F.; Gunsolley, J.C.; Mattison, J.A.; Ingram, D.K.; Novak, M.J. Differential Gender Effects of a Reduced Calorie Diet on Systemic Inflammatory and Immune Parameters in Nonhuman Primates. J. Periodontal Res. 2008, 43, 500–507.
- Reynolds, M.A.; Dawson, D.R.; Novak, K.F.; Ebersole, J.L.; Gunsolley, J.C.; Branch-Mays, G.L.; Holt, S.C.; Mattison, J.A.; Ingram, D.K.; Novak, M.J. Effects of Caloric Restriction on Inflammatory Periodontal Disease. Nutrition 2009, 25, 88–97.
- Pappe, C.L.; Steckhan, N.; Hoedke, D.; Jepsen, S.; Rauch, G.; Keller, T.; Michalsen, A.; Dommisch, H. Prolonged Multimodal Fasting Modulates Periodontal Inflammation in Female Patients with Metabolic Syndrome: A Prospective Cohort Study. J. Clin. Periodontol. 2021, 48, 492–502.
- Monteiro, R.; Azevedo, I. Chronic Inflammation in Obesity and the Metabolic Syndrome. Mediat. Inflamm. 2010, 2010, 289645.
- Pradhan, A.D.; Manson, J.E.; Rifai, N.; Buring, J.E.; Ridker, P.M. C-Reactive Protein, Interleukin 6, and Risk of Developing Type 2 Diabetes Mellitus. JAMA 2001, 286, 327–334.
- Vozarova, B.; Weyer, C.; Hanson, K.; Tataranni, P.A.; Bogardus, C.; Pratley, R.E. Circulating Interleukin-6 in Relation to Adiposity, Insulin Action, and Insulin Secretion. Obes. Res. 2001, 9, 414–417.
- Dandona, P.; Weinstock, R.; Thusu, K.; Abdel-Rahman, E.; Aljada, A.; Wadden, T. Tumor Necrosis Factor-Alpha in Sera of Obese Patients: Fall with Weight Loss. J. Clin. Endocrinol. Metab. 1998, 83, 2907–2910.
- Kern, P.A.; Ranganathan, S.; Li, C.; Wood, L.; Ranganathan, G. Adipose Tissue Tumor Necrosis Factor and Interleukin-6 Expression in Human Obesity and Insulin Resistance. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E745–E751.
- Dandona, P.; Aljada, A.; Bandyopadhyay, A. Inflammation: The Link between Insulin Resistance, Obesity and Diabetes. Trends Immunol. 2004, 25, 4–7.
- Grace, C.S.; Goldrick, R.B. Fibrinolysis and Body Bulid. Interrelationships between Blood Fibrinolysis, Body Composition and Parameters of Lipid and Carbohydrate Metabolism. J. Atheroscler. Res. 1968, 8, 705–719.
- Blood Fibrinolytic Activity in Diabetes Mellitus and Its Bearing on Ischaemic Heart Disease and Obesity. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2122789/ (accessed on 26 July 2021).
- Eggesbø, J.B.; Hjermann, I.; Høstmark, A.T.; Kierulf, P. LPS Induced Release of IL-1 Beta, IL-6, IL-8 and TNF-Alpha in EDTA or Heparin Anticoagulated Whole Blood from Persons with High or Low Levels of Serum HDL. Cytokine 1996, 8, 152–160.
- Salvi, G.E.; Yalda, B.; Collins, J.G.; Jones, B.H.; Smith, F.W.; Arnold, R.R.; Offenbacher, S. Inflammatory Mediator Response as a Potential Risk Marker for Periodontal Diseases in Insulin-Dependent Diabetes Mellitus Patients. J. Periodontol. 1997, 68, 127–135.
- Stashenko, P.; Fujiyoshi, P.; Obernesser, M.S.; Prostak, L.; Haffajee, A.D.; Socransky, S.S. Levels of Interleukin 1 Beta in Tissue from Sites of Active Periodontal Disease. J. Clin. Periodontol. 1991, 18, 548–554.
- Ciampolillo, A.; Guastamacchia, E.; Caragiulo, L.; Lollino, G.; De Robertis, O.; Lattanzi, V.; Giorgino, R. In Vitro Secretion of Interleukin-1 Beta and Interferon-Gamma by Peripheral Blood Lymphomononuclear Cells in Diabetic Patients. Diabetes Res. Clin. Pract. 1993, 21, 87–93.
- Lalla, E.; Papapanou, P.N. Diabetes Mellitus and Periodontitis: A Tale of Two Common Interrelated Diseases. Nat. Rev. Endocrinol. 2011, 7, 738–748.
- Kebschull, M.; Demmer, R.T.; Papapanou, P.N. “Gum Bug, Leave My Heart Alone!”—Epidemiologic and Mechanistic Evidence Linking Periodontal Infections and Atherosclerosis. J. Dent. Res. 2010, 89, 879–902.
- Loos, B.G. Systemic Markers of Inflammation in Periodontitis. J. Periodontol. 2005, 76, 2106–2115.
- Paraskevas, S.; Huizinga, J.D.; Loos, B.G. A Systematic Review and Meta-Analyses on C-Reactive Protein in Relation to Periodontitis. J. Clin. Periodontol. 2008, 35, 277–290.
- Hotamisligil, G.S.; Budavari, A.; Murray, D.; Spiegelman, B.M. Reduced Tyrosine Kinase Activity of the Insulin Receptor in Obesity-Diabetes. Central Role of Tumor Necrosis Factor-Alpha. J. Clin. Investig. 1994, 94, 1543–1549.
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose Expression of Tumor Necrosis Factor-Alpha: Direct Role in Obesity-Linked Insulin Resistance. Science 1993, 259, 87–91.
- Pickup, J.C. Inflammation and Activated Innate Immunity in the Pathogenesis of Type 2 Diabetes. Diabetes Care 2004, 27, 813–823.
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and Insulin Resistance. J. Clin. Investig. 2006, 116, 1793–1801.
- King, G.L. The Role of Inflammatory Cytokines in Diabetes and Its Complications. J. Periodontol. 2008, 79, 1527–1534.
- Abbatecola, A.M.; Ferrucci, L.; Grella, R.; Bandinelli, S.; Bonafè, M.; Barbieri, M.; Corsi, A.M.; Lauretani, F.; Franceschi, C.; Paolisso, G. Diverse Effect of Inflammatory Markers on Insulin Resistance and Insulin-Resistance Syndrome in the Elderly. J. Am. Geriatr. Soc. 2004, 52, 399–404.
- Serum Levels of Soluble Tumor Necrosis Factor-Alpha Receptor 2 Are Linked to Insulin Resistance and Glucose Intolerance in Children—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/15679072/ (accessed on 26 July 2021).
- Engebretson, S.; Chertog, R.; Nichols, A.; Hey-Hadavi, J.; Celenti, R.; Grbic, J. Plasma Levels of Tumour Necrosis Factor-Alpha in Patients with Chronic Periodontitis and Type 2 Diabetes. J. Clin. Periodontol. 2007, 34, 18–24.
- Naguib, G.; Al-Mashat, H.; Desta, T.; Graves, D.T. Diabetes Prolongs the Inflammatory Response to a Bacterial Stimulus through Cytokine Dysregulation. J. Investig. Dermatol. 2004, 123, 87–92.
- Takano, M.; Nishihara, R.; Sugano, N.; Matsumoto, K.; Yamada, Y.; Takane, M.; Fujisaki, Y.; Ito, K. The Effect of Systemic Anti-Tumor Necrosis Factor-Alpha Treatment on Porphyromonas Gingivalis Infection in Type 2 Diabetic Mice. Arch. Oral Biol. 2010, 55, 379–384.
- Santos, V.R.; Lima, J.A.; Gonçalves, T.E.D.; Bastos, M.F.; Figueiredo, L.C.; Shibli, J.A.; Duarte, P.M. Receptor Activator of Nuclear Factor-Kappa B Ligand/Osteoprotegerin Ratio in Sites of Chronic Periodontitis of Subjects with Poorly and Well-Controlled Type 2 Diabetes. J. Periodontol. 2010, 81, 1455–1465.
- Duarte, P.M.; Neto, J.B.C.; Casati, M.Z.; Sallum, E.A.; Nociti, F.H. Diabetes Modulates Gene Expression in the Gingival Tissues of Patients with Chronic Periodontitis. Oral Dis. 2007, 13, 594–599.
- Mahamed, D.A.; Marleau, A.; Alnaeeli, M.; Singh, B.; Zhang, X.; Penninger, J.M.; Teng, Y.-T.A. G(−) Anaerobes–Reactive CD4+ T-Cells Trigger RANKL-Mediated Enhanced Alveolar Bone Loss in Diabetic NOD Mice. Diabetes 2005, 54, 1477–1486.
- He, H.; Liu, R.; Desta, T.; Leone, C.; Gerstenfeld, L.C.; Graves, D.T. Diabetes Causes Decreased Osteoclastogenesis, Reduced Bone Formation, and Enhanced Apoptosis of Osteoblastic Cells in Bacteria Stimulated Bone Loss. Endocrinology 2004, 145, 447–452.
- Liu, R.; Bal, H.S.; Desta, T.; Krothapalli, N.; Alyassi, M.; Luan, Q.; Graves, D.T. Diabetes Enhances Periodontal Bone Loss through Enhanced Resorption and Diminished Bone Formation. J. Dent. Res. 2006, 85, 510–514.
- Liu, R.; Desta, T.; He, H.; Graves, D.T. Diabetes Alters the Response to Bacteria by Enhancing Fibroblast Apoptosis. Endocrinology 2004, 145, 2997–3003.
- Lalla, E.; Lamster, I.B.; Schmidt, A.M. Enhanced Interaction of Advanced Glycation End Products with Their Cellular Receptor RAGE: Implications for the Pathogenesis of Accelerated Periodontal Disease in Diabetes. Ann. Periodontol. 1998, 3, 13–19.
- Lalla, E.; Lamster, I.B.; Feit, M.; Huang, L.; Spessot, A.; Qu, W.; Kislinger, T.; Lu, Y.; Stern, D.M.; Schmidt, A.M. Blockade of RAGE Suppresses Periodontitis-Associated Bone Loss in Diabetic Mice. J. Clin. Investig. 2000, 105, 1117–1124.
- Genco, R.J.; Grossi, S.G.; Ho, A.; Nishimura, F.; Murayama, Y. A Proposed Model Linking Inflammation to Obesity, Diabetes, and Periodontal Infections. J. Periodontol. 2005, 76, 2075–2084.
- Johnson, R.B.; Serio, F.G. Leptin within Healthy and Diseased Human Gingiva. J. Periodontol. 2001, 72, 1254–1257.
- Karthikeyan, B.V.; Pradeep, A.R. Leptin Levels in Gingival Crevicular Fluid in Periodontal Health and Disease. J. Periodontal Res. 2007, 42, 300–304.
- Karthikeyan, B.V.; Pradeep, A.R. Gingival Crevicular Fluid and Serum Leptin: Their Relationship to Periodontal Health and Disease. J. Clin. Periodontol. 2007, 34, 467–472.
- Yamaguchi, N.; Kukita, T.; Li, Y.-J.; Martinez Argueta, J.G.; Saito, T.; Hanazawa, S.; Yamashita, Y. Adiponectin Inhibits Osteoclast Formation Stimulated by Lipopolysaccharide from Actinobacillus Actinomycetemcomitans. FEMS Immunol. Med. Microbiol. 2007, 49, 28–34.
- Iwamoto, Y.; Nishimura, F.; Soga, Y.; Takeuchi, K.; Kurihara, M.; Takashiba, S.; Murayama, Y. Antimicrobial Periodontal Treatment Decreases Serum C-Reactive Protein, Tumor Necrosis Factor-Alpha, but Not Adiponectin Levels in Patients with Chronic Periodontitis. J. Periodontol. 2003, 74, 1231–1236.
- Furugen, R.; Hayashida, H.; Yamaguchi, N.; Yoshihara, A.; Ogawa, H.; Miyazaki, H.; Saito, T. The Relationship between Periodontal Condition and Serum Levels of Resistin and Adiponectin in Elderly Japanese. J. Periodontal Res. 2008, 43, 556–562.
- Saito, T.; Yamaguchi, N.; Shimazaki, Y.; Hayashida, H.; Yonemoto, K.; Doi, Y.; Kiyohara, Y.; Iida, M.; Yamashita, Y. Serum Levels of Resistin and Adiponectin in Women with Periodontitis: The Hisayama Study. J. Dent. Res. 2008, 87, 319–322.
- Bokarewa, M.; Nagaev, I.; Dahlberg, L.; Smith, U.; Tarkowski, A. Resistin, an Adipokine with Potent Proinflammatory Properties. J. Immunol. 2005, 174, 5789–5795.
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Patel, H.R.; Ahima, R.S.; Lazar, M.A. The Hormone Resistin Links Obesity to Diabetes. Nature 2001, 409, 307–312.
- Aksungar, F.B.; Topkaya, A.E.; Akyildiz, M. Interleukin-6, C-Reactive Protein and Biochemical Parameters during Prolonged Intermittent Fasting. Ann. Nutr. Metab. 2007, 51, 88–95.
- Faris, M.A.-I.E.; Kacimi, S.; Al-Kurd, R.A.; Fararjeh, M.A.; Bustanji, Y.K.; Mohammad, M.K.; Salem, M.L. Intermittent Fasting during Ramadan Attenuates Proinflammatory Cytokines and Immune Cells in Healthy Subjects. Nutr. Res. 2012, 32, 947–955.
- Coppack, S.W. Pro-Inflammatory Cytokines and Adipose Tissue. Proc. Nutr. Soc. 2001, 60, 349–356.
- Abd El-Kader, S.M.; Saiem Al-Dahr, M.H. Impact of Weight Loss on Oxidative Stress and Inflammatory Cytokines in Obese Type 2 Diabetic Patients. Afr. Health Sci. 2016, 16, 725–733.
- Montagner, A.; Polizzi, A.; Fouché, E.; Ducheix, S.; Lippi, Y.; Lasserre, F.; Barquissau, V.; Régnier, M.; Lukowicz, C.; Benhamed, F.; et al. Liver PPARα Is Crucial for Whole-Body Fatty Acid Homeostasis and Is Protective against NAFLD. Gut 2016, 65, 1202–1214.
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223.
- Cho, Y.; Hong, N.; Kim, K.-W.; Cho, S.J.; Lee, M.; Lee, Y.-H.; Lee, Y.-H.; Kang, E.S.; Cha, B.-S.; Lee, B.-W. The Effectiveness of Intermittent Fasting to Reduce Body Mass Index and Glucose Metabolism: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 1645.
- Most, J.; Tosti, V.; Redman, L.M.; Fontana, L. Calorie Restriction in Humans: An Update. Aging Res. Rev. 2017, 39, 36–45.
- Zubrzycki, A.; Cierpka-Kmiec, K.; Kmiec, Z.; Wronska, A. The Role of Low-Calorie Diets and Intermittent Fasting in the Treatment of Obesity and Type-2 Diabetes. J. Physiol. Pharmacol. 2018, 69, 663–683.
- Anton, S.D.; Moehl, K.; Donahoo, W.T.; Marosi, K.; Lee, S.; Mainous, A.G.; Leeuwenburgh, C.; Mattson, M.P. Flipping the Metabolic Switch: Understanding and Applying Health Benefits of Fasting. Obesity 2018, 26, 254–268.
- Harvie, M.; Wright, C.; Pegington, M.; McMullan, D.; Mitchell, E.; Martin, B.; Cutler, R.G.; Evans, G.; Whiteside, S.; Maudsley, S.; et al. The Effect of Intermittent Energy and Carbohydrate Restriction v. Daily Energy Restriction on Weight Loss and Metabolic Disease Risk Markers in Overweight Women. Br. J. Nutr. 2013, 110, 1534–1547.
- Catenacci, V.A.; Pan, Z.; Ostendorf, D.; Brannon, S.; Gozansky, W.S.; Mattson, M.P.; Martin, B.; MacLean, P.S.; Melanson, E.L.; Troy Donahoo, W. A Randomized Pilot Study Comparing Zero-Calorie Alternate-Day Fasting to Daily Caloric Restriction in Adults with Obesity. Obesity 2016, 24, 1874–1883.
- Hoddy, K.K.; Kroeger, C.M.; Trepanowski, J.F.; Barnosky, A.; Bhutani, S.; Varady, K.A. Meal Timing during Alternate Day Fasting: Impact on Body Weight and Cardiovascular Disease Risk in Obese Adults. Obesity 2014, 22, 2524–2531.
