1. Introduction: Non-Communicable Disease, the Microbiome, and Symbiosis
The key to understanding the puzzle of non-communicable disease lies in the findings of Denis Burkitt, who carried his surgical skills around Africa in the decades immediately following the end of the Second World War. While describing a transmissible viral disease now known as Burkitt’s lymphoma [
1], he also noted the near complete absence of the non-transmissible diseases of what he described as “Modern Western Civilization” [
2]. Knowing nothing of the microbiome, Burkitt settled for an environmental explanation based on the high consumption of dietary fibre in most of the peoples that he visited. Unfortunately for the full understanding of these diseases, he then assumed that the absence of disease in the Maasai (then known as the Masai) was due to them having become acclimatised to a high-cholesterol meat-based diet over many generations. Sadly, although he provided much evidence of people consuming a high-fibre diet succumbing to disease after moving to low dietary fibre environments, he did not attempt to uncover evidence of Maasai-like steppe-dwelling peoples remaining resilient to these diseases when living in such Modern Western Civilisations [
2]. In addition, although he noted the, to him, inexplicable absence of non-communicable immune system diseases in his African subjects, Burkitt simply failed to mention mental health at all.
The recent emphasis placed upon the intestinal microbiota as a source of B-vitamins and the short-chain fatty acids (SCFAs), including acetate, propionate, and butyrate [
3], has provided an opportunity to revisit Burkitt’s findings, including a potential explanation for immune system disease [
4]. While a highly diverse bacterial microbiome has been associated with health [
5], it was noted that repeated exposure to low-residue diets over several generations of mice caused the apparent disappearance of certain representatives of their normal microbiota [
6]. In addition, essentially the same reduction in diversity following on from industrialisation has been noted among domesticated animals [
7], who also suffer from immune system diseases similar to humans [
8]. Significantly, however, the absence of specific immune-modulating bacteria in captive bred mice was shown to affect the outcome of experiments investigating aspects of amyotrophic lateral sclerosis [
9], thereby posing a major question as to the relevance of disease studies using laboratory animals as a model. Not surprisingly, the potential significance of the microbiome and the microbiota–gut–brain axis for the treatment of psychological diseases has also been recognised, albeit that Ganci et al. describe the sequence as “brain–gut–microbiota”, while recognising the eventual need for a “significant paradigm shift” among practitioners in the field [
10]. Importantly, however, Brüssow has recently pointed out the problems associated with the dysbiotic microbiome concept, specifically the absence of sustained scientific effort to uncover the basic “ecological and evolutionary” rationale for either the existence of the microbiome in the first place, or for the involvement of specific health-enhancing bacteria [
11].
Partly in response to this dilemma, our approach has been to look at the microbiome as if it were a single entity, effectively a “black box”, in which the only measurable factors are input and output, thereby bypassing the complexity of microbe–microbe and microbe–gut interactions. A consequence of this method of analysis is that individual “good” microbes need not be specifically identified. Indeed, the observation of interkingdom signalling amines such as dopamine, serotonin, and histamine within the gut lumen [
12] suggested to us that the microbiome could act as if it were an active entity, rather than just a passive supplier of nutrition [
13]. Note that such agents may be defined as semiochemicals: molecules that convey information from a member of one species to a different species, often between the different “kingdoms” of life, such as bacteria to eukaryotes or vice versa. Furthermore, psychotropic effects may occur without any need for such amines to penetrate the brain if they can activate the gut wall and thereby stimulate the gut–brain axis. A combination of direct and indirect communication is likely. In this context, a new class of gut sensory epithelial cells have recently been uncovered. Called neuropod cells, they directly sense the gut contents and thereby pass information on to the brain via the vagus nerve [
14]. Hormone-like substances produced by gut prokaryotes may activate the gut wall can be described as semiochemicals, prokaryote–eukaryote interkingdom signalling molecules. Serotonin, for example, can be produced both within the gut lumen itself [
15] and also by the adjacent enterochromaffin cells under the influence of SCFAs [
16]. This serotonin produced in the gut has positive direct gastrointestinal effects such as gastrointestinal and pancreatic secretions, or gastrointestinal motility, although it cannot cross the blood–brain barrier (BBB), as its precursor, the amino acid tryptophan (Trp), does. Tryptophan consumed in the diet can either remain in the gut or pass through the BBB, and in either case can be the precursor of serotonin and melatonin in a virtuous pathway, or be a precursor of metabolites of the kynurenine pathway, leading to neuroinflammation in a vicious cycle, which could be the origin of or contribute to various mental impairments [
17]. Colonic cells of the immune system also benefit from such SCFAs [
18]. Bearing in mind Burkitt’s observations, some form of immune system control must also count as an “output” of the microbiome [
2].
On the whole, it seems that the microbiome is sufficiently complex to render a purely bacterial interaction questionable and, indeed, unicellular eukaryotes have been postulated as a “missing link” between the gut and the microbiome [
19]. Normally such entities have been considered to be parasites but, however, some members of the genus Blastocystis have been shown to be consistent with apparently healthy human gut microbiota [
20]. Equally, the contribution of the mycobiome over the first month of life has been investigated [
21]; and the involvement of archaea, bacteriophages, or other viruses in a healthy human gut has also begun to be assessed [
22,
23,
24], although the bulk of both bioinformatic and microbiology techniques are geared towards the detection and classification of bacteria. Interestingly, researchers have previously suggested that the neonate immune system becomes calibrated against the microbial environment of the mother, for which researchers have suggested the presence of unicellular eukaryotic microbial sentinel cells [
25] as a hypothetical precursor of mammalian dendritic cells [
26].
2. Temporary Stimulation of the Gut–Brain Axis
The concept of the placebo has had a long and distinguished history; the hope being that it could allay the symptoms of disease long enough for the underlying condition to right itself. However, the development of the double-blind, placebo controlled, randomised clinical trial let the genie firmly out of the bottle [
92]. Subsequent attempts to unravel exactly why the placebo is so effective merely illustrated the difficulties posed by onion skin-like layers of bias [
93]. Interestingly, of course, the gut–brain axis has evolved to transfer information faster than can be achieved by conscious thought and, as such, it can easily be confused. As an example, the well-known phenomenon of traveller’s diarrhoea is an evolved response to unknown but essentially harmless microbes [
94], presumably constituting a temporary stimulation of both the immune system and the gut–brain axis, albeit with a necessarily negative effect—making you feel bad for a good reason. In a similar fashion, in certain circumstances a subconscious “placebo-like” effect can have a surprisingly negative outcome. Essentially, this nocebo effect is the antithesis of the placebo effect, and may have fatal consequences, at least in hospitalised, vulnerable populations [
95]. In addition, it potentially accounts for some of the non-specific side effects of medication [
96].
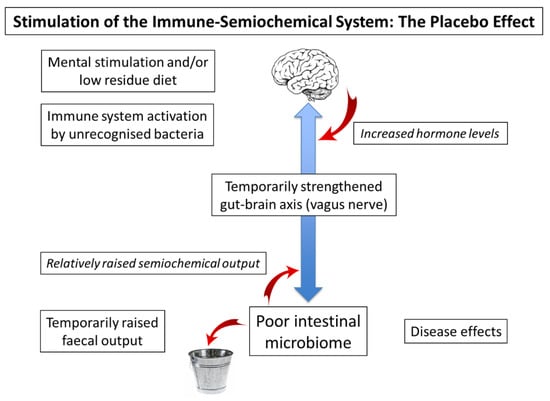
A potential explanation of the value of the placebo effect in the context of a malfunctioning gut–brain axis is illustrated in
Figure 1. The principle here is that a strengthening of this axis from either terminus will affect the other, presumably via the intermediacy of the vagus nerve, which is also known to be involved in inflammatory disorders and a variety of psychiatric conditions [
97]. Indeed, it may be that raised production of semiochemicals has subtle disease-modifying effects, for example, by increased production of dopamine in the periphery [
33]. In the context of a clinical trial, it is possible that discussion with the clinician, followed by the action of taking a mock medicine, stimulates hormone levels in the brain and hence semiochemical levels in the gut. In this context, it is important to note that the placebo effect does not require the recipient to be deceived [
98].
Figure 1. Stimulation of the gut–brain axis (placebo effect). Left hand side: Stimulation of brain, gut, or immune system temporarily strengthens the gut–brain axis, increasing gut motility and allowing more food to pass to the intestine. A rise in semiochemical output is followed by a temporary increase in faecal output. Right hand side: The alert brain may increase hormone levels, while increased semiochemical production may lead to a temporary improvement in physical disease.
It is likely that the primary role of the gut microbiota lies in their initial handshaking function, while any subsequent dietary or probiotic benefits lie within the placebo concept of
Figure 3. Indeed, as the damage done in early childhood is unlikely to be completely reversed, dietary and probiotic interventions must be termed amelioration rather than cure [
99]. As an example of dietary action, the consumption of tea and coffee, both known for their stimulant action, have recently been associated with a reduced risk of stroke and dementia, albeit only by a prospective cohort study that cannot, in itself, determine causality [
100]. Nevertheless, these beverages contain the polyphenolic compounds that seem to benefit health by stimulation of the gut microbiota [
101]. Equally, it has long been known that probiotic preparations containing various bacteria can indeed influence mental health, albeit going under the term psychobiotics [
102,
103]. As described earlier, however, Brüssow has pointed out that the studies involving probiotic bacteria are not sufficiently detailed to be absolutely sure of the exact influence of the various bacteria of interest [
11], and it remains possible that benefits accruing from semiochemical production can be expressed by different classes of bacteria among a sufficiently diverse microbiome [
35]. Alongside this “horizontal gene transfer” approach to microbiome function, the possibility exists that the inadequately studied microeukaryotes carry a significant role in the fully functioning microbiome [
19].
While some studies have revealed hopeful results for significant amelioration of some autistic spectrum features, those are few [
104,
105]. To date, several potential confounding factors have been disregarded, the most important being single- or double-blind trials to precisely assess possible placebo effects. Another problem is obtaining the cooperation of people from diverse ethnicities and nationalities, socioeconomic levels, medical histories of the mothers, etc., all well-known confusing factors for the study of mental health [
106].
3. The Many Variations of Developmental Brain Disease
While the medical model of single diagnosis and appropriate treatment remains the ideal, non-communicable disease tends to present itself as a mix of seemingly very different complaints of variable severity. This is especially so in the context of psychiatric conditions, where the Diagnostic and Statistical Manual of Mental Disorders is in its 5th Edition (DSM-5) [
107], with concomitant debate about the medicalisation of society [
108]. The p-factor mentioned earlier represents an attempt to account for the seeming “inheritance” of multiple varieties of poor mental health in the same individual, often starting at an early age and associated with “compromised brain integrity” [
36], a concept which is entirely consistent with our own view of inappropriate brain growth following poor interoception, inefficient communication between gut and brain as outlined in
Figure 2. It is reasonable to suppose that the relationship of autism spectrum disorder (ASD) with the gut microbiota, the neural, and the immune system will also apply to other non-communicable psychopathological conditions [
46].
3.1. Autism Spectrum Disorder and Attention Deficit Hyperactivity Disorder
Although the division of disease into subtypes may be valuable for the precise application of therapeutic methodology, it makes the critical task of epidemiology much more difficult. Nevertheless, in a recent review Chiarotti and Venerosi have cut through all the variability to show that the incidence of ASD, one of the major forms of poor mental health, has indeed increased across all areas of the world since the establishment of the DSM-5 in 2013, at least until the date of their review in 2020 [
109]. It is likely that this pattern, an overall increase in the incidence of disease combined with massive individual variation, will apply across a number of seemingly different conditions.
A recent review of the nature of ASD illustrates its pathophysiology. Characteristics include cognitive inabilities, impaired communication, and restricted social interaction, along with a wide range of co-morbidities including psychiatric illness, seizures, and gastrointestinal disorders, which are hypothesised to be due to bacterial dysbiosis [
110]. While microbiome disturbances have been implicated in both ASD and attention deficit hyperactivity disorder (ADHD), a systematic literature search revealed twenty-four relevant articles, but with no consistent bacterial variation identified [
111]. It is important to note, however, that once the handshaking process of
Figure 1 has failed in the neonate, the exact nature of the bacterial inhabitants of the intestine may well be irrelevant to the underlying cause of the disease. Thus, as probiotics stimulate the immune system, so the gut–brain axis is temporarily strengthened, perhaps briefly lessening some of its symptoms according to the placebo effect of
Figure 3.
Just as it is important to clearly define and quantify what is “normal microbiota”, it is also important to record not only brain imaging, but also the behaviour of the non-neurotypical individual beyond clinical psychiatric diagnoses that might be subjective to some degree. It is possible, for example, to parameterise the behaviour of an individual with ASD, making quantifiable what is, by definition, a spectrum [
112].
3.2. Dyslexia, Anxiety, Depression, and Other Conditions
Dyslexia is often found to co-occur with ADHD, implying a similar cause and/or mechanism of action [
113], and, as its precise aetiology is not understood, it has recently been suggested to be an extreme case of “specialisation in exploration” [
114]. Interestingly, developmental language disorder (DLD) is presumably related to dyslexia, and striatal changes can be observed by magnetic resonance imaging (MRI) [
115].
While ASD is among the most disabling of the psychiatric disorders and anorexia nervosa the most dangerous [
116], the many manifestations of anxiety are the most prevalent, with a close association with depressive conditions [
117]. Perhaps the most direct explanation of at least some of the various forms of anxiety is related to the phenomenon of traveller’s diarrhoea [
94], in which a malfunctioning gut–brain axis falsely suggests the presence of an infection, leaving the brain uncertain as to whether to trigger the vomit reflex or not. A recent review of studies exploring the role of dietary interventions in the context of anxiety and depression has merely emphasised the complexity of the situation, emphasising the bidirectional nature of the microbiota–gut–brain axis: both being affected by, and affecting, dietary choices [
118]. These complications can, perhaps, be better understood as amelioration rather than cure [
99], albeit only temporarily, following the placebo concept of
Figure 3.
The breakdown of semiochemical and/or neural connection between gut and brain following microbiome–gut dissociation may have consequences for many other conditions. Although the details remain to be discovered, the microbiome is somehow connected to the Parkinson’s disease-related loss of dopamine in the substantia nigra, for example [
119]. Interestingly, dopamine also has a role in dynamic emotion perception [
120], which may be related to the observation of alexithymia, the neurodevelopmental disorder-related difficulty of classifying one’s own emotions [
121]. Probably involving a similar mechanism, people suffering from depressive states have difficulty making the fast, intuitive decisions that are associated with the phrase “gut feeling” [
122]. One significant finding is that the disturbance of the skin microbiota has a depressive effect operating through the gut: an example of a gut–brain–skin axis but, seemingly, with the gut microbiota themselves exerting the major emotional influence on the brain [
123].
6.3. Unexpected Strengths and the Problems of Definition in Psychology
Following an initial failure of the handshaking process in the neonate, the asymmetric development of the brain, clearly revealed by structural magnetic resonance imaging [
124] and convolutional neural networks [
125], probably contributes to a number of developmental issues that may actually be associated with unexpected strengths. One is the above-mentioned “specialisation in exploration” hypothesised to accompany developmental dyslexia [
114], but possibly the most extreme example of disability combined with exceptional skills is that of savant syndrome [
126]. It has even been suggested that ASD may be a variation of “normality” [
127], and that individuals with ASD only need certain specific integration requirements to be functional in society [
128]. Equally, leaving aside the question of the precise definitions of the terms used, the question has been asked as to whether “genius” is related to “madness”, or whether it can also be expressed by “normal” people; it seems that the answer is not clear-cut [
129]. A further study along similar lines has discussed creativity in the context of psychopathology, calling for better definitions and for more methodological rigour [
130], in a similar fashion to Brüssow’s call for greater clarity in the field of probiotic bacteria.
4. Microbiome Measurement: Semiochemicals and Sentinel Cells
Although the human neonatal gut microbiome has been well studied by now, the overall message is that the microbiota transferred by maternal microbial inheritance must be fully functional both in the neonate [
131] and throughout childhood if the worse consequences of adult disease are to be avoided [
35,
39]. As the enclosed intestinal microbiome would have evolved with the vertebrates [
25], so animal studies should be helpful. Equally, it should be possible to obtain information from people living in unpolluted places not suffering from non-communicable disease. In principle, such peoples should still have properly functioning semiochemical messenger systems and also, probably, eukaryote microbial sentinel cells capable of passing environmental antigen information onwards from mother to child [
13]. Peoples such as the African Hadza have been cooperating with medical teams for a number of years, and their microbiomes have been assessed, in terms of the bacteria, at least [
132]. Nevertheless, it is feasible that their microbiota has already been degraded, bearing in mind that any medical consequences may not show up until subsequent generations. Essentially, the same comments apply to the South American Tsimane, except that, to our knowledge, their microbiota have not yet been investigated [
133]. Any information gained from them should be compensated for, perhaps in a similar way as for cancer medicines, for example [
134].
4.1. Semiochemical Measurement: An Ingestible Sensor
Although function is important, it seems that the exact species-level bacterial composition of the maternal microbiome may be irrelevant as a measure of its value to the neonate. In addition to in vitro studies, one option would be to detect potential semiochemicals [
12] in animal faeces under different conditions, such as fed, fasting, or pregnant, bearing in mind previous experience suggesting that laboratory-raised animals may not have the full complement of necessary microbes [
9]. Although the presence of candidate messaging compounds in faeces would provide a suggestion of their activity, significantly more information would be gained after the development of an ingestible sensor, a pill-like device equipped with a detector calibrated for molecules such as dopamine, and a transmitter allowing the readout of real-time information on their ebb and flow under different conditions [
45]. Such studies could also be performed in humans as ingestible sensors are currently considered to be minimally invasive [
135]. Nevertheless, essentially the same strictures apply to humans as to laboratory-raised animals: that even apparently healthy individuals can only be considered to have an intact microbiome if the whole population is free from non-communicable disease [
39].
4.2. Microeukaryotes and Microbial Sentinel Cells
Given the apparent complexity of microbiome function, alongside Brüssow’s observation of the “problems of … gut microbiota dysbiosis” [
11] it would be surprising if unicellular eukaryotes were not critical to the functioning of the microbiome, as has been suggested already [
19]. Granted a co-evolutionary relationship between microbiome and vertebrate animals [
25], and the importance of immune system disease [
35], the possibility of microbial sentinel cells as precursors of the dendritic cells that link the innate and adaptive aspects of our immune system [
26] must be taken into consideration. Eukaryotes such as the Blastocystis genus have been mentioned [
20], but it is possible that such entities can no longer be found in heavily polluted communities suffering from extensive levels of non-communicable disease [
39]. Interestingly, human coprolites have been studied for evidence of now-extinct microbiomes but, as usual, the focus remains on bacteria [
136]. It is important to note, however, that pre-modern communities could still have high levels of pollution due to complex trading societies spreading the results of mining activities [
137]. Although such societies could stretch back into the Stone Age, certainly the Egyptians and Romans used lead extensively and have been confirmed as suffering from non-communicable disease, for example, atherosclerosis [
138] and coeliac disease [
139], respectively. Although it may prove difficult to find examples of immune system-related microeukaryotes associated with undegraded human microbiota, the fact that domesticated animals suffer from immune system diseases implies that wild animal populations could be used as a proxy for human microbial biology [
8].
This entry is adapted from the peer-reviewed paper 10.3390/gidisord4040028