Fibrosis is a pathological process in which parenchymal cells are necrotic and excess extracellular matrix (ECM) is accumulated due to dysregulation of tissue injury repair. Thymosin β4 (Tβ4) is a 43 amino acid multifunctional polypeptide that is involved in wound healing. Prolyl oligopeptidase (POP) is the main enzyme that hydrolyzes Tβ4 to produce its derivative N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) which is found to play a role in the regulation of fibrosis. Accumulating evidence suggests that the Tβ4-POP-Ac-SDKP axis widely exists in various tissues and organs including the liver, kidney, heart, and lung, and participates in the process of fibrogenesis. The Tβ4-POP-Ac-SDKP axis exerts protective effects against organ fibrosis. It is promising that appropriate dosing regimens that rely on this axis could serve as a new therapeutic strategy for alleviating organ fibrosis in the early and late stages.
1. Tβ4-POP-Ac-SDKP Axis
Thymosin β4 (Tβ4) is a water-soluble peptide with a highly conserved structure composed of 43 amino acid residues and was first found in the calf thymus extract [
1]. It is also a multifunctional peptide that can stimulate angiogenesis, promote cell proliferation, inhibit apoptosis, reduce inflammation, and inhibit scar formation and fibrosis [
2]. Tβ4 is crucial in tissue repair and regeneration, and it binds to G-actin and inhibits its polymerization to promote cell migration, including stem/progenitor cell mobilization, migration, and differentiation to form new blood vessels and regenerate tissue [
3,
4]. After the injury, Tβ4 is released by many types of cells such as platelets and macrophages and exerts anti-inflammatory effects by reducing the number of inflammatory cells and downregulating the expression levels of many inflammatory factors such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6, as well as inhibiting nuclear factor-κB (NF-κB) expression [
5]. Studies have found that Tβ4 is involved in the repair and treatment of skin wounds, dry eyes, myocardial infarction (MI), brain injury, and other injuries in various tissues and organs [
6]. It reduces the number of myofibroblasts in wounds, thereby inhibiting scar formation and fibrosis [
7]. Tβ4 has an anti-fibrotic effect and can treat fibrosis of the liver, lung, and kidney [
2].
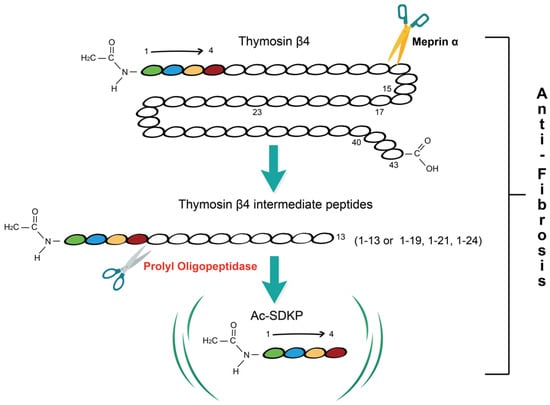
Prolyl oligopeptidase (POP) is a serine protease that can specifically hydrolyze peptide bonds at the carboxyl-terminal of proline residues in polypeptide chains [
8]. After the cleavage of Tβ4 into NH
2-terminal intermediate peptides less than 30 amino acids in length by metalloprotease meprin α, POP hydrolyzes these intermediate peptides to ultimately release N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) [
9] (
Figure 1). Studies have shown that POP is widely distributed in mammalian organs and has an anti-fibrotic effect. It generates Ac-SDKP, which has been demonstrated to alleviate liver fibrosis in carbon tetrachloride (CCl
4)-induced fibrosis in a rat model and attenuate the activation of primary hepatic stellate cells (HSCs) isolated from rats in vitro [
10,
11]. In the kidney and heart, POP inhibitor S17092 treatment decreased the endogenous Ac-SDKP and increased collagen deposition in vivo [
12], suggesting that POP is involved in alleviating fibrosis through Tβ4-POP-Ac-SDKP axis.
Figure 1. Overview of Tβ4-POP-Ac-SDKP axis. Thymosin β4 (Tβ4), a 43-amino-acid peptide, is firstly cleaved by meprin α into Tβ4 intermediate peptides shorter than 30 amino acids in length and these peptides are hydrolyzed by prolyl oligopeptidase (POP) to produce the N-terminal tetrapeptide N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP). Tβ4 intermediate peptides 1-13, 1-19, 1-21, and 1-24 are products of Tβ4 hydrolysis at four specific meprin α cleavage sites. The Tβ4-POP-Ac-SDKP axis has an anti-fibrotic effect.
Ac-SDKP, an N-terminal derivative of Tβ4, is a tetrapeptide generated by the hydrolysis of its precursor Tβ4 by meprin α and POP [
9]. In vivo, Ac-SDKP is degraded by the N-terminal catalytic sites of angiotensin-converting enzyme (ACE) to become inactive, and its plasma concentration is increased five times when given ACE inhibitors [
13]. Initial studies believed that Ac-SDKP prevents hematopoietic stem cells from entering the S phase spontaneously, keeping them in the G0/G1 phase and results in the inhibition of hematopoietic stem cell proliferation [
14,
15]. More and more research in recent years has discovered that it can inhibit fibroblast proliferation and fibrosis in various organs and tissues such as the liver, kidney, heart, and lung. In the liver, heart, and lung, Ac-SDKP reduces collagen synthesis by downregulating the transforming growth factor (TGF)-β1 and reducing the differentiation of fibroblasts into active myofibroblasts [
16,
17,
18]. In the kidney, Ac-SDKP attenuates renal dysfunction and fibrosis after anti-glomerular basement membrane nephritis is developed, which is associated with inhibiting the infiltration of macrophages and the TGF-β/Smad pathway [
19].
The Tβ4-POP-Ac-SDKP axis is widely present in various tissues and organs including the liver, kidney, heart, and lung, and it is crucial for the regulation of tissue and organ fibrosis.
2. Tissue and Organ Fibrosis
Tissue and organ fibrosis refers to a pathological process in which connective tissue components are excessively accumulated and it is the result of the dysregulated repair response after tissue injury [
20]. Fibrosis is a significant factor in the occurrence and progression of diseases in major organs of the human body, such as the liver, kidney, heart, and lung [
21]. Continued progression of fibrosis can lead to organ structural damage, loss of function, and ultimately failure. Tissue and organ fibrosis are the main causes of many diseases and deaths [
22]. The essence of fibrosis is the repair response of tissues after injury to protect the relative integrity of tissues and organs. Tissue injury can lead to tissue cell degeneration, necrosis, and inflammatory response. If the damage is small, normal parenchymal cells around the damaged cells will undergo proliferation and repair, which can completely restore normal structure and function [
23]. However, if the damage is serious or the repeated damage exceeds the regeneration capacity of the parenchymal cells around the injury site, the extracellular matrix (ECM) will proliferate to repair the defect tissue, that is, the pathological changes of fibrosis will occur [
24]. Excessive deposition of collagen and other extracellular matrix proteins repair the defect but do not have the structure and function of the original organ parenchymal cells. It can result in fibrosis and decreased organ function if the healing reaction is excessive and out of control [
25].
Fibrogenesis is a highly organized process that is regulated by various chemical signals and cells. Parenchymal cells are damaged following injury while immune cells dominated by macrophages are activated. Large amounts of biological mediators such as IL-4, IL-13, IL-25, IL-33, platelet-derived growth factor (PDGF), and other cytokines and chemokines secreted by these immune cells cause mesenchymal cells to become active locally which transform fibroblasts, vascular smooth muscle cells, pericytes, mesothelial cells, fibrocytes and many other types of cells into myofibroblasts, which express α-smooth muscle actin (α-SMA) [
26,
27]. Myofibroblasts involve in extracellular matrix production leading to scar formation and the destruction of tissue and organ structure [
28]. Moreover, recent studies showed that scleroderma-associated fibroblasts (ScAFs) expressing the LGR5 receptor and circulating fibrocytes are associated with skin fibrosis progression [
29,
30]. In addition to producing ECM proteins, myofibroblasts aid in repair by producing contractile forces that are conveyed to the surrounding ECM and activate TGF-β, a crucial cytokine in fibrosis [
31]. Furthermore, it has been demonstrated that the activity of fibroblast and the process of fibrosis are regulated by the synergistic or antagonistic action of other various cytokines such as C-C motif chemokine 2 (CCL2), monocyte chemoattractant protein-1 (MCP-1), interleukins, TNF-α, reactive oxygen species (ROS) [
32].
Many studies have reported that Tβ4-POP-Ac-SDKP axis plays an important role in the development of tissue and organ fibrosis. The research on the regulatory mechanism of Tβ4-POP-Ac-SDKP is conducive to further understanding the relationship between tissue damage repair imbalance and the occurrence and development of tissue and organ fibrosis, as well as underlying pathophysiological mechanisms. We will summarize the effect of Tβ4-POP-Ac-SDKP axis on fibrosis of different organs including the liver, kidney, lung, heart, and the underlying mechanisms of fibrogenesis.
This entry is adapted from the peer-reviewed paper 10.3390/ijms232113282