Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Green & Sustainable Science & Technology
Research has explored new recycling methods to convert spent SCR catalysts into products rather than raw materials, such as a pigment and ceramic opacifier. The idea of recycling spent SCR catalysts as photocatalysts for pollution treatment is in-line with the concept of “treating waste with waste”.
- SCR
- Photocatalyst
- Vanadium
- Tungsten
- Titanium
- Resource utilization
1. Introduction
Spent SCR catalysts have a high recovery value with components of over 80wt% consisting of V2O5, WO3 and TiO2 and should, therefore, be regenerated or recovered in order to recycle the resource [15,16,17]. Although spent SCR catalysts may contain oxides such as SiO2, Al2O3 and CaO that constitute the ceramics, as well as compounds of elements including As and Pb deposited from the flue gas, the extraction of these materials is not reviewed in this article. Regeneration methods can extend the life of SCR catalysts by focusing on detoxification and reactivation. The major problems to be addressed during regeneration are the deactivation and loss of active sites as well as blocking of pores [4,18,19,20]. Specific regeneration methods for different types of deactivations can achieve promising results [21]. However, current regeneration processes are unable to achieve the expected results when the catalyst is severely deactivated or has undergone several regenerations. In this case, the SCR catalyst will reach the end of its life and be recycled by recovery [22]. Conventional recovery methods focused on the extraction and purification of metals (V, W and Ti) [23]. However, recent research has increasingly turned to the added value of the product with a view to increasing the economic benefits of recycling. For instance, after separation, NH4VO3, ammonium paratungstate and anatase TiO2 are produced, respectively [24].
Under light conditions, photocatalysts produce photogenerated electrons and holes and, further, form active species such as hydroxyl (·OH) and oxygen (O2−) radicals. Due to the strong redox properties of the active species, photocatalysts can address pollutions caused by heavy metals, organics and other substances [25]. Anatase TiO2 is a classical photocatalyst with excellent photocatalytic activity. Studies have often used doping to build heterostructures to extend the wavelength range of light and to suppress the separation of photogenerated carriers [26,27]. On the other hand, to inhibit the agglomeration of nano TiO2 and to assist recycling, the preference is to use carrier-loaded nano TiO2 [28]. Spent SCR catalysts contain a large amount of anatase TiO2 and are cost-effective; therefore, recovery as photocatalysts not only increases the recovery benefit, but also overcomes the high cost of conventional photocatalysts. In addition, V [29,30] and W [31,32] in spent SCR catalysts have the potential to build heterostructures with TiO2.
2. Catalyst Carriers
Photocatalytic degradation is a promising technology to address environmental pollution with economic and environmental efficiency [64]. However, as most photocatalysts are nanoparticles, they are difficult to recycle and, therefore, present the potential risk of secondary pollution [65]. On the other hand, the tendency of nanoparticles to agglomerate can also limit their catalytic activity [66]. To solve these problems, photocatalysts can be loaded onto carriers, such as fly ash [67] and activated carbon [68]. The carriers not only provide dispersion and easier recycling, but specific carriers can also improve the catalytic activity of the catalyst by forming a specific heterostructure [69].
Spent SCR catalysts contain a variety of oxides with catalytic activity, which can be used as carriers to enhance catalytic activity. However, it is important to ensure that components such as V2O5 and As2O5 do not enter the environment during utilization and cause secondary contamination. Jin et al. [70] investigated spent SCR catalysts by grinding into powder and adding Al2O3, diatomite and agglomerant to calcine at 1000 °C to obtain ceramics. This was followed by impregnation and sintering in an 8wt% Ni(NO3)2 solution to obtain NiO loading. The prepared NiO-based catalysts were used for the reforming of formaldehyde and water vapour for hydrogen production with a selectivity of 100% for H2, 31.9% for CO and 53.2% for CO2 at 500 °C, and a conversion of formaldehyde above 93.0%. The analysis of the XPS data and the mechanism of the reforming reaction revealed that the presence of oxides on the surface had a positive effect on the performance of the catalysis. Based on the work of Jin et al., it can be demonstrated that spent SCR catalysts have potential as catalyst carriers. Particularly, TiO2 shows typical photocatalytic activity and research on recycling spent SCR catalysts as photocatalyst carriers for this feature awaits further exploration.
3. Photocatalysts
V2O5, WO3 and TiO2 from spent SCR catalysts can be used for the preparation of photocatalysts. V2O5 and WO3 can form crystals with cations such as Bi3+ in the form of VO43− and WO66−, respectively [71], while TiO2 exists as anatase TiO2 mostly [72]. Zhang et al. [57] used a solution of H2SO4 and Na2SO3 to leach out V from spent SCR catalyst, followed by dissolving the Ti-containing residue in HF solution to obtain WO3-TiO2 photocatalyst after a hydrothermal reaction. In another case, V and W were leached out using a NaOH solution, followed by the addition of Bi(NO3)3 for a hydrothermal reaction to obtain BiVO4/Bi2WO6 photocatalysts [71]. Wang et al. [73] also used NaOH to retain Ti in the leach residue for separation. Subsequently, Na3PO4 and Mg(NO3)2 were used to precipitate Ca2+, SiO44−, and PO43−, respectively. This was followed by the precipitation of Al3+ and Mg2+ using HNO3 and NaOH to adjust the pH. The solution retains VO3− and WO42−, but additional NH4VO3 is required due to the low V. The addition of Zn(NO3)2 eventually leads to a visible light responsive Zn3(VO4)2/ZnWO4 photocatalyst by hydrothermal reaction.
Qian et al. [74] used NaOH to leach the V element from the catalyst, followed by a hydrothermal reaction using 96% H2SO4 to convert the TiO2 to TiOSO4 after roasting at 200 °C. V is removed after reaction with NaOH, while W remains in the residue. This method overcomes the difficulty of separating Ti and W and makes effective use of Ti. The prepared photocatalyst exhibits similar catalytic activity to P25 at a lower Ti content and demonstrates the feasibility of dissolving and converting Ti to nano TiO2. Furthermore, loading TiO2 onto the fly-ash surface also overcomes the shortcomings of nano TiO2, which is difficult to recover and prone to agglomeration.
Current research has focused on the separation of TiO2 from V2O5 and WO3 with some progress being achieved. Figure 1 illustrates the transformation relationships of substances in these works. Since the goal is no longer to separate and purify V, W and Ti, the process can be simplified based on current separation processes. New extraction processes can also be developed, such as the dissolution of TiO2 with sulphuric acid under hydrothermal conditions [75]. Mechanochemical methods also have potential applications in the preparation of TiO2 [76]. Ball milling as a pre-treatment has proven to be effective in the recycling of spent SCR catalysts [59]. On the other hand, both BiVO4 [77] and Bi2WO6 [78] can form corresponding heterostructures with TiO2. Moreover, V and W can be extracted directly into solution as NH4VO3 and (NH4)2WO4 [45], which would assist in the further synthesis of bismuth salt.
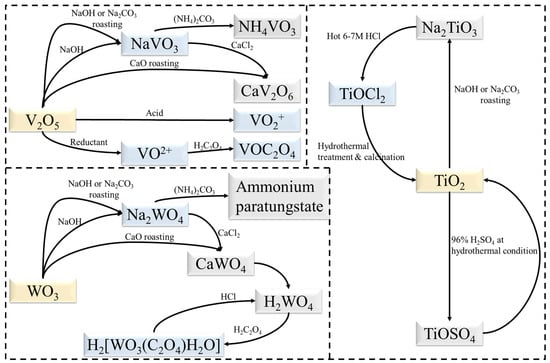
Figure 1. Transformation relationships of substances during extraction.
This entry is adapted from the peer-reviewed paper 10.3390/ma15227984
This entry is offline, you can click here to edit this entry!
