Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
African American (AA) men have 2.4 times higher mortality rate due to prostate cancer than White men in the United States. Evidence implicates circadian rhythm disruption (CRD) as a potential driver of prostate cancer risk and progression. AA men are particularly vulnerable to CRDs due to greater exposure to night shift work, artificial light at night, noise pollution, racial discrimination, and socioeconomic disadvantages.
- prostate cancer
- circadian genes
- night shift work
- artificial light at night
- jet lag
1. Introduction
Prostate cancer (PCa) is the second leading cause of cancer-related mortality for men in the United States [1][2]. Significant racial disparities exist at all stages of PCa treatment, including diagnosis, management, and follow-up care [3]. African American (AA) men are 1.6 times as likely to be diagnosed with PCa and at a 2.4 times higher risk to die of the disease compared to White men [4]. The racial disparities in PCa may be due to lower rates of health literacy, comorbidities such as diabetes and obesity, and behavioral factors such as smoking; in addition, racial bias and barriers to healthcare access impact PCa outcomes of AA men [3]. While the current risk factors for PCa are age, race, and family history, increased epidemiological evidence points to the role of circadian rhythm disorder (CRD) in cancer progression [5]. In addition to cancer metastasis, CRD is a risk factor for various other conditions, such as chronic sleep deprivation, obesity, metabolic syndrome, cardiovascular diseases, and psychiatric diseases [6]. CRD is characterized by the lack of synchrony between the endogenous master circadian clock and the external light–dark cycles [7].
AA men and women may be at heightened vulnerability for developing CRD due to a greater prevalence of night shift work, environmental factors (e.g., living in neighborhoods characterized by high noise pollution, exposure to low daytime light levels or too much nighttime light), and chronic conditions (e.g., diabetes, obesity, long-term stress, cardiovascular disease) [8][9][10][11][12][13]. The underdiagnosis of sleep disorders, including obstructive sleep apnea (OSA), within predominately AA communities contributes to abnormal sleep architecture, which puts OSA patients at high risk for CRD. Further research is required to understand the exact relationship between CRD on OSA [14][15][16][17]. Epidemiological evidence so far points to the connections between PCa and CRD. Meta-analyses on the health outcomes of airplane pilots [18][19] and studies on female night shift nurses [20] have demonstrated the contribution of CRD (due to jetlag or night shift work) to both prostate and breast cancer [21]. Sleep disruption and light-induced melatonin suppression, both related outcomes of CRD, are associated with an increased risk for advanced PCa [22][23]. Lastly, the consequences of CRD—including circadian gene polymorphisms and conditions such as diabetes, obesity, and depression—contribute to an increased PCa risk [24][25][26][27][28].
Taken together, the unique impact CRD has on the AA population and its role in PCa, elucidating the biological mechanisms through which CRD contributes to PCa, may be critical to mitigating the racial disparities in PCa outcomes. Compelling evidence suggests that CRD may contribute to PCa progression through (a) circadian-gene variants [29][30][31] (b) stress and obesity-related biological pathways [32][33][34], and (c) melatonin inhibition [35]. As a result of CRDs, circadian clock genes no longer function as tumor suppressors, contributing to worse PCa outcomes. The complex interplay between stress, obesity, and circadian disruption may have detrimental effects on the tumor microenvironment and could enhance the stress-related PCa growth pathway, otherwise known as the glucocorticoid-mediated androgen receptor signaling pathway. Considering these findings, targeting the melatonin pathway and the glucocorticoid receptor, both of which are implicated in CRD, may provide new opportunities to impair PCa growth and overcome therapeutic resistance, respectively.
2. Regulation of the Circadian Clock System
Driven by the 24 h rotation of the planet, almost all organisms have adapted on earth by developing an internal biological clock system known as the circadian system, which rhythmically synchronizes sleep, metabolic, and dietary behavior to light/dark cycles [36]. In any given tissue, around 10–20% of the genome is expressed in a circadian manner [37]. Circadian rhythms play a significant role in the sleep/wake cycle, metabolic function, and gene expression [38]. Disruption of circadian rhythms has major consequences on the body’s ability to regulate metabolic homeostasis [39].
The central circadian clock, located in the suprachiasmatic nuclei (SCN) of the anterior hypothalamus, regulates the timing of activities of the peripheral clocks [40]. Light–dark patterns synchronize the SCN with the external environment, assuring that the body does the “right thing at the right time” [41]. In diurnal species, the light phase is associated with an increase in body temperature, heart rate, and blood pressure. During the dark phase, melatonin production increases, body temperature declines, heart rate slows down, and blood pressure lowers [42]. Within individual cells, the circadian clock is self-regulated by transcriptional–translational feedback loops (TTFLs) [43]. TTFLs are comprised of a positive arm with a heterodimeric complex at its core that behaves as the activator of the system, promoting the transcription of one or more components of the negative arm, which, when translated, inhibits the activity of the positive arm. Transcription activator factors CLOCK and BMAL1 make up one arm of the feedback loop, and repressor proteins PER and CRY, made from Per1/2/3 and Cry1/2 genes, make up the other arm. Accessory TTFLs regulate the primary TTFL. The first accessory loop is made up of RORs and nuclear REV-ERB receptors, while the second accessory loop is composed of D-box-related genes and transcription factors, including albumin D-binding protein (DBP), thyrotroph embryonic factor (TEF), and hepatic leukemia factor (HLF) [7][44].
In humans, during the light phase (morning), transcription activators BMAL1 and CLOCK form heterodimers, bind to the E-box (5′-CACGTG-3′), and signal the transcription of target genes Period (Per 1/2/3) and Cryptochrome (Cry 1/2) [45]. These target genes encode transcriptional repressors, PER and CRY proteins. During the light phase, PER and CRY transcription is high, and PER and CRY proteins accumulate in the cytoplasm. During the dark phase (evening), PER and CRY proteins dimerize to form PER–CRY complexes, with subsequent nuclear translocation and inactivation of the CLOCK/BMAL1-mediated transcription, reprising their own transcription and closing the loop. As the dark phase progresses, PER and CRY complexes are gradually phosphorylated by casein kinase I (CkIδ and CkIε) and 5′ AMP-activated protein kinase (AMPK), and subsequently realize their degradation through the proteasome pathway [46]. Degradation of the PER and CRY repressor proteins allows for CLOCK-BMAL1 transcription to resume, thus initiating a new transcriptional cycle [47]. In addition to the primary feedback loop, accessory loops are formed from nuclear orphan receptors, retinoid-related orphan receptors (RORs), and REV-ERBα/β, which target BMAL1 production through binding to ROR- binding elements (ROREs) [7]. REV-ERBα/β inhibits BMAL1 transcription upon binding, while ROR, acting as a positive regulator, initiates BMAL1 transcription [45] (Figure 1).
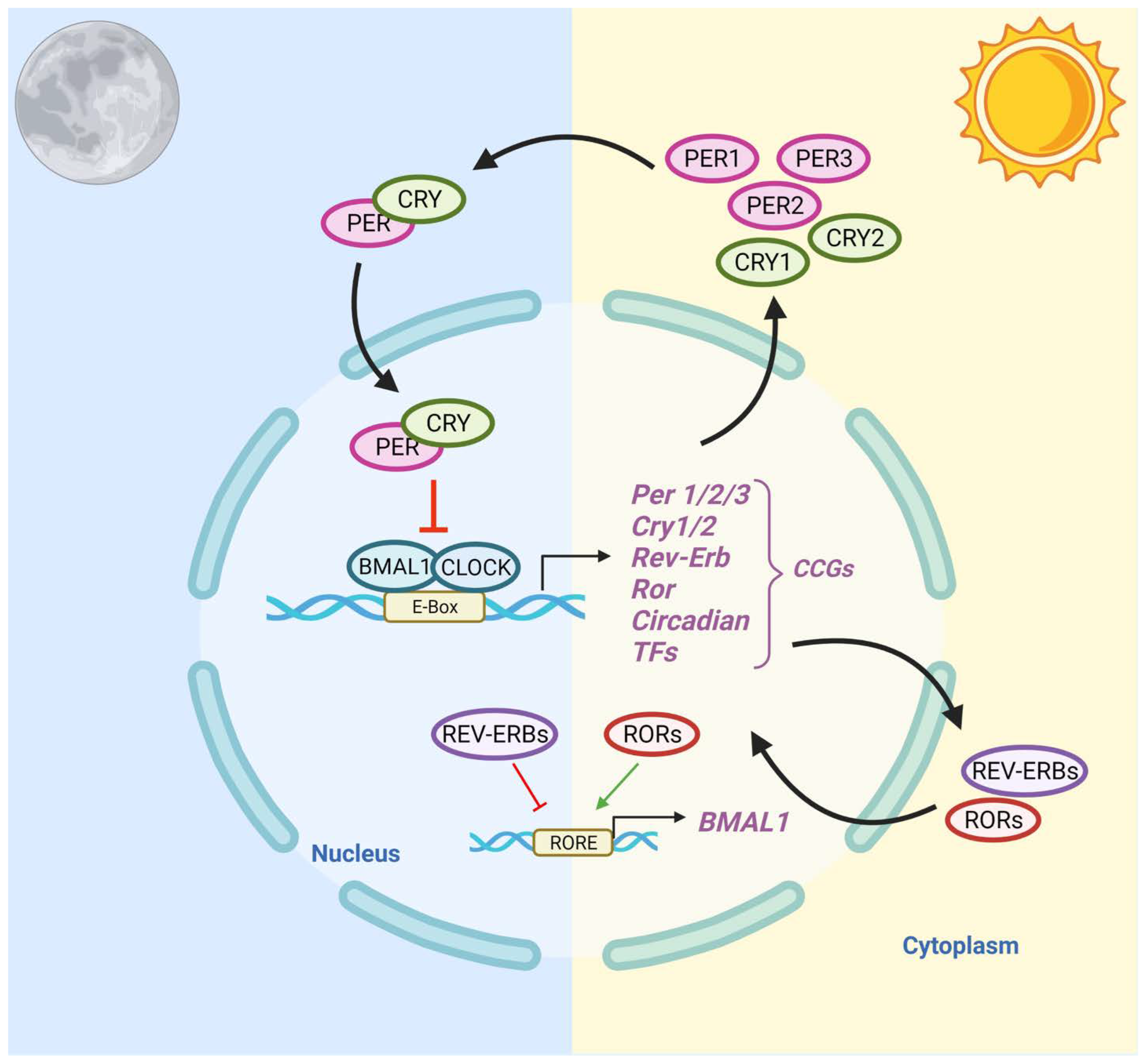
Figure 1. Schematic Representation of the Circadian Clock Transcriptional–Translational Feedback Loops. The main feedback loop is comprised of activator proteins Brain and Muscle ARNT-Like 1 (BMAL1) and Circadian Locomotor Output Cycles Kaput (CLOCK), and two repressor proteins, Period (PER) and Cryptochrome (CRY). BMAL1 and CLOCK heterodimerize and bind to the E-box, which activates the transcription of CRY (1-2), PER (1-3), RORα, REV-ERBα, and Clock transcription factors (TFs). The primary negative feedback occurs when CRY and PER accumulate and dimerize in the cytoplasm and translocate to the nucleus to inhibit the BMAL1: CLOCK. In the secondary feedback loop, RORα and REV-ERBα activate and inhibit the transcription of BMAL1.
While the SCN mainly relies on cues from light–dark cycles to entrain the biological clock, peripheral clocks, such as the ones in the reproductive, endocrine, and immune systems, receive timing signals from the SCN as well as from feeding patterns, temperature, and hormones [48][49][50]. The feed/fasting cycle dictates nutrient intake within specific periods during the day: the periodic phosphorylation of energy sensors such as AMP-activated protein kinase (AMPK) promotes ATP production in response to exercise/fasting and encourages the breakdown of fatty acids, glucose, and triglycerides after eating a meal. AMPK destabilizes CRY1 in peripheral proteins and interacts with SIRTUIN 1(SIRT1), which modulates transcription factors including PER2 [51]. In addition to nutrient-sensing molecules, ligand-activated transcription factors can have several effects on clock genes; REV-ERB regulates gluconeogenesis and the lipid metabolism while repressing Bmal1 transcription, while transcription factor ROR, its competitive inhibitor, induces Bmal1 expression upon binding. PPAR is activated by fatty acids and plays a role in lipid homeostasis through a positive feedback loop with BMAL1 protein [52]. Thus, peripheral clocks are involved in several important metabolic functions, including digestion, hormone secretion, lipid homeostasis, and the immune system response [53].
3. Epidemiological Evidence—The Link between CRDs and Prostate Cancer Risk
Disruption of circadian rhythms is thought to be caused by environmental noise pollution [54][55], jet lag [56][57], night shift work [58], and artificial light at night (ALAN) [59]. CRD is associated with various health consequences, including premature death, metabolic syndrome, obesity, immune dysregulation, reproductive problems, stress, and depression [6]. Compelling evidence demonstrates that PCa is linked with both the causes and consequences of CRDs [60].
3.1. Causes of CRD
Noise pollution: Nocturnal environmental noise, such as noise from transportation or industrial plants, can induce disturbances in sleep quality, metabolic and psychiatric changes, and alterations in sleep architecture [61]. As a consequence of noise exposure, the redistribution of time spent in different sleep stages—increasing wakefulness and stage one sleep, and decreasing slow-wave sleep and REM sleep—negatively impacts cognitive performance, mood, and energy restoration while increasing daytime sleepiness [62]. Epidemiological studies have found that exposure to traffic noise at night increases the risk of hypertension, heart disease, and stroke. Nocturnal noise contributes to an increased risk for cardiovascular comorbidity through the greater secretion of endocrine hormones, including cortisol, noradrenaline, and adrenaline [61]. Nocturnal ambient noise exposure is associated with dysregulation of the central circadian clock, and may be involved in alternations in peripheral clock genes [63].
Jetlag: CRD may contribute to PCa through jetlag. Studies involving US pilots and astronauts have determined there is an increased risk of developing PCa, yet not PCa mortality [64][65][66]. Longer air hours, number of employment years, and radiation exposure positively correlate with increased PCa risk [19][67]. Male pilots are at least twice as likely to develop PCa than men in the general population, and several subgroups, including AA pilots and military pilots, were found to have an increased risk of PCa [18][68].
Night shift work: In addition to jetlag, numerous studies have established the impact of night shift work on cancer risk. In 2017, the International Agency for Research on Cancer identified rotating shift work, in association with circadian disruption, as a probable human carcinogen, placing it in the same risk category as ultraviolet radiation, benzo(a)pyrene, and acrylamide [69]. Co-exposures within night shift workplaces, including noise levels and light at night, may additionally be linked with CRD [70]. A longitudinal study found that female nurses who engaged in nightshift work for over thirty years displayed a 36% increase in the relative risk of breast cancer [71]. The established evidence for the effect of night shift work on the prevalence of breast cancer has incited the investigation of night shift work in relation to PCa [72]. Several meta-analyses, including cohort-based studies in Japan, Canada, and Spain, found an association between night shift work and PCa [21][72][73][74][75]. In the Spanish cohort, workers with a longer duration of work hours were more likely to have tumors with a worse prognosis, while the Canadian population study determined that night-workers were at increased risk for developing PCa, regardless of work duration [74][75]. Additionally, a fixed vs. rotating night shift work has a differential effect on PCa risk, with rotating shift workers having a 20% higher risk for developing PCa than fixed schedule night shift workers [76]. Greater PCa risk has been reported in firefighters, health practitioners, and police, all of which typically require some degree of night shift work [77]. AA firefighters, machinery maintenance workers, and railroad workers are particularly more at risk for developing PCa [65]. Despite evidence implicating a positive correlation between night shift work and PCa, some reports found no such association [78][79]. These diverse findings may be related to the size of the cohort, differences in fixed schedules vs. rotating schedules, and duration of occupation.
Artificial light at night: While the current literature on the effects of ALAN as an environmental risk factor is limited, there is evidence for the association between ALAN and PCa incidence [80][81]. The established literature on the link between ALAN exposure and the risk of breast cancer has promoted further exploration of the effects on other hormone-dependent cancers, including PCa [80][82][83][84][85]. Several cross-geographic studies have found a significant positive association between population exposure to ALAN and the incidence of prostate, breast, colorectal, and lung cancers individually, after adjusting for population size, electricity consumption, air pollution, and total area of land covered by forest [86][87]. A case–control study in Spain found that night shift workers had a slightly higher prostate cancer risk compared to non-night shift workers; the risk increased with longer light exposure and was more pronounced for high-risk prostate tumors [75]. Exposure to ALAN affects melatonin levels, a potential mechanism linking shift work with increased PCa risk [88]. Lower melatonin serum levels have been associated with exposure to ALAN [89]. Thus, the lack of melatonin, a circadian hormone with potential anti-cancer effects, may be correlated with enhanced tumor development. A direct link between ALAN, melatonin suppression, and increased risk for cancer, however, has not been established, mainly due to a lack of measurements of ALAN using calibrated personal light-measuring devices.
3.2. Consequences of CRD
Behavioral stress: Behavioral stress and psychosocial factors, which has a bidirectional relationship with CRD, have an impact on PCa outcomes [90]. PCa patients report the highest levels of stress and anxiety on average compared to other cancer patients. Findings from studies on patient anxiety, stress, and prostate specific antigen (PSA) levels are heterogeneous [91][92][93]. Clinical studies have found that participants with high cortisol levels had a positive correlation with high prostate specific antigen (PSA) values, indicating high PCa risk [94]. Among a cohort of World Trade Center responders during the 9/11 terrorist attacks, re-experiencing a traumatic event was correlated with increased PCa incidence [95]. Greater perceived stress is associated with increased PCa-specific mortality, grieving and sleep loss, and a lack of adequate social support [96]. Patients utilizing high-effort coping (a coping mechanism used for race-based discrimination and mistreatment) had a slightly greater PCa risk relative to men who had decreased levels of high-effort coping, demonstrating how race-based discrimination may be a contributing factor to the stress-related PCa pathway [97]. Lastly, glucocorticoid (GR) signaling, which is overstimulated in chronic stress conditions, contributes to the progression of metastatic-castration-resistant prostate cancer (mCRPC), by promoting AR-target genes in the absence of androgens [98].
Obesity: Obesity is associated with multiple chronic problems as well as hormonal changes, which have an impact on the progression of PCa. Studies investigating the potential role of obesity in PCa risk, progression, and mortality yield heterogeneous results. Measures of obesity, such as body mass index (BMI) > 30, waist–hip ratio, and waist circumference (WC), are positively correlated with risk for advanced PCa and mortality due to PCa [99][100][101][102]. Obesity has a stronger association with PCa risk among AA men compared to White men [103]. Among a racially diverse cohort, AA men had the greatest obesity rates (BMI > 35), and obese AA men were more likely to have positive surgical margins, higher-grade tumors, and higher rates of biochemical failure after a radical prostatectomy [104]. A higher BMI has been associated with increased progression towards mCRPC, a 3-fold risk of developing metastases, and PC-specific mortality [105]. These findings provide new insights into the role of CRD and the consequences of CRD (e.g., obesity and stress) for PCa progression.
This entry is adapted from the peer-reviewed paper 10.3390/cancers14205116
References
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89.
- Society, A.C. Estimated New Cases and Deaths. Available online: https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html#:~:text=Prostate%20cancer%20is%20the%20second,do%20not%20die%20from%20it (accessed on 15 October 2021).
- Jiang, S.; Narayan, V.; Warlick, C. Racial disparities and considerations for active surveillance of prostate cancer. Transl. Androl. Urol. 2018, 7, 214–220.
- McGrowder, D.A.; Jackson, L.A.; Crawford, T.V. Prostate cancer and metabolic syndrome: Is there a link? Asian Pac. J. Cancer Prev. 2012, 13, 1–13.
- Zuniga, K.B.; Chan, J.M.; Ryan, C.J.; Kenfield, S.A. Diet and lifestyle considerations for patients with prostate cancer. Urol. Oncol. 2020, 38, 105–117.
- Evans, J.A.; Davidson, A.J. Health consequences of circadian disruption in humans and animal models. Prog. Mol. Biol. Transl. Sci. 2013, 119, 283–323.
- Xie, Y.; Tang, Q.; Chen, G.; Xie, M.; Yu, S.; Zhao, J.; Chen, L. New Insights Into the Circadian Rhythm and Its Related Diseases. Front. Physiol. 2019, 10, 682.
- James, S.M.; Honn, K.A.; Gaddameedhi, S.; Van Dongen, H.P.A. Shift Work: Disrupted Circadian Rhythms and Sleep-Implications for Health and Well-Being. Curr. Sleep Med. Rep. 2017, 3, 104–112.
- Cooksey-Stowers, K.; Schwartz, M.B.; Brownell, K.D. Food Swamps Predict Obesity Rates Better Than Food Deserts in the United States. Int. J. Environ. Res. Public Health 2017, 14, 1366.
- Seltenrich, N. Inequality of Noise Exposures: A Portrait of the United States. Environ. Health Perspect. 2017, 125, 094003.
- Bower, K.M.; Thorpe, R.J., Jr.; Rohde, C.; Gaskin, D.J. The intersection of neighborhood racial segregation, poverty, and urbanicity and its impact on food store availability in the United States. Prev. Med. 2014, 58, 33–39.
- Diez Roux, A.V. Neighborhoods and Health: What Do We Know? What Should We Do? Am. J. Public Health 2016, 106, 430–431.
- Casey, J.A.; Morello-Frosch, R.; Mennitt, D.J.; Fristrup, K.; Ogburn, E.L.; James, P. Race/Ethnicity, Socioeconomic Status, Residential Segregation, and Spatial Variation in Noise Exposure in the Contiguous United States. Environ. Health Perspect. 2017, 125, 077017.
- Von Allmen, D.C.; Francey, L.J.; Rogers, G.M.; Ruben, M.D.; Cohen, A.P.; Wu, G.; Schmidt, R.E.; Ishman, S.L.; Amin, R.S.; Hogenesch, J.B.; et al. Circadian Dysregulation: The Next Frontier in Obstructive Sleep Apnea Research. Otolaryngol. Head Neck Surg. 2018, 159, 948–955.
- Roenneberg, T.; Merrow, M. The Circadian Clock and Human Health. Curr. Biol. 2016, 26, R432–R443.
- Koritala, B.S.C.; Conroy, Z.; Smith, D.F. Circadian Biology in Obstructive Sleep Apnea. Diagnostics 2021, 11, 1082.
- Gabryelska, A.; Turkiewicz, S.; Karuga, F.F.; Sochal, M.; Strzelecki, D.; Białasiewicz, P. Disruption of Circadian Rhythm Genes in Obstructive Sleep Apnea Patients—Possible Mechanisms Involved and Clinical Implication. Int. J. Mol. Sci. 2022, 23, 709.
- Raslau, D.; Summerfield, D.T.; Abu Dabrh, A.M.; Steinkraus, L.W.; Murad, M.H. The risk of prostate cancer in pilots: A meta-analysis. Aerosp. Med. Hum. Perform. 2015, 86, 112–117.
- Gudmundsdottir, E.M.; Hrafnkelsson, J.; Rafnsson, V. Incidence of cancer among licenced commercial pilots flying North Atlantic routes. Environ. Health 2017, 16, 86.
- Wang, F.; Yeung, K.L.; Chan, W.C.; Kwok, C.C.; Leung, S.L.; Wu, C.; Chan, E.Y.; Yu, I.T.; Yang, X.R.; Tse, L.A. A meta-analysis on dose-response relationship between night shift work and the risk of breast cancer. Ann. Oncol. 2013, 24, 2724–2732.
- Rao, D.; Yu, H.; Bai, Y.; Zheng, X.; Xie, L. Does night-shift work increase the risk of prostate cancer? a systematic review and meta-analysis. Oncotargets Ther. 2015, 8, 2817–2826.
- Sigurdardottir, L.G.; Valdimarsdottir, U.A.; Mucci, L.A.; Fall, K.; Rider, J.R.; Schernhammer, E.; Czeisler, C.A.; Launer, L.; Harris, T.; Stampfer, M.J.; et al. Sleep disruption among older men and risk of prostate cancer. Cancer Epidemiol. Biomark. Prev. 2013, 22, 872–879.
- Markt, S.C.; Grotta, A.; Nyren, O.; Adami, H.O.; Mucci, L.A.; Valdimarsdottir, U.A.; Stattin, P.; Bellocco, R.; Lagerros, Y.T. Insufficient Sleep and Risk of Prostate Cancer in a Large Swedish Cohort. Sleep 2015, 38, 1405–1410.
- Wendeu-Foyet, M.G.; Cénée, S.; Koudou, Y.; Trétarre, B.; Rébillard, X.; Cancel-Tassin, G.; Cussenot, O.; Boland, A.; Olaso, R.; Deleuze, J.F.; et al. Circadian genes polymorphisms, night work and prostate cancer risk: Findings from the EPICAP study. Int. J. Cancer 2020, 147, 3119–3129.
- Freedland, S.J.; Aronson, W.J. Examining the relationship between obesity and prostate cancer. Rev. Urol. 2004, 6, 73–81.
- Lavalette, C.; Trétarre, B.; Rebillard, X.; Lamy, P.J.; Cénée, S.; Menegaux, F. Abdominal obesity and prostate cancer risk: Epidemiological evidence from the EPICAP study. Oncotarget 2018, 9, 34485–34494.
- Hassan, S.; Karpova, Y.; Baiz, D.; Yancey, D.; Pullikuth, A.; Flores, A.; Register, T.; Cline, J.M.; D’Agostino, R., Jr.; Danial, N.; et al. Behavioral stress accelerates prostate cancer development in mice. J. Clin. Investig. 2013, 123, 874–886.
- Jayadevappa, R.; Malkowicz, S.B.; Chhatre, S.; Johnson, J.C.; Gallo, J.J. The burden of depression in prostate cancer. Psychooncology 2012, 21, 1338–1345.
- Zhu, Y.; Stevens, R.G.; Hoffman, A.E.; Fitzgerald, L.M.; Kwon, E.M.; Ostrander, E.A.; Davis, S.; Zheng, T.; Stanford, J.L. Testing the circadian gene hypothesis in prostate cancer: A population-based case-control study. Cancer Res. 2009, 69, 9315–9322.
- Shafi, A.A.; McNair, C.M.; McCann, J.J.; Alshalalfa, M.; Shostak, A.; Severson, T.M.; Zhu, Y.; Bergman, A.; Gordon, N.; Mandigo, A.C.; et al. The circadian cryptochrome, CRY1, is a pro-tumorigenic factor that rhythmically modulates DNA repair. Nat. Commun. 2021, 12, 401.
- Li, Q.; Xia, D.; Wang, Z.; Liu, B.; Zhang, J.; Peng, P.; Tang, Q.; Dong, J.; Guo, J.; Kuang, D.; et al. Circadian Rhythm Gene PER3 Negatively Regulates Stemness of Prostate Cancer Stem Cells via WNT/β-Catenin Signaling in Tumor Microenvironment. Front. Cell Dev. Biol. 2021, 9, 656981.
- Arora, V.K.; Schenkein, E.; Murali, R.; Subudhi, S.K.; Wongvipat, J.; Balbas, M.D.; Shah, N.; Cai, L.; Efstathiou, E.; Logothetis, C.; et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell 2013, 155, 1309–1322.
- Woods-Burnham, L.; Cajigas-Du Ross, C.K.; Love, A.; Basu, A.; Sanchez-Hernandez, E.S.; Martinez, S.R.; Ortiz-Hernández, G.L.; Stiel, L.; Durán, A.M.; Wilson, C.; et al. Glucocorticoids Induce Stress Oncoproteins Associated with Therapy-Resistance in African American and European American Prostate Cancer Cells. Sci. Rep. 2018, 8, 15063.
- Frankenberry, K.A.; Somasundar, P.; McFadden, D.W.; Vona-Davis, L.C. Leptin induces cell migration and the expression of growth factors in human prostate cancer cells. Am. J. Surg. 2004, 188, 560–565.
- Calastretti, A.; Gatti, G.; Lucini, V.; Dugnani, S.; Canti, G.; Scaglione, F.; Bevilacqua, A. Melatonin Analogue Antiproliferative and Cytotoxic Effects on Human Prostate Cancer Cells. Int. J. Mol. Sci. 2018, 19, 1505.
- Potter, G.D.; Skene, D.J.; Arendt, J.; Cade, J.E.; Grant, P.J.; Hardie, L.J. Circadian Rhythm and Sleep Disruption: Causes, Metabolic Consequences, and Countermeasures. Endocr. Rev. 2016, 37, 584–608.
- Rosenwasser, A.M.; Turek, F.W. Neurobiology of Circadian Rhythm Regulation. Sleep Med. Clin. 2015, 10, 403–412.
- Rivkees, S.A. The Development of Circadian Rhythms: From Animals To Humans. Sleep Med. Clin. 2007, 2, 331–341.
- Shi, S.Q.; Ansari, T.S.; McGuinness, O.P.; Wasserman, D.H.; Johnson, C.H. Circadian disruption leads to insulin resistance and obesity. Curr. Biol. 2013, 23, 372–381.
- Astiz, M.; Heyde, I.; Oster, H. Mechanisms of Communication in the Mammalian Circadian Timing System. Int. J. Mol. Sci. 2019, 20, 343.
- Jagannath, A.; Taylor, L.; Wakaf, Z.; Vasudevan, S.R.; Foster, R.G. The genetics of circadian rhythms, sleep and health. Hum. Mol. Genet. 2017, 26, R128–R138.
- Carmona-Alcocer, V.; Rohr, K.E.; Joye, D.A.M.; Evans, J.A. Circuit development in the master clock network of mammals. Eur. J. Neurosci. 2020, 51, 82–108.
- Bellet, M.M.; Sassone-Corsi, P. Mammalian circadian clock and metabolism—The epigenetic link. J. Cell Sci. 2010, 123, 3837–3848.
- Pacheco-Bernal, I.; Becerril-Pérez, F.; Aguilar-Arnal, L. Circadian rhythms in the three-dimensional genome: Implications of chromatin interactions for cyclic transcription. Clin. Epigenetics 2019, 11, 79.
- Milev, N.B.; Reddy, A.B. Circadian redox oscillations and metabolism. Trends Endocrinol. Metab. 2015, 26, 430–437.
- Shostak, A. Circadian Clock, Cell Division, and Cancer: From Molecules to Organism. Int. J. Mol. Sci. 2017, 18, 873.
- Masri, S.; Sassone-Corsi, P. The emerging link between cancer, metabolism, and circadian rhythms. Nat. Med. 2018, 24, 1795–1803.
- Albrecht, U. Timing to perfection: The biology of central and peripheral circadian clocks. Neuron 2012, 74, 246–260.
- Richards, J.; Gumz, M.L. Advances in understanding the peripheral circadian clocks. FASEB J. 2012, 26, 3602–3613.
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012, 35, 445–462.
- Potter, G.D.; Cade, J.E.; Grant, P.J.; Hardie, L.J. Nutrition and the circadian system. Br. J. Nutr. 2016, 116, 434–442.
- Huang, W.; Ramsey, K.M.; Marcheva, B.; Bass, J. Circadian rhythms, sleep, and metabolism. J. Clin. Investig. 2011, 121, 2133–2141.
- Oike, H.; Oishi, K.; Kobori, M. Nutrients, Clock Genes, and Chrononutrition. Curr. Nutr. Rep. 2014, 3, 204–212.
- Daiber, A.; Frenis, K.; Kuntic, M.; Li, H.; Wolf, E.; Kilgallen, A.B.; Lecour, S.; Van Laake, L.W.; Schulz, R.; Hahad, O.; et al. Redox Regulatory Changes of Circadian Rhythm by the Environmental Risk Factors Traffic Noise and Air Pollution. Antioxid. Redox Signal. 2022, 37, 679–703.
- Eze, I.C.; Imboden, M.; Foraster, M.; Schaffner, E.; Kumar, A.; Vienneau, D.; Héritier, H.; Rudzik, F.; Thiesse, L.; Pieren, R.; et al. Exposure to Night-Time Traffic Noise, Melatonin-Regulating Gene Variants and Change in Glycemia in Adults. Int. J. Environ. Res. Public Health 2017, 14, 1492.
- Comperatore, C.A.; Krueger, G.P. Circadian rhythm desynchronosis, jet lag, shift lag, and coping strategies. Occup. Med. 1990, 5, 323–341.
- Gander, P.; Mulrine, H.M.; van den Berg, M.J.; Wu, L.; Smith, A.; Signal, L.; Mangie, J. Does the circadian clock drift when pilots fly multiple transpacific flights with 1- to 2-day layovers? Chronobiol. Int. 2016, 33, 982–994.
- Arendt, J. Shift work: Coping with the biological clock. Occup. Med. 2010, 60, 10–20.
- Lunn, R.M.; Blask, D.E.; Coogan, A.N.; Figueiro, M.G.; Gorman, M.R.; Hall, J.E.; Hansen, J.; Nelson, R.J.; Panda, S.; Smolensky, M.H.; et al. Health consequences of electric lighting practices in the modern world: A report on the National Toxicology Program’s workshop on shift work at night, artificial light at night, and circadian disruption. Sci. Total Environ. 2017, 607–608, 1073–1084.
- Sigurdardottir, L.G.; Valdimarsdottir, U.A.; Fall, K.; Rider, J.R.; Lockley, S.W.; Schernhammer, E.; Mucci, L.A. Circadian disruption, sleep loss, and prostate cancer risk: A systematic review of epidemiologic studies. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1002–1011.
- Halperin, D. Environmental noise and sleep disturbances: A threat to health? Sleep Sci. 2014, 7, 209–212.
- Muzet, A. Environmental noise, sleep and health. Sleep Med. Rev. 2007, 11, 135–142.
- Patrick, D.M.; Harrison, D.G. Nocturnal noise knocks NOS by Nox: Mechanisms underlying cardiovascular dysfunction in response to noise pollution. Eur. Heart J. 2018, 39, 3540–3542.
- Raslau, D.; Abu Dabrh, A.M.; Summerfield, D.T.; Wang, Z.; Steinkraus, L.W.; Murad, M.H. Prostate Cancer in Pilots. Aerosp. Med. Hum. Perform. 2016, 87, 565–570.
- Krstev, S.; Baris, D.; Stewart, P.A.; Hayes, R.B.; Blair, A.; Dosemeci, M. Risk for prostate cancer by occupation and industry: A 24-state death certificate study. Am. J. Ind. Med. 1998, 34, 413–420.
- Reynolds, R.; Little, M.P.; Day, S.; Charvat, J.; Blattnig, S.; Huff, J.; Patel, Z.S. Cancer incidence and mortality in the USA Astronaut Corps, 1959-2017. Occup. Environ. Med. 2021, 78, 869–875.
- Buja, A.; Lange, J.H.; Perissinotto, E.; Rausa, G.; Grigoletto, F.; Canova, C.; Mastrangelo, G. Cancer incidence among male military and civil pilots and flight attendants: An analysis on published data. Toxicol. Ind. Health 2005, 21, 273–282.
- Webber, B.J.; Tacke, C.D.; Wolff, G.G.; Rutherford, A.E.; Erwin, W.J.; Escobar, J.D.; Simon, A.A.; Reed, B.H.; Whitaker, J.G.; Gambino-Shirley, K.J.; et al. Cancer Incidence and Mortality Among Fighter Aviators in the United States Air Force. J. Occup. Environ. Med. 2021, 64, 71.
- Erren, T.C.; Falaturi, P.; Morfeld, P.; Knauth, P.; Reiter, R.J.; Piekarski, C. Shift work and cancer: The evidence and the challenge. Dtsch. Arztebl. Int. 2010, 107, 657–662.
- Pepłońska, B.; Burdelak, W.; Bukowska, A.; Krysicka, J.; Konieczko, K. Night shift work characteristics and occupational co-exposures in industrial plants in Łódź, Poland. Int. J. Occup. Med. Environ. Health 2013, 26, 522–534.
- Schernhammer, E.S.; Laden, F.; Speizer, F.E.; Willett, W.C.; Hunter, D.J.; Kawachi, I.; Colditz, G.A. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J. Natl. Cancer Inst. 2001, 93, 1563–1568.
- Conlon, M.; Lightfoot, N.; Kreiger, N. Rotating shift work and risk of prostate cancer. Epidemiology 2007, 18, 182–183.
- Kubo, T.; Oyama, I.; Nakamura, T.; Kunimoto, M.; Kadowaki, K.; Otomo, H.; Fujino, Y.; Fujimoto, N.; Matsumoto, T.; Matsuda, S. Industry-based retrospective cohort study of the risk of prostate cancer among rotating-shift workers. Int. J. Urol. 2011, 18, 206–211.
- Parent, M.; El-Zein, M.; Rousseau, M.C.; Pintos, J.; Siemiatycki, J. Night work and the risk of cancer among men. Am. J. Epidemiol. 2012, 176, 751–759.
- Papantoniou, K.; Castaño-Vinyals, G.; Espinosa, A.; Aragonés, N.; Pérez-Gómez, B.; Burgos, J.; Gómez-Acebo, I.; Llorca, J.; Peiró, R.; Jimenez-Moleón, J.J.; et al. Night shift work, chronotype and prostate cancer risk in the MCC-Spain case-control study. Int. J. Cancer 2015, 137, 1147–1157.
- Mancio, J.; Leal, C.; Ferreira, M.; Norton, P.; Lunet, N. Does the association of prostate cancer with night-shift work differ according to rotating vs. fixed schedule? A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2018, 21, 337–344.
- Davis, S.; Mirick, D.K. Circadian disruption, shift work and the risk of cancer: A summary of the evidence and studies in Seattle. Cancer Causes Control 2006, 17, 539–545.
- Yong, M.; Blettner, M.; Emrich, K.; Nasterlack, M.; Oberlinner, C.; Hammer, G.P. A retrospective cohort study of shift work and risk of incident cancer among German male chemical workers. Scand. J. Work Environ. Health 2014, 40, 502–510.
- Barul, C.; Richard, H.; Parent, M.E. Night-Shift Work and Risk of Prostate Cancer: Results From a Canadian Case-Control Study, the Prostate Cancer and Environment Study. Am. J. Epidemiol. 2019, 188, 1801–1811.
- Rybnikova, N.A.; Haim, A.; Portnov, B.A. Is prostate cancer incidence worldwide linked to artificial light at night exposures? Review of earlier findings and analysis of current trends. Arch. Environ. Occup. Health 2017, 72, 111–122.
- Kim, K.Y.; Lee, E.; Kim, Y.J.; Kim, J. The association between artificial light at night and prostate cancer in Gwangju City and South Jeolla Province of South Korea. Chronobiol. Int. 2017, 34, 203–211.
- Stevens, R.G. Light-at-night, circadian disruption and breast cancer: Assessment of existing evidence. Int. J. Epidemiol. 2009, 38, 963–970.
- Bauer, S.E.; Wagner, S.E.; Burch, J.; Bayakly, R.; Vena, J.E. A case-referent study: Light at night and breast cancer risk in Georgia. Int. J. Health Geogr. 2013, 12, 23.
- Hurley, S.; Goldberg, D.; Nelson, D.; Hertz, A.; Horn-Ross, P.L.; Bernstein, L.; Reynolds, P. Light at night and breast cancer risk among California teachers. Epidemiology 2014, 25, 697–706.
- Haim, A.; Portnov, B.A. Light-at-Night (LAN) as a General Stressor. In Light Pollution as a New Risk Factor for Human Breast and Prostate Cancers, Haim, A., Portnov, B.A., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 67–70.
- Kloog, I.; Haim, A.; Stevens, R.G.; Portnov, B.A. Global co-distribution of light at night (LAN) and cancers of prostate, colon, and lung in men. Chronobiol. Int. 2009, 26, 108–125.
- Al-Naggar, R.A.; Anil, S. Artificial Light at Night and Cancer: Global Study. Asian Pac. J. Cancer Prev. 2016, 17, 4661–4664.
- Samanta, S. The Potential Oncostatic Effects of Melatonin against Prostate Cancer. Crit. Rev. Oncog. 2021, 26, 53–67.
- Schernhammer, E.S.; Schulmeister, K. Melatonin and cancer risk: Does light at night compromise physiologic cancer protection by lowering serum melatonin levels? Br. J. Cancer 2004, 90, 941–943.
- Zorn, J.V.; Schür, R.R.; Boks, M.P.; Kahn, R.S.; Joëls, M.; Vinkers, C.H. Cortisol stress reactivity across psychiatric disorders: A systematic review and meta-analysis. Psychoneuroendocrinology 2017, 77, 25–36.
- Ullrich, P.M.; Carson, M.R.; Lutgendorf, S.K.; Williams, R.D. Cancer fear and mood disturbance after radical prostatectomy: Consequences of biochemical evidence of recurrence. J. Urol. 2003, 169, 1449–1452.
- Kotwal, A.A.; Schumm, P.; Mohile, S.G.; Dale, W. The influence of stress, depression, and anxiety on PSA screening rates in a nationally representative sample. Med. Care 2012, 50, 1037–1044.
- Fabre, B.; Grosman, H.; Gonzalez, D.; Machulsky, N.F.; Repetto, E.M.; Mesch, V.; Lopez, M.A.; Mazza, O.; Berg, G. Prostate Cancer, High Cortisol Levels and Complex Hormonal Interaction. Asian Pac. J. Cancer Prev. 2016, 17, 3167–3171.
- Gidron, Y.; Fabre, B.; Grosman, H.; Nolazco, C.; Mesch, V.; Mazza, O.; Berg, G. Life events, cortisol and levels of prostate specific antigen: A story of synergism. Psychoneuroendocrinology 2011, 36, 874–880.
- Clouston, S.A.P.; Kuan, P.; Kotov, R.; Mukherjee, S.; Thompson-Carino, P.; Bromet, E.J.; Luft, B.J. Risk factors for incident prostate cancer in a cohort of world trade center responders. BMC Psychiatry 2019, 19, 389.
- Jan, M.; Bonn, S.E.; Sjölander, A.; Wiklund, F.; Stattin, P.; Holmberg, E.; Grönberg, H.; Bälter, K. The roles of stress and social support in prostate cancer mortality. Scand. J. Urol. 2016, 50, 47–55.
- Coker, A.L.; Sanderson, M.; Ellison, G.L.; Fadden, M.K. Stress, coping, social support, and prostate cancer risk among older African American and Caucasian men. Ethn. Dis. 2006, 16, 978–987.
- Isikbay, M.; Otto, K.; Kregel, S.; Kach, J.; Cai, Y.; Vander Griend, D.J.; Conzen, S.D.; Szmulewitz, R.Z. Glucocorticoid receptor activity contributes to resistance to androgen-targeted therapy in prostate cancer. Horm. Cancer 2014, 5, 72–89.
- Møller, H.; Roswall, N.; Van Hemelrijck, M.; Larsen, S.B.; Cuzick, J.; Holmberg, L.; Overvad, K.; Tjønneland, A. Prostate cancer incidence, clinical stage and survival in relation to obesity: A prospective cohort study in Denmark. Int. J. Cancer 2015, 136, 1940–1947.
- Fowke, J.H.; Motley, S.S.; Concepcion, R.S.; Penson, D.F.; Barocas, D.A. Obesity, body composition, and prostate cancer. BMC Cancer 2012, 12, 23.
- Pischon, T.; Boeing, H.; Weikert, S.; Allen, N.; Key, T.; Johnsen, N.F.; Tjønneland, A.; Severinsen, M.T.; Overvad, K.; Rohrmann, S.; et al. Body size and risk of prostate cancer in the European prospective investigation into cancer and nutrition. Cancer Epidemiol. Biomark. Prev. 2008, 17, 3252–3261.
- Engeland, A.; Tretli, S.; Bjørge, T. Height, body mass index, and prostate cancer: A follow-up of 950,000 Norwegian men. Br. J. Cancer 2003, 89, 1237–1242.
- Barrington, W.E.; Schenk, J.M.; Etzioni, R.; Arnold, K.B.; Neuhouser, M.L.; Thompson, I.M., Jr.; Lucia, M.S.; Kristal, A.R. Difference in Association of Obesity With Prostate Cancer Risk Between US African American and Non-Hispanic White Men in the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA Oncol. 2015, 1, 342–349.
- Freedland, S.J.; Giovannucci, E.; Platz, E.A. Are findings from studies of obesity and prostate cancer really in conflict? Cancer Causes Control 2006, 17, 5–9.
- Keto, C.J.; Aronson, W.J.; Terris, M.K.; Presti, J.C.; Kane, C.J.; Amling, C.L.; Freedland, S.J. Obesity is associated with castration-resistant disease and metastasis in men treated with androgen deprivation therapy after radical prostatectomy: Results from the SEARCH database. BJU Int. 2012, 110, 492–498.
This entry is offline, you can click here to edit this entry!
