Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The need for renewable and sustainable fuel and energy storage sources is pressing. Biohydrogen has the potential to be a storable energy carrier, a direct fuel and a diverse building block for various downstream products. Utilizing microbial electrolysis cells (MECs) to produce biohydrogen from residue streams, such as the organic fraction of municipal solid waste (OFMSW), agricultural residues and wastewater facilitate utilization and energy recovery from these streams, paving the path for a circular economy.
- MEC
- green hydrogen
- OFMSW
- wastewater
1. Introduction
Energy demand has been increasing alongside a growing human population [1] and a growing number of consumables purchased, shipped, used and discharged [2]. The access and availability of products have led to an increase in both types of waste and the quantity generated. Waste generated by humans is the third largest source of methane emissions after industrial and agricultural sources. Although CO2 remains the most significant contributor to global warming, methane has a more considerable comparative impact due to its increased efficiency at trapping CO2 radiation, thus causing dire consequences for climate change [3][4]. As the industrial, agricultural and transportation sectors formulate and implement their respective strategies in combating climate change, the utilization of waste as a resource transcends sectoral boundaries. Reducing emissions is a retrogressive action that addresses the challenges cumulated due to inaction over the past decades [5]. Some contemporary waste streams create value using energy generation, and others might serve as product precursors.
1.1. Hydrogen as the Product
Hydrogen is a synthetic energy carrier, implying that it is a direct energy source and constitutes a possibility for energy storage in the form of chemical potential. These abilities make hydrogen a promising key player in the future of renewable energy systems [6].
The demand for hydrogen has continuously increased since 1975, with ammonia production accounting for the bulk of the demand. However, global hydrogen consumption is expected to increase drastically due to its properties, making it a central building block in mitigating fossil-derived energy sources used in hard-to-eliminate emission sectors [7].
Hydrogen can be produced by various methods, including electrolysis, thermal water splitting, gasification, dark fermentation, photoelectrochemical cells, fossil fuel reforming, artificial photosynthesis, aqueous phase reforming and conventional reforming methods [8][9][10]. These production techniques may be divided into categories based on their CO2 emissions and environmental impact. The four main sections in which hydrogen production has been categorized are brown, grey, blue and green [11].
These color codes are designated in terms of the degree of CO2 emissions and capture, as seen in Figure 1. The brown hydrogen category includes carbon and fossil-fuel production methods, such as gasification by coal, which emits the highest levels of CO2. The grey hydrogen category includes industrially produced hydrogen, e.g., through steam reforming using natural gas, but with no carbon capture and high CO2 emissions. Blue hydrogen is produced during the carbon capture process and is, therefore, the category that emits the least CO2. Green hydrogen is the ideal climate-neutral production method, i.e., it is produced through renewable electricity and electrolysis, emitting zero CO2 [8][12].
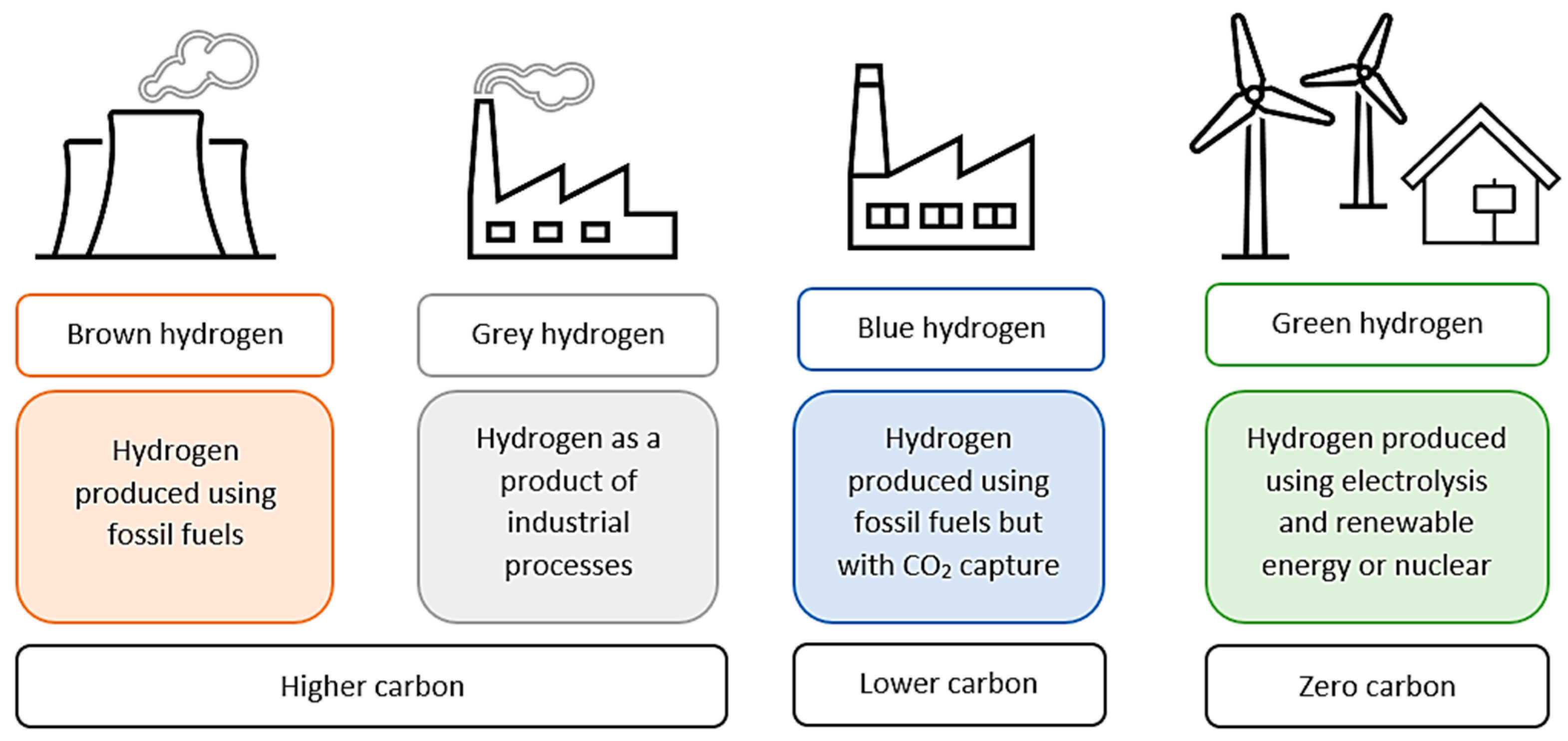
Figure 1. Differences in hydrogen production methods and their carbon emission impact.
Green hydrogen possesses a higher purity than blue hydrogen, which needs further purification if it is to be implemented in a vehicle’s fuel cell. However, blue hydrogen is suitable for industrial use without purification, and the production of blue hydrogen eliminates the industry’s emissions by reducing CO2 on a large scale and with a low cost [13].
In 2019, hydrogen production was 75 million tons per year and was used primarily in refining and in the chemical industry [14]. It is estimated that, in 2070, hydrogen and hydrogen-based fuels will account for 13% of all energy needs, compared to 1% in 2019, with most of the hydrogen being used in the transportation and chemical industries [14]. In 2070, the transportation industry will account for 70% of hydrogen consumption, divided into shipping (52%), aviation (40%) and the remaining road transport [14]. In 2019, hydrogen production was responsible for greenhouse gas (GHG) emissions of more than 800 million tons of CO2, corresponding to around 2% of the global CO2 emissions from the energy sector [14]. Of the many developing strategies and projects working on a low-carbon hydrogen value chain, fuel cells in transportation and electrolysis production are among the more advanced methods. However, hydrogen-fueled engines for road transportation (mainly cars), ships and ammonia-fueled ships are currently only in the prototype stage [14][15].
1.1.1. Power-to-X and the Role of Hydrogen in Denmark
The use of hydrogen already extends to several parts of the global energy sector, and production is projected to grow six-fold from 2021 to 2050 to meet 10% of the total energy consumption [16]. As part of meeting the European Green Deal, renewable hydrogen production is expected to reach 10 million tons in Europe by 2030 [17]. The energy directive promotes using hydrogen to decarbonize hard-to-eliminate emission industries and heavy-duty and long-distance transport.
Power-to-X (PtX or P2X) is a collective term for renewable technologies in which electricity production is achieved through renewable resources, which convert into hydrogen, synthetic gases, fuels or chemicals. These conversion technologies allow for the storage and utilization of excess renewable energy via the production of green fuels and chemicals while also providing low capital-intensive decarbonization [13][16][18]. The benefits of implementing PtX technology in the energy sector include decentralized production, seasonal energy storage, decarbonization and grid stability, which become increasingly important as contributions from the wind and solar sectors steadily grow [13].
1.1.2. From Hydrogen to Ammonia
The future of hydrogen as an energy source seems promising; however, there are drawbacks to the technology. The low volumetric energy density of hydrogen, both in gaseous and liquid forms, makes hydrogen challenging to store, thus decreasing its accessibility by making it harder to deliver and distribute. The low energy density of hydrogen is potentially one of the most significant barriers to implementing hydrogen-fueled energy [19]. To utilize and exploit the full potential of hydrogen as an emission-free fuel source, the shipping and storing of hydrogen may lean on another often-used chemical asset—ammonia.
Ammonia has a high hydrogen density of 17% due to having three bound hydrogen atoms, enabling its use as a fuel for combustion systems directly or as a fuel in fuel cells [20]. Thus, ammonia has been gaining increased interest due to its potential as a carbon-free fuel for shipping because of its high liquefaction temperature and energy density compared with hydrogen [21]. The volumetric energy density of ammonia is 11.5 MJ · L−1 and is therefore superior to liquid hydrogen with 8.491 MJ · L−1 and compressed hydrogen with 4.5 MJ · L−1 at 690 bar and 25 °C [22]. Moreover, ammonia can be stored and transported at −33 °C compared with cryogenic hydrogen storage at −253 °C, significantly reducing the economic cost of being less energy intensive [23]. Additionally, ammonia allows for safer handling and distribution, as it is less hazardous than hydrogen. Although toxic, its smell can be detected even at safe concentration levels (<1 ppm). Ammonia has a narrower flammable range than hydrogen and is often considered nonflammable when transported, whereas hydrogen burns with an invisible flame [24]. Similar to hydrogen, ammonia is ultimately carbon free, although CO2 emissions during ammonia production depend on the energy source. Both hydrogen and ammonia utilize energy sources for production, such as fossil fuels, biomass, renewable electricity and nuclear and solar energy [25].
Exploring different alternatives to storing and shipping hydrogen has led to extensive research and numerous studies [26][27][28][29][30][31][32][33][34][35][36]. However, compared with much of the work performed, the key advantage of exploiting liquid ammonia is its ease of storage and distribution, thereby overcoming the issues of hydrogen infrastructure [33]. For hydrogen production to become sustainable, environmentally friendly and carbon neutral, emphasis must be given to production from renewable sources. This can be achieved by utilizing a microbial electrolysis cell (MEC) assisted with a renewable energy source for external power and waste to ensure and optimize a circular bioeconomy [37].
2. Current State-of-the-Art for MEC Technology
2.1. Operating Principle
Microbial electrolysis cell (MEC) is an anaerobic biological process used to convert organic compounds into hydrogen or methane. MEC technology is related to traditional electrolysis, such as alkaline water electrolysis (AWE) or proton exchange membrane (PEM) electrolysis. The main difference is that the hydrogen evolution reaction (HER) is catalyzed by electroactive bacteria under anaerobic conditions. Moreover, MEC technology resembles another hydrogen production technology: the microbial fuel cell (MFC). The technologies differ, as MEC produces hydrogen from anaerobic electrogenic bacteria with an applied voltage, whereas MFC produces an electrical current from aerobic electrogenic bacteria [38][39][40][41]. As MEC and MFC are structurally similar, MFC is representable in many experimental studies and is therefore included in the coming sections.
An MEC consists of an anode and cathode coupled to a power source and submerged in a suitable electrolyte, facilitating electron transport via microorganisms pre-inoculated in the anaerobic container (cf. Figure 2). The two electrodes can be submerged into the electrolyte in the same container, or they may be separated by a membrane or placed in separate containers with different electrolytes, providing several configuration options [39]. MEC technology utilizes bacteria for the catalysis of the electrochemical oxidation or reduction reactions used to produce current [41].
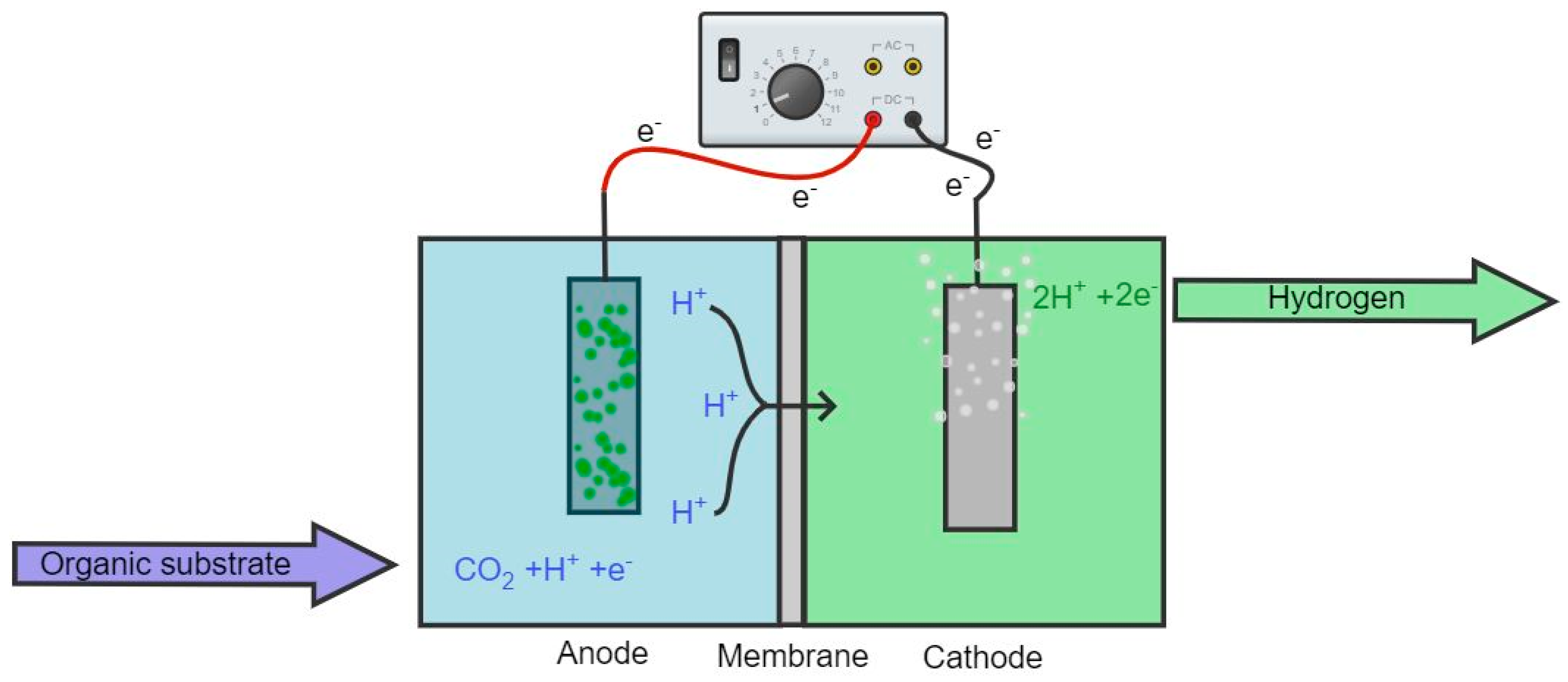
Figure 2. Simple construction scheme of two-chamber MEC separated by a membrane. The green attachment on the anode represents the microbial presence.
The electrons and protons generated at the anode during oxidation are utilized at the cathode to produce biohydrogen but require additional voltage to trigger biohydrogen production [42]. The optimally applied voltage lies in the range of 0.5–1.0 V [43], which is lower than the voltage required for water electrolysis (1.23–1.8 V) [39], which indicates an advantage to MEC technology. In theory, small voltage can be supplied by renewable energy sources, such as wind or solar energy, during hours of low demand [44].
The mechanism in MEC, as seen in Figure 3, can be summarized as the breakdown of organic matter by exoelectrogenic bacteria into ions, which the bacteria can transport outside of their cell to the surface of the anode inside the MEC chamber. The exoelectrogenic bacteria are attached to the surface of the anode, where they oxidize the organic material into CO2, releasing protons to the solution and electrons to the anode. When exoelectrogenic or anode-respiring bacteria consume organic matter under anaerobic conditions, they donate their final electron as part of their electron transport chain to the terminal electron acceptor (TEA) at the electrode [10][39].
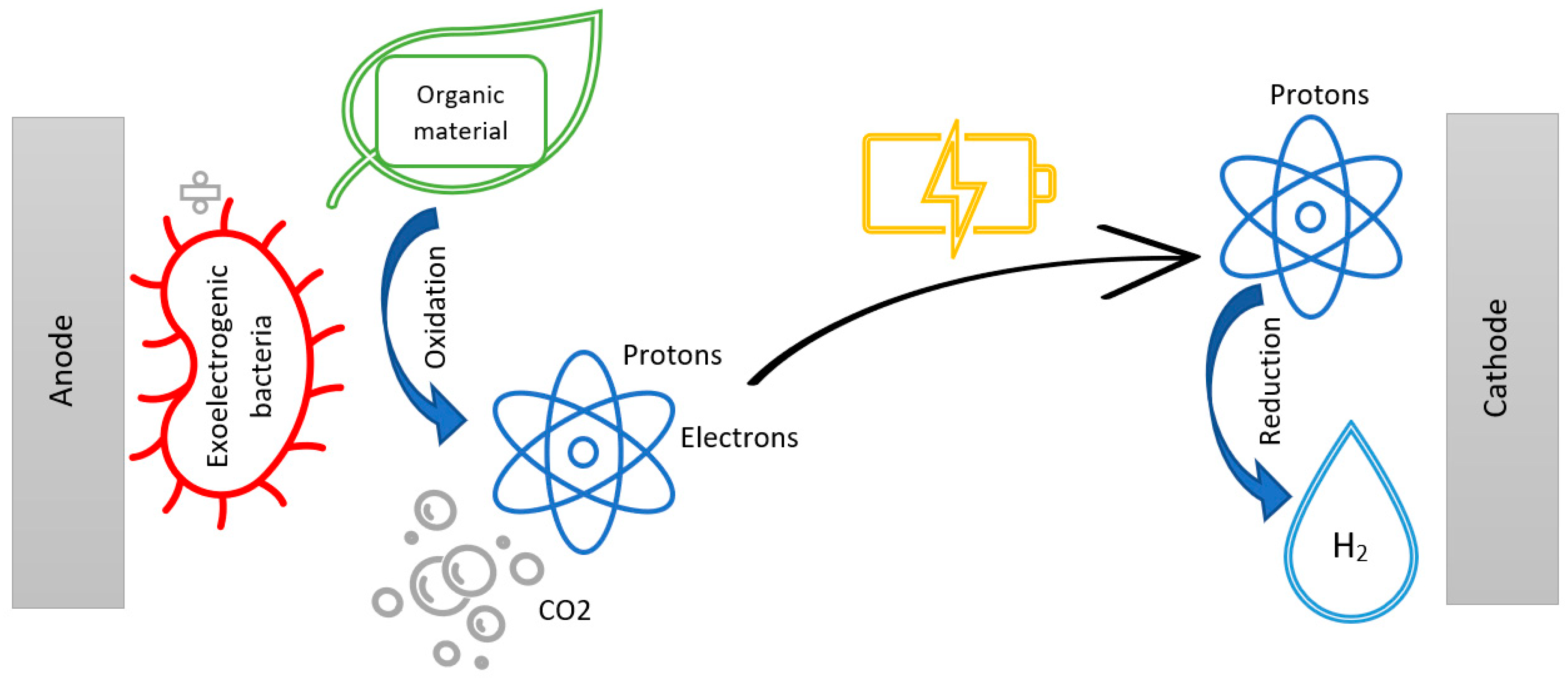
Figure 3. An illustration of the mechanism in the MEC chamber during electron transfer.
The applied small current ensures the transfer of electrons from the anode to the cathode being fused to produce biohydrogen and reducing protons. The system should be kept under strictly anaerobic conditions at both the anode and cathode, since aerobic conditions at the cathode produce water and electricity, thus converting the setup into an MFC [37][39].
Electrogenic bacteria are bacteria capable of transferring electrical charges from or to an electrode [45]. Electrogenic bacteria possess the most critical role in hydrogen production through MEC technology. Thus, the choice and type of exoelectrogenic bacteria are essential to ensure ideal conditions for optimized hydrogen production. Several microbial species have been documented as electrogenic bacteria, which are useful in MEC systems. Mixed microbial communities are often utilized for MECs, since studies have proven that their electroactive efficiency compared with pure bacterial cultures is higher when used in mixed cultures [46][47].
Methanogens are another niche of microbes that are encountered and found in MECs with mixed cultures. Methanogens facilitate biofilm formation at the cathode and use the hydrogen and electrons to produce methane, decreasing the yield and contaminating the hydrogen. As the MEC is operated anaerobically, the presence of methanogens such as the Methanobrevibacter species is inevitable when using mixed cultures [39][48]. However, although methanogens are often found in MECs, especially during biofilm formation, electrogenic bacteria capable of producing current densities are more relevant and desired when aiming to produce hydrogen through MEC.
Table 1 shows and summarizes some commonly found electrogenic species in MEC systems. The table presents relevant information on the bacterial description and its function in MEC.
Table 1. Overview of commonly found bacteria in MECs and MFCs, including several electrogenic bacteria, each with its biological description and function in MEC.
| Bacteria | Biological Description | Function in Relation to MECs | Electrogenic Properties | Source | |||
|---|---|---|---|---|---|---|---|
| Gram (+/−) | Oxygen | Other | Proteo Bacteria | ||||
| Geobacter genus | − | Anaerobic | Chemo-lithotrophic; Iron-reducing |
Delta- probact |
Geobacter transfers electrons onto the surface of electrodes, which can produce electricity out of waste organic matter. Commonly found species in MECs. | Present | [45][49][50][51][52][53] |
| Shewanella genus | − | Facultative anaerobic | Found in aquatic or marine life | Gamma- probact |
The Shewanella species use a variety of compounds as electron acceptors, including oxygen, iron, manganese, uranium and nitrate. Commonly found species in MECs. |
Present | [45][49][54][55][56] |
| Desulfovibrio genus | − | Anaerobic | Sulphate-reducing | Delta- probact |
The Desulfovibrio species are identified in MFCs and MECs when the biocathode is enriched as an anode fed with H2 and acetate. Multiple articles suggest that the Desulfovibrio species may be a significant player in biohydrogen production. Consume and produce H2. | Present | [57][58] |
| Clostridium genus | + | Anaerobic | Mesophilic Known to produce H2 | - | Researchers have studied MFCthe power generation performance using Clostridium species of Gram-positive bacteria. | N/A | [53][55][59][60] |
| Firmicutes phylum | + (−) | Aerobic or facultative anaerobic | Phylum divided into clostridium and bacillus | - | Studies have shown that Bacillus subtilis and Bacillus megaterium are among the best-performing electrogenic bacteria. | Present | [61][62] |
| Pseudomonas genus | − | Aerobic and anaerobic | Rod-shaped | Gamma- probact |
Transfer electrons to the electrode via self-produced phenazine-based mediators. | Present | [53][63][64] |
| Nitrospirota phylum | +/− | Aerobic | Chemo-litho-autotrophic, found in marine life |
- | Nitrite-oxidizing. Nitrospirae spp. Are distantly related to the thermophilic and sulfate-reducing thermosdesulfovibrio spp. | N/A | [51][65][66] |
| Actinomycetota phylum | + | Anaerobic or aerobic. | Either terrestrial or aquatic | - | The marine actinobacterial strain Actinoalloteichus spp. is capable of generating bioelectricity. | N/A | [51][67][68] |
| Rhodobacter genus | − | Anaerobic or aerobic | Found in freshwater or marine life | Alpha-probact | Rhodobacter sphaeroides is known for its capability of using a wide variety of substrates and its high activity in hydrogen production under anaerobic conditions, including high electricity production in MFC. | N/A | [53][69][70][71][72] |
| Desulforomonas genus | − | Anaerobic | Sulfur/sulphate-reducing | Delta- probact |
Desulfuromonas is a distinct phylogenetic cluster (one of three, the others being Geobacter and Dulfuromusa). | N/A | [65][73] |
| Bacteroidetes phylum | − | Anaerobic or aerobic | Rod-shaped | - | Bacteroides is one of the most abundant genera found in MEC and possesses extracellular electron transfer abilities. | Present | [51][53][55][56][74] |
The most abundant bacteria observed in MEC are proteobacteria, firmicutes and bacteroides. The microbial analyses of several studies have shown that proteobacteria are abundant in 20% to 46% of the total microbial communities found in MFCs and MECs. One study investigated the distribution of different proteobacteria in MFC inoculated with a sediment–water slurry and found that the most abundant classes were the gamma- (62.4%), epsilon- (19.3%) and beta-proteobacteria (17.6%), whereas alpha- (0.65%) and delta-paroteobacteria (<0.1%) were also present in smaller degrees [75]. Another similar study observed that the distribution of proteobacteria consisted of gamma-proteobacteria (19%), epsilon-proteobacteria (2%), delta-proteobacteria (1%) and alpha-proteobacteria (0.9%) [76]. These findings indicate that gamma-proteobacteria are common electrogenic bacteria in MEC and MFC. Firmicutes have been observed in the range of 25% to 32%, whereas bacteroides have been observed in the range 17% to 36% in MFCs and MECs [49][50][51][52][53][54][55][56][59][60][63][65][70][72][74][75][77][78].
Electrogenic bacteria, such as Geobacter and Shewanella, are characterized as extracellular electron transferors (EETs), i.e., they are capable of donating or accepting electrons. This ability to transfer electrons is utilized in MEC for hydrogen production, as the electrons utilized in those reactions are delivered by electrogenic bacteria [79][80].
The chemical description of biohydrogen production at the electrodes includes the hydrogen evolution reaction (HER). The HER comprises multiple reactions in which the first step is the electrochemical Volmer reaction, followed by a chemical Tafel reaction or an electrochemical Heyrovský reaction [81]. The HER starts with the Volmer reaction, where protons are coupled to electrons on the electrode’s metal surface (MS), producing hydrogen ions bound to the surface.
The next step may involve either the Tafel reaction or the Heyrovský reaction. The Tafel reaction utilizes two different molecules of hydrogen bound to a metal surface, which are liberated into hydrogen gas (H2).
The alternative Heyrovský reaction utilizes protons soluble in the substrate and combines them with the hydrogen attached to the metal surface to produce hydrogen gas (H2) [82][83].
The productivity can be affected by numerous factors, including the MEC design; however, the choice and profile of the substrate and feedstock might be underestimated, since the type and accessibility of carbohydrates have proven essential to the efficiency and operation of a MEC [84][85].
2.2. Anode Material
The material used for the anode can heavily affect the MEC’s performance. MEC has three active materials responsible for converting feedstock to biohydrogen, i.e., the anode, cathode and membrane. These active materials can be configured to accommodate a wide range of feedstock and microbial cultures. Most research suggests that the limiting parameter is coupled to the bacterial activity at the anode [86] or the catalysis and availability of protons at the cathode [87].
The common factor of anodes is the coupling of the anode to a more conductive metal, thereby allowing a concentrated flow of electrons, ensuring bonding and thriving microbes. The bonding is facilitated when the right functional groups are attached to the anode [88]. Attachment to the electrode mainly occurs via the adsorption or entrapment of the microbial species. Both can be affected by the anode’s surface properties, roughness and porosity [89]. Furthermore, a successful anode requires a strong and resilient construction with a developed surface area, still allowing for substrate flow and high proton and electron productivity [90][91]. However, biocompatibility is vital for achieving the required microbial adhesion. Therefore, the presence of molecular affinity, chemical bonding or electrostatic forces is necessary [88].
Carbon and metal-based anodes are commonly used in MECs as an optional mix in which the metal is used to conduct the current [57][92][93]. Carbon-based anodes comprise carbon felt [94][95][96], carbon cloth [97][98], graphite (as felt, plate or fiber) [99][100][101], carbon fiber, carbon brushes, carbon mesh, carbon paper, activated carbon (granules), graphite granules, etc. (as seen in Table 2). When investigating the differences between carbon and graphite materials, it should be noted that some studies state that graphite materials are carbon, as it is an allotrope of carbon. Purely metal-based anodes consists of stainless steel (mesh [102], sheets [103] and scrubbers [104]), nickel [105], silver [106], titanium [107], copper [108], etc.
Table 2. Pictures of most applied carbon-based materials used in MEC.
Carbon felt exhibits a high porous structure while being an excellent electric conductor. Carbon felt is also a low-cost anode with a sizeable flexible surface area when considering its flat-plate surface [109][115]. Carbon cloth possesses high mechanical strength, but as it is a dense structure, clogging is a potential hazard [116]. Carbon cloth is also an expensive carbon-based anode, especially for larger-scale MECs. A carbon brush is a carbon-based anode with a large surface area per unit of volume. These brushes are often based on a titanium wire, which increases the conductivity compared with other carbon materials and increases the overall price. The carbon brush has been proposed as a next-generation anode since its open structure allows for a high surface area with a lower chance of clogging [90][116]. Carbon mesh can be used as an alternative to carbon cloth, which can be an expensive anode. Carbon mesh has a low cost but possesses many of the same properties as a carbon cloth. Carbon mesh is less prone to clogging due to its more open structure [117]. Carbon paper has a paper-like structure, which offers a slightly porous structure, but it has not been assessed to be an optimal solution for larger-scale operations, as the material is fragile [118][119]. Activated carbon granules have a large surface area and porous structure, promoting biofilm formation. Carbon granules are also a low-cost material but have the potential to clog. The conductivity of carbon granules is often relatively low, as the granules have a poor connection to the current collector. However, the granules have another property that can be exploited, as the structure allows for easier entrapment of pollutants, such as heavy metals [120].
Stainless steel is another material used as an anode; however, the material is constricted, as the surface area is reduced compared to carbon-based anodes. Stainless steel can be used with other materials such as graphene to increase biofilm formation [121]. Stainless steel is one of the cheapest materials used for anodes and has excellent electric conduction, exhibiting strong mechanical strength and corrosion resistance and having a long lifetime. Other materials such as nickel [105], silver [106], titanium [122] and copper [123] have also been tested as anode materials, but in their pure forms, these materials lack the ability of microbial adhesion. However, most can use a combination of materials, such as a carbon-based anodes modified with a nickel coating [105]. Both copper and nickel ions can be poisonous for microbes and are undesirable when released into, e.g., wastewater, which makes these materials less ideal, as it constrains the environmental aspect of the MEC design.
Most bacteria’s outer membrane consists of proteins and amino acids which are negatively charged. Thus if the anode material can be configured to be more positively charged by embedding nitrogen molecules in the structure by nitrogen doping, biofilm formation can be enhanced and promoted [124]. The chemical bonding between C-, N-, O- and S-containing functional groups can be exploited when optimizing the anode.
Y. Zhau et al. (2018) [115] investigated the modification of carbon felt anodes by double oxidant HNO3/H2O2 and found it to decrease the inoculation period and to increase electron production for the anodes [115]. Another study by S. Rozenfeld et al. (2019) [86] investigated how to improve anode activity by utilizing two different anode materials: plasma-pretreated carbon cloth and stainless steel. The study found that plasma pretreated anodes, with the introduction of nitrogen doping atoms, increased -C-OH, -COOH, and =C=O groups, and this relationship was found to increase both biofilm formation and the current in biohydrogen production.
Already known surface modifications have proven to increase the productivity of electrolysis cells with methods as simple as utilizing the heating of the material with ammonia, giving currents of 2400 mW · m−2 [125] and hydrogen production of 7.4 L H2 · L−1 · day−1 [126].
When comparing anode materials with and without surface modification, increased productivity was found for surface-modified anodes, cf. Table 3. As surface modification substantially increases productivity, future studies should investigate optimal treatments allowing for high productivity for a long duration, which can feed into scalability for both the anodes and optimized surface modification.
Table 3. Summary of anode materials, their advantages/disadvantages, their modifications and their performance in an MEC/MFC setting.
| Advantages | Disadvantages | Surface Modification | MEC/MFC Performance | Reference | |
|---|---|---|---|---|---|
| Carbon felt | Good conductivity, large porosity | Expensive, potential for clogging | Polyaniline doped Isopropanol and biohydrogen peroxide treatment |
460 mW · m2 COD removal: 69 mg · L−1 · day−1 0.44 mmol CH4 · L−1 · day−1 |
[109] [96] |
| Carbon cloth | Flexible, large surface area, high mechanical strength, porous | Potential for clogging | Heated with ammonia gas None Heated with ammonia gas |
988 mW · m−2 0.69 L H2 · L−1 · day−1, 14 A · m−2 7.4 L H2 · L−1 · day−1, 607 A · m−2 |
[117] [127] [126] |
| Graphite felt | Good conductivity, large porosity, chemical resistance | Expensive, potential for clogging | None Not stated |
3.3 A · m−2 1.85 L H2 · L−1 · day−1 |
[128] [129] |
| Graphite plate | Good electric conductor, chemical resistance | Smooth surface, relatively expensive, low surface area | Treated with sandpaper and soaked in HCl 5.5–7.5% manganese incorporated |
120 A · m−3 17.9 L biogas · L−1 |
[130] [131] |
| Graphite fiber (brush) | Porous, high surface area, good current collection, chemical resistance | Must be integrated correctly due to fiber distances, which can create dead zones, and due to electrode spacing, which can be hard to make sufficiently small, expensive | Heated with ammonia gas Heated with ammonia gas Heated with ammonia gas |
2400 mW · m−2 3.12 L H2 · L−1 · day−1, 292 A · m−3 17.8 L H2 · L−1 · day−1, 1830 A · m−3 |
[125] [132] [126] |
| Carbon brush | Porous, high surface area, good current collection | Must be integrated correctly due to fiber distances, which can create dead zones, and due to electrode spacing, which can be hard to make sufficiently small, relatively expensive | None Heat-treated Soaked in an alkaline solution |
0.64 L biohythate · day−1 1270 mW · m−2 7.62 A · m−2 |
[133] [134] [135] |
| Carbon mesh | Cheaper than carbon cloth but has many of the same properties, less potential for clogging | Pretreatment can be necessary | Heated with ammonia gas | 1015 mW · m−2 | [117] |
| Carbon paper | Porous, thin | Fragile, can be expensive, smooth surface | Nitrogen-doped carbon dots-supported Electrodeposition of cobalt |
0.32 mW 710 mA · m−2 |
[118] [136] |
| Activated carbon granules | High surface area, relatively cheap, can be used for bioremediation | Potential for clogging, current collection can be challenging | None | 0.9 mW power output | [137] |
| Graphite granules | High surface area (smaller than carbon granules), relatively cheap, can be used for bioremediation | Potential for clogging, current collection can be challenging | Washed with diluted HCl | 137.37 mW · m−2 | [138] |
| Stainless steel | Can be combined with other materials for enhanced efficiency, a low cost, excellent conductivity, and resistance | Low surface area, smooth surface, low bacterial adhesion, surface treatment is necessary | None—microstructure stainless steel plate was used Anodized in mesh form |
21.5 A · m−2 430 mW · m−2 |
[139] [140] |
For MEC to become commercially successful, the anode material must have a low cost with stable operation over time. The anode must be environmentally friendly and have strong mechanical strength [90][120]. The most commonly used anode material capable of complying with the success criteria is the carbon-based anode, which has been used in many studies [90][120][141]. Carbon-based anodes have the advantage of good attachment for electrogenic microbial species, and some exhibit a large surface area in a small volume due to their 3D structure. However, this can also be a problem, as the pores can be affected by clogging, thereby restricting flow. This decreases electron transfer and biohydrogen production, resulting in the usage of 2D carbon anodes.
Anodes are typically defined as 2D or 3D structures depending on the surface. Three-dimensional structures have been preferred in a laboratory scale and have shown the highest biohydrogen production and current density, as seen in Table 3. However, a study conducted by E. Blanchet et al. (2016) [142] mimicked an industrial MEC operation based on food waste and wastewater, where 3D (carbon felt) and 2D (carbon cloth) anodes were used for comparison. The study found that the 3D structure performed worse than the 2D structure, as there was no clogging in the 2D structure. This contradicts previous studies, suggesting that 3D anodes are not always the preferred choice [142]. This could especially be relevant for the upscaling process, as larger facilities are able to implement 2D anodes with a faster flow, ultimately creating the potential for higher production of protons and electrons. Therefore, 2D structures show an interesting approach when dealing with larger particle substrates, as the chance of clogging decreases.
An investigation into 2D structured anodes was conducted by D. Pocaznoi et al. (2012) [139], comparing the following anode materials: stainless steel, flat-plate graphite and carbon cloth. The study found that carbon-based materials increased biofilm formation compared with stainless steel. Comparing the flat-plate graphite with stainless steel, no difference in biofilm formation was found, but the stainless steel had twice the current density. Comparing the stainless steel with the carbon cloth, a higher current density was exhibited by the carbon cloth. The study concluded that the topography of stainless steel prevented it from being the preferred anode material, but surface treatment can be used to increase the current density [139]. Therefore, combining the electric properties of stainless steel with a biofilm-promoting carbon material can provide anodes with higher hydrogen productivity.
No single anode material has been able to satisfy all criteria for an excellent industrial and environmentally friendly anode while still being economically favorable. Carbon-based materials with a metal current collector outperform their solely metal-based counterparts, but few have provided sufficient biohydrogen production. Almost all studies have used surface modification on their anode in one form or another, and it seems generally accepted that surface modifications increase the performance of almost all materials, as shown in Table 3 [90][117][120][143]. Surface treatments are worth exploring, where treatments with low environmental effects and prices can bring the anodes closer to a viable industrial MEC. Additionally, surface treatment and combinations with other anode materials could make the metals a viable candidate in the future, especially considering the costs and operation times of anodes.
2.3. Cathode Material
To drive the HER reaction in an MEC, a catalyst is needed, predominantly implemented as a catalyst coating/catalytic material or by using microbes to drive catalysis [39][43][144][145]. Like the anode, the cathode must exhibit different properties to be a viable candidate for an industrially applicable MEC. These properties include fast catalysis, low overpotential, a low cost and a long operation time [146].
Cathodes can be based on current-collecting materials or carbon-based materials, used as a basis for the structure and electron transportation. Materials used as an anode can be used as a base; the difference is denoted by incorporating a catalyst. Traditional catalysts, such as noble metals, e.g., platinum or palladium, incorporated as a coating can also be used due to stable catalytic activity. Platinum-based cathodes are well established and known for their good catalytic properties and low degree of overpotential [55][56]. However, using platinum has the drawbacks of a negative impact on the environment and a high cost; however, it is often used as a baseline for comparison [147]. Platinum can be a viable option for lab-scale MECs, but the use of complex substrates for larger operations would require protection from sulfide poisoning from platinum, which reduces its catalytic effectiveness [147][148]. Palladium-based catalysts have been explored as an alternative used as a coating, as palladium is more resilient and can be acquired at a lower cost [149].
Nickel has been proposed as an alternative to platinum, as it exhibits good catalytic activities and high corrosion resistance at a low cost [150][151]. Nickel is often used as an alloy, as it increases the active surface area and lowers the overpotential, as explained in a study by A. Jeremiasse et al. (2010) [150]. The study found that nickel foam decreased the overpotential and achieved production of 50 L H2 · L−1 · day−1 [150]. Nickel foam also showed potential as a cathode base utilized with a platinum coating [152]. Nickel foam can be used as a versatile cathode at a low cost and can be a cheap alternative to platinum cathodes as a baseline comparison for future studies. This was investigated by Z. Yan et al. (2012) [153], who combined a low concentration of platinum and nickel nanoparticles and compared the performance to a Pt/C catalyst. The study found that the new nickel alloy was stable and could produce current densities in the same range at a quarter of the price [153]. This tendency aligns with other findings [154][155], as shown in Table 4, but the toxicity of nickel ions should be investigated before upscaling. Nickel foam has the advantage of easier scalability, as the material can be found in nickel metal hydride (NIMH) batteries produced on a large scale, and the material is therefore readily available.
Many of the highest-performing MECs have been constructed based on expensive catalysts, which are rarely viable in an industrial setting. However, research with biotic cathodes has started to increase HER productivity and current density, enabling the use of microbial species instead of a catalyst [68][90]. Biotic cathodes are an inexpensive catalyst and can be used for industrial settings with a low maintenance cost, as the biofilm driving the biotic catalysis is replenished by itself. However, using a biotic cathode, especially in a one-chamber configuration, also creates the possibility of cultivating methanogens. Methanogens can be eliminated or decreased by utilizing some of the following tactics: using a pure culture, exposing electrodes to oxygen before each batch, temperature shock, the addition of beryllium sulfide or by modifying the MEC design [68]. Furthermore, biocathodes have thus far not been reviewed as the highest-producing MEC [147], and thus, a cost–benefit analysis should be implemented to ensure their viability.
Evaluating the optimal cathode material and catalyst is a complex decision, but it should focus on cost, efficiency and scalability. The basis of the cathode has not been proven to be the deciding factor and does not affect hydrogen production in the same manner as the catalyst. As the base material constitutes a large fraction of the total cathode, investigation into the structural effect when scaling from small MECs to larger systems should be investigated, as larger MECs would require a more robust system. The focus aims to find new catalysts or to optimize previous catalysts to decrease costs and improve catalytic properties.
If a biotic cathode is utilized, this enables the production of pure biohydrogen, where methanogens can also be cultivated, leading to hydrogen loss. The presence of methanogens can be prevented, and it should not be the deciding factor. Nickel alloys provide a cheaper alternative to platinum catalysts coupled with HER-catalyzing microbial species. Nickel alloys outperform platinum-based alternatives and are becoming well studied at the laboratory-scale, as seen in Table 4. Thus far, biotic cathodes have experienced lower hydrogen productivity than platinum-based cathodes, and methods enabling higher productivity, either through biofilms, cultures or material optimization, must be explored.
Table 4. Summary of cathode materials, their advantages/disadvantages, catalytic loading and their performance in an MEC/MFC setting.
| Cathode Material | Catalyst Loading In mg · cm−2 |
Advantages | Disadvantages | MEC/MFC Performance | Reference |
|---|---|---|---|---|---|
| Pt-Co/G (15 wt.% Pt) on carbon cloth | 2.5 | Less Pt needed compared with Pt/C coating | Pt is expensive and would need repleting | 1378 mW · m−2 | [153] |
| FePC-supported multiwalled carbon nanotubes on carbon cloth | 1 | Alternative to Pt, cheaper than Pt | Carbon nanotubes need further investigation to become a stable cathode | 601 mW · m−2 | [156] |
| MnOx on carbon paper | 0.1 | Alternative to Pt and cheaper than Pt, low catalyst loading needed | - | 772.8 mW · m−3 | [157] |
| Stainless steel 306 (12% Ni) | - | Ni incorporated into stainless steel enables the catalysis of HER | Investigated for water electrolysis | - | [158] |
| Ni/AC/PTFE-coated stainless steel mesh | 6.5 | Ni can substitute noble catalysts with high activity, high porosity | Ni ions can be poisonous | 1.88 L H2 · L−1 · day−1 | [151] |
| Ni2P/C coated on stainless steel mesh | 0.5 | Large chemical stability, HPR as high as Pt-based cathodes | 0.29 L H2 · L−1 · day−1 | [159] | |
| Pt-coated carbon cloth Pt-coated nickel foam |
0.5 0.5 |
Reliable, efficient, long operation time Cheaper and higher HPR than similar Pt-coated carbon cloth, porous |
Week base, expensive catalyst Not stable in the same duration as Pt-coated carbon cloth, relatively expensive |
0.67 L H2 · L−1 · day−1 0.71 L H2 · L−1 · day−1 |
[152] |
| Nickel foam | - | High productivity, large surface area enables fast catalysis. | Problems connected to scaling, quick decrease in MEC performance | 50 L H2 · L−1 · day−1 | [150] |
| Biotic based on wastewater incorporated on stainless steel mesh | - | Cheap, environmentally friendly, long operation time | Not as effective as Pt or similar catalysts | 240 A · m−3 | [130] |
| Biotic based on urban wastewater and MFC inoculum incorporated on granular graphite | - | Cheap, environmentally friendly, long operation time, found to be as effective or more effective than carbon-based cathodes | Relatively low HPR | 0.9 L H2 · L−1 NCC · day−1 | [144] |
| Pd/GO-C incorporated on carbon paper | 0.25 | Cheap compared to Pt, efficient catalyst | Expensive compared to nickel or stainless steel | 901 mW · m−2 | [149] |
This entry is adapted from the peer-reviewed paper 10.3390/en15228396
References
- He, Y. How Population Growth Impacts Energy Consumption in Guangdong in China; Research Features: Stonehouse, UK, 2019.
- Hopfenberg, R.; Pimentel, D. Human Population Numbers as a Function of Food Supply. Environ. Dev. Sustain. 2001, 3, 1–15.
- Shindell, D. Global Methane Assessment: Benefits and Costs of Mitigating Methane Emissions; Climate & Clean Air Coalition: Paris, France, 2021.
- Solomon, S.; Qin, D.; Manning, M.; Chen, Z.; Marquis, M.; Averyt, K.B.; Tignor, M.M.B.; Miller, H.L., Jr. Climate Change the Physical Science Basis; IPCC: Geneva, Switzerland, 2007.
- Malinauskaite, J.; Jouhara, H.; Czajczyńska, D.; Stanchev, P.; Katsou, E.; Rostkowski, P.; Thorne, R.J.; Colón, J.; Ponsá, S.; Al-Mansour, F.; et al. Municipal Solid Waste Management and Waste-to-Energy in the Context of a Circular Economy and Energy Recycling in Europe. Energy 2017, 141, 2013–2044.
- Acar, C.; Dincer, I. 4.24 Hydrogen Energy Conversion Systems. In Comprehensive Energy Systems; Elsevier: Oxford, UK, 2018; Volume 4, pp. 947–984. ISBN 978-0-12-814925-6.
- International Energy Agency. The Future of Hydrogen; International Energy Agency: Paris, France, 2019.
- Dincer, I.; Acar, C. Review and Evaluation of Hydrogen Production Methods for Better Sustainability. Int. J. Hydrogen Energy 2015, 40, 11094–11111.
- Pipitone, G.; Zoppi, G.; Pirone, R.; Bensaid, S. A Critical Review on Catalyst Design for Aqueous Phase Reforming. Int. J. Hydrogen Energy 2022, 47, 151–180.
- Tak, S.S.; Shetye, O.; Muley, O.; Jaiswal, H.; Malik, S.N. Emerging Technologies for Hydrogen Production from Wastewater. Int. J. Hydrogen Energy, 2022; in press.
- Brown, F.; Roberts, D. Green, Blue, Brown: The Colours of Hydrogen Explained. Available online: https://blog.csiro.au/green-blue-brown-hydrogen-explained/ (accessed on 5 October 2021).
- Nikolaidis, P.; Poullikkas, A. A Comparative Overview of Hydrogen Production Processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611.
- Daiyan, R.; MacGill, I.; Amal, R. Opportunities and Challenges for Renewable Power-to-X. ACS Energy Lett. 2020, 5, 3843–3847.
- International Energy Agency. Energy Technology Perspectivies 2020; International Energy Agency: Paris, France, 2020.
- Lindstrand, N. Unlocking Ammonia’s Potential for Shipping. Available online: https://www.man-es.com/discover/two-stroke-ammonia-engine (accessed on 5 October 2021).
- International Energy Agency. Global Hydrogen Review 2021; International Energy Agency: Paris, France, 2021.
- European Union 2030 Climate Target Plan. Available online: https://ec.europa.eu/clima/eu-action/european-green-deal/2030-climate-target-plan_en (accessed on 7 October 2021).
- de Vasconcelos, B.; Lavoie, J.-M. Recent Advances in Power-to-X Technology for the Production of Fuels and Chemicals. Front. Chem. 2019, 7.
- Thomas, G.; Parks, G. Potential Roles of Ammonia in a Hydrogen Economy; Office of Energy Efficiency & Renewable Energy: Washington, DC, USA, 2006.
- Ghavam, S.; Vahdati, M.; Wilson, I.A.G.; Styring, P. Sustainable Ammonia Production Processes. Front. Energy Res. 2021, 9, 34.
- Smith, C.; Hill, A.K.; Torrente-Murciano, L. Current and Future Role of Haber–Bosch Ammonia in a Carbon-Free Energy Landscape. Energy Environ. Sci. 2020, 13, 331–344.
- Amar, I.A.; Lan, R.; Petit, C.T.G.; Tao, S. Solid-State Electrochemical Synthesis of Ammonia: A Review. J. Solid State Electrochem. 2011, 15, 1845.
- Tullo, A.H. Is Ammonia the Fuel of the Future? Chem. Eng. News 2021, 99, 20–22.
- Minic, D.C.E.-D. Ammonia as a Hydrogen Source for Fuel Cells: A Review; IntechOpen: Rijeka, Croatia, 2012; p. 13.
- Lan, R.; Irvine, J.T.S.; Tao, S. Ammonia and Related Chemicals as Potential Indirect Hydrogen Storage Materials. Int. J. Hydrogen Energy 2012, 37, 1482–1494.
- Eberle, U.; Felderhoff, M.; Schüth, F. Chemical and Physical Solutions for Hydrogen Storage. Angew. Chemie Int. Ed. 2009, 48, 6608–6630.
- Grochala, W.; Edwards, P.P. Thermal Decomposition of the Non-Interstitial Hydrides for the Storage and Production of Hydrogen. Chem. Rev. 2004, 104, 1283–1316.
- Satyapal, S.; Petrovic, J.; Read, C.; Thomas, G.; Ordaz, G. The U.S. Department of Energy’s National Hydrogen Storage Project: Progress towards Meeting Hydrogen-Powered Vehicle Requirements. Catal. Today 2007, 120, 246–256.
- Sartbaeva, A.; Kuznetsov, V.L.; Wells, S.A.; Edwards, P.P. Hydrogen Nexus in a Sustainable Energy Future. Energy Environ. Sci. 2008, 1, 79–85.
- Jain, I.P.; Jain, P.; Jain, A. Novel Hydrogen Storage Materials: A Review of Lightweight Complex Hydrides. J. Alloys Compd. 2010, 503, 303–339.
- Hu, Y.H.; Zhang, L. Hydrogen Storage in Metal–Organic Frameworks. Adv. Mater. 2010, 22, E117–E130.
- Graetz, J. New Approaches to Hydrogen Storage. Chem. Soc. Rev. 2009, 38, 73–82.
- Makepeace, J.W.; He, T.; Weidenthaler, C.; Jensen, T.R.; Chang, F.; Vegge, T.; Ngene, P.; Kojima, Y.; de Jongh, P.E.; Chen, P.; et al. Reversible Ammonia-Based and Liquid Organic Hydrogen Carriers for High-Density Hydrogen Storage: Recent Progress. Int. J. Hydrogen Energy 2019, 44, 7746–7767.
- Zamfirescu, C.; Dincer, I. Using Ammonia as a Sustainable Fuel. J. Power Sources 2008, 185, 459–465.
- Christensen, C.H.; Johannessen, T.; Sørensen, R.Z.; Nørskov, J.K. Towards an Ammonia-Mediated Hydrogen Economy? Catal. Today 2006, 111, 140–144.
- Zamfirescu, C.; Dincer, I. Ammonia as a Green Fuel and Hydrogen Source for Vehicular Applications. Fuel Process. Technol. 2009, 90, 729–737.
- Cotterill, S.; Heidrich, E.; Curtis, T. Microbial electrolysis cells for hydrogen production. Microb. Electrochem. Fuel Cells 2016, 287–319.
- Rahimnejad, M.; Adhami, A.; Darvari, S.; Zirepour, A.; Oh, S.-E. Microbial Fuel Cell as New Technology for Bioelectricity Generation: A Review. Alexandria Eng. J. 2015, 54, 745–756.
- Kadier, A.; Simayi, Y.; Abdeshahian, P.; Azman, N.F.; Chandrasekhar, K.; Kalil, M.S. A Comprehensive Review of Microbial Electrolysis Cells (MEC) Reactor Designs and Configurations for Sustainable Hydrogen Gas Production. Alexandria Eng. J. 2016, 55, 427–443.
- Kadier, A.; Yuliasni, R.; Sapuan, S.; Ilyas, R.A.; Rai, P.K.; Ma, P.-C.; Rajasekar, A.; Alabbosh, K.; Hamid, A.; Abu Hasan, H. The Role of Microbial Electrolysis Cell in Bioenergy Production: Current Applications and Pilot Plant Experiences. In Bioelectrochemical Systems; Springer: Singapore, 2021; pp. 323–342.
- Adekunle, A.; Raghavan, V.; Tartakovsky, B. A Comparison of Microbial Fuel Cell and Microbial Electrolysis Cell Biosensors for Real-Time Environmental Monitoring. Bioelectrochemistry 2019, 126, 105–112.
- Kadier, A.; Chaurasia, A.K.; Sapuan, S.M.; Ilyas, R.A.; Ma, P.C.; Alabbosh, K.F.S.; Rai, P.K.; Logroño, W.; Hamid, A.A.; Abu Hasan, H. Essential Factors for Performance Improvement and the Implementation of Microbial Electrolysis Cells (MECs). In Bioelectrochemical Systems; Springer: Singapore, 2021; pp. 139–168. ISBN 978-981-15-6871-8.
- Lim, S.S.; Fontmorin, J.-M.; Izadi, P.; Wan Daud, W.R.; Scott, K.; Yu, E.H. Impact of Applied Cell Voltage on the Performance of a Microbial Electrolysis Cell Fully Catalysed by Microorganisms. Int. J. Hydrogen Energy 2020, 45, 2557–2568.
- Katuri, K.P.; Ali, M.; Saikaly, P.E. The Role of Microbial Electrolysis Cell in Urban Wastewater Treatment: Integration Options, Challenges, and Prospects. Curr. Opin. Biotechnol. 2019, 57, 101–110.
- Sacco, N.J.; Bonetto, M.C.; Cortón, E. Isolation and Characterization of a Novel Electrogenic Bacterium, Dietzia Sp. RNV-4. PLoS ONE 2017, 12, e0169955.
- Chabert, N.; Amin Ali, O.; Achouak, W. All Ecosystems Potentially Host Electrogenic Bacteria. Bioelectrochemistry 2015, 106, 88–96.
- Call, D.F.; Wagner, R.C.; Logan, B.E. Hydrogen Production by Geobacter Species and a Mixed Consortium in a Microbial Electrolysis Cell. Appl. Environ. Microbiol. 2009, 75, 7579–7587.
- Cusick, R.D.; Bryan, B.; Parker, D.S.; Merrill, M.D.; Mehanna, M.; Kiely, P.D.; Liu, G.; Logan, B.E. Performance of a Pilot-Scale Continuous Flow Microbial Electrolysis Cell Fed Winery Wastewater. Appl. Microbiol. Biotechnol. 2011, 89, 2053–2063.
- Almatouq, A.; Babatunde, A.O.; Khajah, M.; Webster, G.; Alfodari, M. Microbial Community Structure of Anode Electrodes in Microbial Fuel Cells and Microbial Electrolysis Cells. J. Water Process Eng. 2020, 34, 101140.
- UniProt Proteomes-Geobacter Sp. (Strain M18). Available online: https://www.uniprot.org/proteomes/UP000001442 (accessed on 8 March 2021).
- Guo, Y.; Wang, J.; Shinde, S.; Wang, X.; Li, Y.; Dai, Y.; Ren, J.; Zhang, P.; Liu, X. Simultaneous Wastewater Treatment and Energy Harvesting in Microbial Fuel Cells: An Update on the Biocatalysts. RSC Adv. 2020, 10, 25874–25887.
- Babauta, J.; Renslow, R.; Lewandowski, Z.; Beyenal, H. Electrochemically Active Biofilms: Facts and Fiction. A Review. Biofouling 2012, 28, 789–812.
- Hari, A.R.; Venkidusamy, K.; Katuri, K.P.; Bagchi, S.; Saikaly, P.E. Temporal Microbial Community Dynamics in Microbial Electrolysis Cells–Influence of Acetate and Propionate Concentration. Front. Microbiol. 2017, 8, 1371.
- Proteomes-Shewanella Sp. (Strain ANA-3). Available online: https://www.uniprot.org/proteomes/UP000002589 (accessed on 8 March 2021).
- Hodgson, D.M.; Smith, A.; Dahale, S.; Stratford, J.P.; Li, J.V.; Grüning, A.; Bushell, M.E.; Marchesi, J.R.; Avignone Rossa, C. Segregation of the Anodic Microbial Communities in a Microbial Fuel Cell Cascade. Front. Microbiol. 2016, 7, 699.
- Tahernia, M.; Plotkin-Kaye, E.; Mohammadifar, M.; Gao, Y.; Oefelein, M.R.; Cook, L.C.; Choi, S. Characterization of Electrogenic Gut Bacteria. ACS Omega 2020, 5, 29439–29446.
- Croese, E.; Jeremiasse, A.W.; Marshall, I.P.G.; Spormann, A.M.; Euverink, G.-J.W.; Geelhoed, J.S.; Stams, A.J.M.; Plugge, C.M. Influence of Setup and Carbon Source on the Bacterial Community of Biocathodes in Microbial Electrolysis Cells. Enzym. Microb. Technol. 2014, 61–62, 67–75.
- Walker, C.B.; He, Z.; Yang, Z.K.; Ringbauer, J.A.; He, Q.; Zhou, J.; Voordouw, G.; Wall, J.D.; Arkin, A.P.; Hazen, T.C.; et al. The Electron Transfer System of Syntrophically Grown Desulfovibrio vulgaris. J. Bacteriol. 2009, 191, 5793–5801.
- Figueiredo, G.G.O.; Lopes, V.R.; Romano, T.; Camara, M.C. Clostridium. In Beneficial Microbes in Agro-Ecology; Academic Press: Cambridge, MA, USA, 2020; pp. 477–491. ISBN 978-0-12-823414-3.
- Juang, D.F.; Yang, P.C.; Lee, C.H.; Hsueh, S.C.; Kuo, T.H. Electrogenic Capabilities of Gram Negative and Gram Positive Bacteria in Microbial Fuel Cell Combined with Biological Wastewater Treatment. Int. J. Environ. Sci. Technol. 2011, 8, 781–792.
- Milner, D.A.; Pecora, N.; Solomon, I.; Soong, T.R. (Eds.) Bacillus Species Infections. In Diagnostic Pathology: Infectious Diseases; Elsevier: Philadelphia, PA, USA, 2015; pp. II-2-2–II-2-5. ISBN 978-0-323-37677-8.
- Kamaraj, Y. Electrogenicity Assessment of Bacillus Subtilis and Bacillus Megaterium Using Microbial Fuel Cell Technology. Int. J. Appl. Res. 2015, 1, 435–438.
- Logan, B.E.; Call, D.; Cheng, S.; Hamelers, H.V.M.; Sleutels, T.H.J.A.; Jeremiasse, A.W.; Rozendal, R.A. Microbial Electrolysis Cells for High Yield Hydrogen Gas Production from Organic Matter. Environ. Sci. Technol. 2008, 42, 8630–8640.
- Pham, T.H.; Boon, N.; Aelterman, P.; Clauwaert, P.; De Schamphelaire, L.; Vanhaecke, L.; De Maeyer, K.; Höfte, M.; Verstraete, W.; Rabaey, K. Metabolites Produced by Pseudomonas Sp. Enable a Gram-Positive Bacterium to Achieve Extracellular Electron Transfer. Appl. Microbiol. Biotechnol. 2008, 77, 1119–1129.
- Gatidou, G.; Samanides, C.G.; Fountoulakis, M.S.; Vyrides, I. Microbial Electrolysis Cell Coupled with Anaerobic Granular Sludge: A Novel Technology for Real Bilge Water Treatment. Chemosphere 2022, 296, 133988.
- Zecchin, S.; Mueller, R.C.; Seifert, J.; Stingl, U.; Anantharaman, K.; von Bergen, M.; Cavalca, L.; Pester, M. Rice Paddy Nitrospirae Carry and Express Genes Related to Sulfate Respiration: Proposal of the New Genus “Candidatus Sulfobium”. Appl. Environ. Microbiol. 2018, 84, e02224-17.
- Gobalakrishnan, R.; Bhuvaneswari, R. Microbial fuel cells potential of marine actinobacteria Actinoalloteichus sp. MHA15 from the Havelock island of the Andamans, India. Biotechnol. Res. Innov. 2019, 3, 144–158.
- Hasany, M.; Mardanpour, M.M.; Yaghmaei, S. Biocatalysts in Microbial Electrolysis Cells: A Review. Int. J. Hydrogen Energy 2016, 41, 1477–1493.
- Ye, J.-Y.; Pan, Y.; Wang, Y.; Wang, Y.-C. Enhanced Hydrogen Production of Rhodobacter Sphaeroides Promoted by Extracellular H+ of Halobacterium Salinarum. Ann. Microbiol. 2021, 71, 15.
- Salar-Garcia, M.J.; Obata, O.; Kurt, H.; Chandran, K.; Greenman, J.; Ieropoulos, I.A. Impact of Inoculum Type on the Microbial Community and Power Performance of Urine-Fed Microbial Fuel Cells. Microorganisms 2020, 8, 1921.
- Cadirci, B.H. An electricity production study by Rhodobacter sphaeroides. Int. J. Hydrogen Energy 2018, 43, 18001–18006.
- Cho, Y.K.; Donohue, T.J.; Tejedor, I.; Anderson, M.A.; McMahon, K.D.; Noguera, D.R. Development of a Solar-Powered Microbial Fuel Cell. J. Appl. Microbiol. 2008, 104, 640–650.
- Holmes, D.E.; Nevin, K.P.; Lovley, D.R. Comparison of 16S RRNA, NifD, RecA, GyrB, RpoB and FusA Genes within the Family Geobacteraceae Fam. Nov. Int. J. Syst. Evol. Microbiol. 2004, 54, 1591–1599.
- Lu, L.; Xing, D.; Ren, N. Pyrosequencing Reveals Highly Diverse Microbial Communities in Microbial Electrolysis Cells Involved in Enhanced H2 Production from Waste Activated Sludge. Water Res. 2012, 46, 2425–2434.
- Uria, N.; Ferrera, I.; Mas, J. Electrochemical Performance and Microbial Community Profiles in Microbial Fuel Cells in Relation to Electron Transfer Mechanisms. BMC Microbiol. 2017, 17, 208.
- Cao, Y.; Mu, H.; Liu, W.; Zhang, R.; Guo, J.; Xian, M.; Liu, H. Electricigens in the Anode of Microbial Fuel Cells: Pure Cultures versus Mixed Communities. Microb. Cell Fact. 2019, 18, 39.
- Xie, X.; Yu, G.; Liu, N.; Bao, Z.; Criddle, C.S.; Cui, Y. Graphene–Sponges as High-Performance Low-Cost Anodes for Microbial Fuel Cells. Energy Environ. Sci. 2012, 5, 6862–6866.
- Khilyas, I.V.; Sorokin, A.A.; Kiseleva, L.; Simpson, D.J.W.; Fedorovich, V.; Sharipova, M.R.; Kainuma, M.; Cohen, M.F.; Goryanin, I. Comparative Metagenomic Analysis of Electrogenic Microbial Communities in Differentially Inoculated Swine Wastewater-Fed Microbial Fuel Cells. Scientifica (Cairo) 2017, 2017, 7616359.
- Yasri, N.; Roberts, E.P.L.; Gunasekaran, S. The Electrochemical Perspective of Bioelectrocatalytic Activities in Microbial Electrolysis and Microbial Fuel Cells. Energy Rep. 2019, 5, 1116–1136.
- Aiyer, K.S. How Does Electron Transfer Occur in Microbial Fuel Cells? World J. Microbiol. Biotechnol. 2020, 36, 19.
- Kadier, A.; Al-Shorgani, N.K.N.; Jadhav, D.A.; Sonawane, J.M.; Mathuriya, A.S.; Kalil, M.S.; Hasan, H.A.; Alabbosh, K.F.S. Microbial Electrolysis Cell (MEC): An Innovative Waste to Bioenergy and Value-Added By-product Technology. In Bioelectrosynthesis: Principles and Technologies for Value-Added Products; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2020; pp. 95–128.
- Bajracharya, S.; ElMekawy, A.; Srikanth, S.; Pant, D. Cathodes for Microbial Fuel Cells. In Microbial Electrochemical and Fuel Cells; Woodhead Publishing: Boston, MA, USA, 2016; pp. 179–213. ISBN 978-1-78242-375-1.
- Jeremiasse, A.W. Cathode Innovations for Enhanced H2 Production through Microbial Electrolysis; Wageningen University: Wageningen, The Netherlands, 2011.
- Velasquez-Orta, S.B.; Yu, E.; Katuri, K.P.; Head, I.M.; Curtis, T.P.; Scott, K. Evaluation of Hydrolysis and Fermentation Rates in Microbial Fuel Cells. Appl. Microbiol. Biotechnol. 2011, 90, 789–798.
- Montpart, N.; Baeza, M.; Baeza, J.A.; Guisasola, A. Low-Cost Fuel-Cell Based Sensor of Hydrogen Production in Lab Scale Microbial Electrolysis Cells. Int. J. Hydrogen Energy 2016, 41, 20465–20472.
- Rozenfeld, S.; Hirsch, L.O.; Gandu, B.; Farber, R.; Schechter, A.; Cahan, R. Improvement of Microbial Electrolysis Cell Activity by Using Anode Based on Combined Plasma-Pretreated Carbon Cloth and Stainless Steel. Energies 2019, 12, 1968.
- Borole, A.P.; Lewis, A.J. Proton Transfer in Microbial Electrolysis Cells. Sustain. Energy Fuels 2017, 1, 725–736.
- Li, C.; Cheng, S. Functional Group Surface Modifications for Enhancing the Formation and Performance of Exoelectrogenic Biofilms on the Anode of a Bioelectrochemical System. Crit. Rev. Biotechnol. 2019, 39, 1015–1030.
- Böl, M.; Ehret, A.E.; Bolea Albero, A.; Hellriegel, J.; Krull, R. Recent Advances in Mechanical Characterisation of Biofilm and Their Significance for Material Modelling. Crit. Rev. Biotechnol. 2013, 33, 145–171.
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial Fuel Cells: From Fundamentals to Applications. A Review. J. Power Sources 2017, 356, 225–244.
- Rinaldi, A.; Mecheri, B.; Garavaglia, V.; Licoccia, S.; Di Nardo, P.; Traversa, E. Engineering Materials and Biology to Boost Performance of Microbial Fuel Cells: A Critical Review. Energy Environ. Sci. 2008, 1, 417–429.
- Zhen, G.; Lu, X.; Kumar, G.; Bakonyi, P.; Xu, K.; Zhao, Y. Microbial Electrolysis Cell Platform for Simultaneous Waste Biorefinery and Clean Electrofuels Generation: Current Situation, Challenges and Future Perspectives. Prog. Energy Combust. Sci. 2017, 63, 119–145.
- Feng, Y.; Yang, Q.; Wang, X.; Liu, Y.; Lee, H.; Ren, N. Treatment of Biodiesel Production Wastes with Simultaneous Electricity Generation Using a Single-Chamber Microbial Fuel Cell. Bioresour. Technol. 2011, 102, 411–415.
- Zheng, D.; Gu, W.; Zhou, Q.; Zhang, L.; Wei, C.; Yang, Q.; Li, D. Ammonia Oxidation and Denitrification in a Bio-Anode Single-Chambered Microbial Electrolysis Cell. Bioresour. Technol. 2020, 310, 123466.
- Tartakovsky, B.; Manuel, M.-F.; Wang, H.; Guiot, S.R. High Rate Membrane-Less Microbial Electrolysis Cell for Continuous Hydrogen Production. Int. J. Hydrogen Energy 2009, 34, 672–677.
- Spiess, S.; Kucera, J.; Seelajaroen, H.; Sasiain, A.; Thallner, S.; Kremser, K.; Novak, D.; Guebitz, G.; Haberbauer, M. Impact of Carbon Felt Electrode Pretreatment on Anodic Biofilm Composition in Microbial Electrolysis Cells. Biosensors 2021, 11, 170.
- Gandu, B.; Rozenfeld, S.; Ouaknin Hirsch, L.; Schechter, A.; Cahan, R. Immobilization of Bacterial Cells on Carbon-Cloth Anode Using Alginate for Hydrogen Generation in a Microbial Electrolysis Cell. J. Power Sources 2020, 455, 227986.
- Chang, S.-H.; Huang, B.-Y.; Wan, T.-H.; Chen, J.-Z.; Chen, B.-Y. Surface Modification of Carbon Cloth Anodes for Microbial Fuel Cells Using Atmospheric-Pressure Plasma Jet Processed Reduced Graphene Oxides. RSC Adv. 2017, 7, 56433–56439.
- Roubaud, E.; Lacroix, R.; Da Silva, S.; Etcheverry, L.; Bergel, A.; Basséguy, R.; Erable, B. Benchmarking of Industrial Synthetic Graphite Grades, Carbon Felt, and Carbon Cloth as Cost-Efficient Bioanode Materials for Domestic Wastewater Fed Microbial Electrolysis Cells. Front. Energy Res. 2019, 7, 106.
- Clauwaert, P.; Verstraete, W. Methanogenesis in Membraneless Microbial Electrolysis Cells. Appl. Microbiol. Biotechnol. 2009, 82, 829–836.
- Croese, E.; Keesman, K.J.; Widjaja-Greefkes, H.C.A.; Geelhoed, J.S.; Plugge, C.M.; Sleutels, T.H.J.A.; Stams, A.J.M.; Euverink, G.-J.W. Relating MEC Population Dynamics to Anode Performance from DGGE and Electrical Data. Syst. Appl. Microbiol. 2013, 36, 408–416.
- Masoudi, M.; Rahimnejad, M.; Mashkour, M. Fabrication of Anode Electrode by a Novel Acrylic Based Graphite Paint on Stainless Steel Mesh and Investigating Biofilm Effect on Electrochemical Behavior of Anode in a Single Chamber Microbial Fuel Cell. Electrochim. Acta 2020, 344, 136168.
- Todoroki, N.; Wadayama, T. Electrochemical Stability of Stainless-Steel-Made Anode for Alkaline Water Electrolysis: Surface Catalyst Nanostructures and Oxygen Evolution Overpotentials under Applying Potential Cycle Loading. Electrochem. Commun. 2021, 122, 106902.
- Sonawane, J.M.; Patil, S.A.; Ghosh, P.C.; Adeloju, S.B. Low-Cost Stainless-Steel Wool Anodes Modified with Polyaniline and Polypyrrole for High-Performance Microbial Fuel Cells. J. Power Sources 2018, 379, 103–114.
- Tahir, K.; Miran, W.; Jang, J.; Maile, N.; Shahzad, A.; Moztahida, M.; Ghani, A.A.; Kim, B.; Jeon, H.; Lim, S.-R.; et al. Nickel Ferrite/MXene-Coated Carbon Felt Anodes for Enhanced Microbial Fuel Cell Performance. Chemosphere 2021, 268, 128784.
- Zakaria, B.S.; Barua, S.; Sharaf, A.; Liu, Y.; Dhar, B.R. Impact of Antimicrobial Silver Nanoparticles on Anode Respiring Bacteria in a Microbial Electrolysis Cell. Chemosphere 2018, 213, 259–267.
- Banerjee, A.; Calay, R.K.; Mustafa, M. Review on Material and Design of Anode for Microbial Fuel Cell. Energies 2022, 15, 2283.
- Zhu, X.; Logan, B.E. Copper anode corrosion affects power generation in microbial fuel cells. J. Chem. Technol. Biotechnol. 2013, 89, 471–474.
- Hidalgo, D.; Tommasi, T.; Bocchini, S.; Chiolerio, A.; Chiodoni, A.; Mazzarino, I.; Ruggeri, B. Surface Modification of Commercial Carbon Felt Used as Anode for Microbial Fuel Cells. Energy 2016, 99, 193–201.
- Purushotham, K.G.; Sendilvelan, S. Analysis of Microbial Fuel Cell for Energy Harvesting with Waste Water and Molasses. Int. J. Pharma Bio Sci. 2017, 8, 63–69.
- Fuel Cell Store. Available online: https://www.fuelcellstore.com/isomolded-plate-024 (accessed on 7 October 2021).
- Delord, B.; Neri, W.; Bertaux, K.; Derre, A.; Ly, I.; Mano, N.; Poulin, P. Carbon Nanotube Fiber Mats for Microbial Fuel Cell Electrodes. Bioresour. Technol. 2017, 243, 1227–1231.
- Amazon Microbial Fuel Cell Carbon Brush. Available online: https://www.amazon.com/Microbial-carbon-brush-conductive-5cm10cm15cm(LHW)/dp/B08154Z4C4 (accessed on 7 October 2021).
- Wei, J.; Liang, P.; Huang, X. Recent Progress in Electrodes for Microbial Fuel Cells. Bioresour. Technol. 2011, 102, 9335–9344.
- Zhao, Y.; Ma, Y.; Li, T.; Dong, Z.; Wang, Y. Modification of Carbon Felt Anodes Using Double-Oxidant HNO3/H2O2 for Application in Microbial Fuel Cells. RSC Adv. 2018, 8, 2059–2064.
- Baek, G.; Saikaly, P.E.; Logan, B.E. Addition of a Carbon Fiber Brush Improves Anaerobic Digestion Compared to External Voltage Application. Water Res. 2021, 188, 116575.
- Wang, X.; Cheng, S.; Feng, Y.; Merrill, M.D.; Saito, T.; Logan, B.E. Use of Carbon Mesh Anodes and the Effect of Different Pretreatment Methods on Power Production in Microbial Fuel Cells. Environ. Sci. Technol. 2009, 43, 6870–6874.
- Guan, Y.-F.; Zhang, F.; Huang, B.-C.; Yu, H.-Q. Enhancing Electricity Generation of Microbial Fuel Cell for Wastewater Treatment Using Nitrogen-Doped Carbon Dots-Supported Carbon Paper Anode. J. Clean. Prod. 2019, 229, 412–419.
- Santoro, C.; Guilizzoni, M.; Correa Baena, J.P.; Pasaogullari, U.; Casalegno, A.; Li, B.; Babanova, S.; Artyushkova, K.; Atanassov, P. The Effects of Carbon Electrode Surface Properties on Bacteria Attachment and Start up Time of Microbial Fuel Cells. Carbon N. Y. 2014, 67, 128–139.
- Escapa, A.; Mateos, R.; Martínez, E.J.; Blanes, J. Microbial Electrolysis Cells: An Emerging Technology for Wastewater Treatment and Energy Recovery. From Laboratory to Pilot Plant and Beyond. Renew. Sustain. Energy Rev. 2016, 55, 942–956.
- Saravanan, A.; Karishma, S.; Kumar, P.S.; Yaashikaa, P.R.; Jeevanantham, S.; Gayathri, B. Microbial Electrolysis Cells and Microbial Fuel Cells for Biohydrogen Production: Current Advances and Emerging Challenges. Biomass Convers. Biorefinery 2020.
- Michaelidou, U.; ter Heijne, A.; Euverink, G.J.W.; Hamelers, H.V.M.; Stams, A.J.M.; Geelhoed, J.S. Microbial Communities and Electrochemical Performance of Titanium-Based Anodic Electrodes in a Microbial Fuel Cell. Appl. Environ. Microbiol. 2011, 77, 1069–1075.
- Jeon, Y.; Kim, J.H.; Koo, K.; Kim, S. A Photo-Assisted Microbial Electrolysis Cell for the Exclusive Biohydrogen Production Using a MoS2-Coated p-Type Copper Oxide. J. Power Sources 2018, 373, 79–84.
- Wu, X.; Qiao, Y.; Guo, C.; Shi, Z.; Li, C.M. Nitrogen Doping to Atomically Match Reaction Sites in Microbial Fuel Cells. Commun. Chem. 2020, 3, 68.
- Logan, B.; Cheng, S.; Watson, V.; Estadt, G. Graphite Fiber Brush Anodes for Increased Power Production in Air-Cathode Microbial Fuel Cells. Environ. Sci. Technol. 2007, 41, 3341–3346.
- Cheng, S.; Logan, B.E. High Hydrogen Production Rate of Microbial Electrolysis Cell (MEC) with Reduced Electrode Spacing. Bioresour. Technol. 2011, 102, 3571–3574.
- Hu, H.; Fan, Y.; Liu, H. Hydrogen Production Using Single-Chamber Membrane-Free Microbial Electrolysis Cells. Water Res. 2008, 42, 4172–4178.
- Jeremiasse, A.W.; Hamelers, H.V.M.; Buisman, C.J.N. Microbial Electrolysis Cell with a Microbial Biocathode. Bioelectrochemistry 2010, 78, 39–43.
- Jafary, T.; Daud, W.R.W.; Ghasemi, M.; Kim, B.H.; Carmona-Martínez, A.A.; Bakar, M.H.A.; Jahim, J.M.; Ismail, M. A Comprehensive Study on Development of a Biocathode for Cleaner Production of Hydrogen in a Microbial Electrolysis Cell. J. Clean. Prod. 2017, 164, 1135–1144.
- Call, D.; Logan, B. A Method for High Throughput Bioelectrochemical Research Based on Small Scale Microbial Electrolysis Cells. Biosens. Bioelectron. 2011, 26, 4526–4531.
- Hassanein, A.; Witarsa, F.; Guo, X.; Yong, L.; Lansing, S.; Qiu, L. Next Generation Digestion: Complementing Anaerobic Digestion (AD) with a Novel Microbial Electrolysis Cell (MEC) Design. Int. J. Hydrogen Energy 2017, 42, 28681–28689.
- Call, D.; Logan, B.E. Hydrogen Production in a Single Chamber Microbial Electrolysis Cell Lacking a Membrane. Environ. Sci. Technol. 2008, 42, 3401–3406.
- Luo, S.; Jain, A.; Aguilera, A.; He, Z. Effective Control of Biohythane Composition through Operational Strategies in an Innovative Microbial Electrolysis Cell. Appl. Energy 2017, 206, 879–886.
- Lanas, V.; Ahn, Y.; Logan, B.E. Effects of Carbon Brush Anode Size and Loading on Microbial Fuel Cell Performance in Batch and Continuous Mode. J. Power Sources 2014, 247, 228–234.
- Li, L.; Jiang, B.; Tang, D.; Zhang, X.; Yuan, K.; Zhang, Q. Alkaline Treatment of Used Carbon-Brush Anodes for Restoring Power Generation of Microbial Fuel Cells. RSC Adv. 2018, 8, 36754–36760.
- Mohamed, H.O.; Sayed, E.T.; Obaid, M.; Choi, Y.-J.; Park, S.-G.; Al-Qaradawi, S.; Chae, K.-J. Transition Metal Nanoparticles Doped Carbon Paper as a Cost-Effective Anode in a Microbial Fuel Cell Powered by Pure and Mixed Biocatalyst Cultures. Int. J. Hydrogen Energy 2018, 43, 21560–21571.
- Jiang, D.; Li, B. Granular Activated Carbon Single-Chamber Microbial Fuel Cells (GAC-SCMFCs): A Design Suitable for Large-Scale Wastewater Treatment Processes. Biochem. Eng. J. 2009, 47, 31–37.
- Mu, Y.; Rabaey, K.; Rozendal, R.A.; Yuan, Z.; Keller, J. Decolorization of Azo Dyes in Bioelectrochemical Systems. Environ. Sci. Technol. 2009, 43, 5137–5143.
- Pocaznoi, D.; Calmet, A.; Etcheverry, L.; Erable, B.; Bergel, A. Stainless Steel Is a Promising Electrode Material for Anodes of Microbial Fuel Cells. Energy Environ. Sci. 2012, 5, 9645–9652.
- Abbas, A.A.; Farrag, H.H.; El-Sawy, E.; Allam, N.K. Microbial Fuel Cells with Enhanced Bacterial Catalytic Activity and Stability Using 3D Nanoporous Stainless Steel Anode. J. Clean. Prod. 2021, 285, 124816.
- Kumar, G.G.; Sarathi, V.G.S.; Nahm, K.S. Recent Advances and Challenges in the Anode Architecture and Their Modifications for the Applications of Microbial Fuel Cells. Biosens. Bioelectron. 2013, 43, 461–475.
- Blanchet, E.; Erable, B.; De Solan, M.-L.; Bergel, A. Two-Dimensional Carbon Cloth and Three-Dimensional Carbon Felt Perform Similarly to Form Bioanode Fed with Food Waste. Electrochem. Commun. 2016, 66, 38–41.
- Cheng, S.; Logan, B.E. Ammonia Treatment of Carbon Cloth Anodes to Enhance Power Generation of Microbial Fuel Cells. Electrochem. Commun. 2007, 9, 492–496.
- Batlle-Vilanova, P.; Puig, S.; Gonzalez-Olmos, R.; Vilajeliu-Pons, A.; Bañeras, L.; Balaguer, M.D.; Colprim, J. Assessment of Biotic and Abiotic Graphite Cathodes for Hydrogen Production in Microbial Electrolysis Cells. Int. J. Hydrogen Energy 2014, 39, 1297–1305.
- Croese, E.; Pereira, M.A.; Euverink, G.-J.W.; Stams, A.J.M.; Geelhoed, J.S. Analysis of the Microbial Community of the Biocathode of a Hydrogen-Producing Microbial Electrolysis Cell. Appl. Microbiol. Biotechnol. 2011, 92, 1083–1093.
- Dange, P.; Pandit, S.; Jadhav, D.; Shanmugam, P.; Gupta, P.K.; Kumar, S.; Kumar, M.; Yang, Y.-H.; Bhatia, S.K. Recent Developments in Microbial Electrolysis Cell-Based Biohydrogen Production Utilizing Wastewater as a Feedstock. Sustainability 2021, 13, 8796.
- Tang, J.; Bian, Y.; Jin, S.; Sun, D.; Ren, Z.J. Cathode Material Development in the Past Decade for H2 Production from Microbial Electrolysis Cells. ACS Environ. Au 2022, 2, 20–29.
- Kundu, A.; Sahu, J.N.; Redzwan, G.; Hashim, M.A. An Overview of Cathode Material and Catalysts Suitable for Generating Hydrogen in Microbial Electrolysis Cell. Int. J. Hydrogen Energy 2013, 38, 1745–1757.
- Wang, J.; Mu, K.; Zhao, X.; Luo, D.; Yu, X.; Li, W.; Chu, J.; Yang, J.; Yang, Q. Uniform Distribution of Pd on GO-C Catalysts for Enhancing the Performance of Air Cathode Microbial Fuel Cell. Catalysts 2021, 11, 888.
- Jeremiasse, A.W.; Hamelers, H.V.M.; Saakes, M.; Buisman, C.J.N. Ni Foam Cathode Enables High Volumetric H2 Production in a Microbial Electrolysis Cell. Int. J. Hydrogen Energy 2010, 35, 12716–12723.
- Son, S.; Koo, B.; Chai, H.; Tran, H.V.H.; Pandit, S.; Jung, S.P. Comparison of Hydrogen Production and System Performance in a Microbial Electrolysis Cell Containing Cathodes Made of Non-Platinum Catalysts and Binders. J. Water Process Eng. 2021, 40, 101844.
- Wang, L.; Liu, W.; He, Z.; Guo, Z.; Zhou, A.; Wang, A. Cathodic Hydrogen Recovery and Methane Conversion Using Pt Coating 3D Nickel Foam Instead of Pt-Carbon Cloth in Microbial Electrolysis Cells. Int. J. Hydrogen Energy 2017, 42, 19604–19610.
- Yan, Z.H.; Wang, M.; Huang, B.X.; Zhao, J.S.; Liu, R.M. Carboxyl Multi-Wall Carbon Nanotubes Supported Pt-Ni Alloy Nanoparticles as Cathode Catalyst for Microbial Fuel Cells. Int. J. Electrochem. Sci. 2012, 7, 10825–10834.
- Wang, H.; Wang, G.; Ling, Y.; Qian, F.; Song, Y.; Lu, X.; Chen, S.; Tong, Y.; Li, Y. High Power Density Microbial Fuel Cell with Flexible 3D Graphene–Nickel Foam as Anode. Nanoscale 2013, 5, 10283–10290.
- Karthikeyan, R.; Krishnaraj, N.; Selvam, A.; Wong, J.W.-C.; Lee, P.K.H.; Leung, M.K.; Berchmans, S. Effect of composites based nickel foam anode in microbial fuel cell using Acetobacter aceti and Gluconobacter roseus as a biocatalysts. Bioresour. Technol. 2016, 217, 113–120.
- Yuan, Y.; Zhao, B.; Jeon, Y.; Zhong, S.; Zhou, S.; Kim, S. Iron Phthalocyanine Supported on Amino-Functionalized Multi-Walled Carbon Nanotube as an Alternative Cathodic Oxygen Catalyst in Microbial Fuel Cells. Bioresour. Technol. 2011, 102, 5849–5854.
- Liu, X.-W.; Sun, X.-F.; Huang, Y.-X.; Sheng, G.-P.; Zhou, K.; Zeng, R.J.; Dong, F.; Wang, S.-G.; Xu, A.-W.; Tong, Z.-H.; et al. Nano-Structured Manganese Oxide as a Cathodic Catalyst for Enhanced Oxygen Reduction in a Microbial Fuel Cell Fed with a Synthetic Wastewater. Water Res. 2010, 44, 5298–5305.
- Olivares-Ramírez, J.M.; Campos-Cornelio, M.L.; Uribe Godínez, J.; Borja-Arco, E.; Castellanos, R.H. Studies on the Hydrogen Evolution Reaction on Different Stainless Steels. Int. J. Hydrogen Energy 2007, 32, 3170–3173.
- Kim, K.-Y.; Habas, S.E.; Schaidle, J.A.; Logan, B.E. Application of Phase-Pure Nickel Phosphide Nanoparticles as Cathode Catalysts for Hydrogen Production in Microbial Electrolysis Cells. Bioresour. Technol. 2019, 293, 122067.
This entry is offline, you can click here to edit this entry!








