Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Polymer Science
Metal-organic frameworks (MOFs) , or metal-organic frameworks, are a new form of a porous coordination polymer. Novel materials have been developed because of technological advancements combined with material research. MOFs technology has been investigated for biomedical applications in this line.
- MOF technology
- biomedical applications
- biomolecules
- drug
- toxicity
1. Introduction
MOFs, or metal-organic frameworks, are a new form of a porous coordination polymer. Due to their considerable amount of diversity in their structure and increased surface area, these exciting materials have a significant impact on a various field such as academics, industries, and so on. Today several companies like BASF (Sigma-Aldrich, St. Louis, MO, USA, 2020), NuMat Technologies (NuMat Technologies, Skokie, IL, USA, 2020), MOFapps (MOFapps, Oslo, Norway 2020), framergy (Framergy, Wilmington, NC, USA, 2020), metalorganic-frameworks.EU (Materials Center, Leoben, Austria, 2020), ImmondoTech, and MOF Technologies (MOF Technologies, Belfast, UK, 2020) produce MOFs on an industrial scale. MOFs have a diverse range of applications, namely, separation, gas storage, sensing, proton conduction, catalysis, etc. [1]. Due to their unique properties as hybrid composite systems, MOFs have been thoroughly investigated in a diverse application, particularly in biological fields.
MOFs have also been referred to as materials made of organic–inorganic hybrids, coordination polymers, porous coordination networks, and metal organic polymers in the literature. In the area of nanoporous material research, MOFs were among the most alluring substances. MOFs are smart alternatives to conventional nanoporous materials in a wide range of scientific and industrial fields due to their excellent combination of high porosity, absence of inaccessible bulk volume, incredibly wide range of topologies and pore sizes, vast surface areas, and a wide range of potential structure viability. The synthesis of MOFs based on the reticular design concept in late 1999 sparked interest in this field even though they have been known about since 1965 [2].
Although around 25,000 MOFs have been synthesized and characterized, BioMOFs is a new subclass of MOFs composed of biomolecules as linkers [3]. They are defined as the MOF, which constitutes at least one biomolecule as a linker [4]. The common biological organic linkers include amino acids, proteins, nucleobases, polypeptides, saccharides, and cyclodextrins [4]. Often, it has been observed that some auxiliary linkers like dicarboxylates can also be used as organic linkers. BioMOFs are either two-dimensional or three-dimensional structures with excellent porosity generated using coordination bonding between transition metal ions and biomolecules rather than a conventional organic linker [5]. The potential of BioMOFs have been demonstrated for several biological and medical applications such as sensing, imaging, and drug delivery [5]. To maintain a good host–guest response towards the biological systems, BioMOFs should necessarily be biocompatible [3][5]. To address the fundamental performances and characteristics of MOFs for biological applications, there are a variety of methods that may be applied, including (i) covalent bonding, (ii) non-covalent attachment, (iii) polymer coordination, and (iv) encapsulation. For example, Cu and Eu-TCA (H3TCA = tricarboxytriphenyl amine) MOFs have a luminescent property to detect (highly toxic and carcinogenic) in an aqueous solution and living cells [6]. The first context of MOFs in the biological application was being used as vectors for drug delivery; their large pore volumes, and their diverse structure makes MOFs a suitable candidate for a drug delivery system (DDS) [5][7][8]. Pore chemistry, crystal size, and stable framework inflect characteristics; controlled release rates, biocompatibility, and loading capacities ensure MOFs as carriers for a drug in recent reports. For instance, MOFs have been used as encapsulated macromolecules, which can post synthetically infiltrated micro peroxidase MP-11 and cytochrome-C into Tb-based MOFs [9][10]. It has been reported that the potential application of MOFs can be achieved by precipitation of a porous framework by enzymes and proteins in areas such as bio catalysis and bio-banking [11][12]. Water is a non-toxic medium, and it is an essential and important solvent system for biological application. Non-toxicity, biocompatibility, green recyclable, and low cost are important features for MOF technology. On the other hand, water stability and solubility have been majorly considered for membrane separation, adsorption, sensing, catalysis, drug delivery, and imaging.
2. Classification of MOFs
Based on solubility, MOFs have been classified into two primary classes of MOFs, i.e., (a) Water-stable MOFs and (b) Water-soluble MOFs.
2.1. Water-Soluble MOFs
These types of MOFs have low thermodynamic and kinetic stabilities. They showed faster hydrolysis processes due to the high water ligand exchange rate [13]. Water-soluble MOFs also have a wide role in various aspects. The addition of strong polar groups such as -OH, -COOH, and -NH2 on MOF surface transforms it from non-polar to polar, thereby enhances membrane hydrophilicity [14]. MOFs are generally stable in water. However, modern medical and drug release approaches are required to gain the water-solubility phenomenon. The solubility of MOFs promptly depends on their crystallinity. It has been found that MOFs having lower crystallinity are generally water-soluble. Since hydrophilicity and hydrophobicity is related with the contact angle, a contact angle less than 90 °C indicates the hydrophilicity of compounds [15]. The addition of a strong polar group increased the polarity of MOF and decreased the contact angle [16], and it enhanced the hydrophilicity.
2.2. Water-Stable MOFs
The scientific community is interested in water-stable MOFs because the majority of industrial processes contain some level of water or moisture in various forms of preparation, storage, transportation, and applications. Water-stable MOFs are stable in the presence of water to make them more viable commercially and industrially in water. Water stability is the most important factor for real-world applications because our environment has a lot of water as moisture or many other forms to degrade them and decrease their functional activities like adsorption, degradation, etc. Large-scale production of MOFs is still hard because of their different properties, different ways of making them, and quality control, high production cost, lack of standardization, etc. [17]. Some commercially available water-stable MOFs are BasoliteRF300 (Fe-BTC), BasoliteRA100 (MIL-53(Al)), and BasoliteRZ1200 (ZIF-8), etc., which are manufactured by Sigma-Aldrich [17].
It is often possible to determine whether the MOF structure is either stable or unstable in the water. Generally, it is discovered by comparing the chemical properties of pre- and post-exposure samples. After the comparison of powder X-ray diffraction (PXRD) data and gas adsorption isotherm base BET surface area, they could present a convincing argument for whether the crystallinity or structural porosity of the MOF were lost or not after coming into contact with water. Typically, water exposure causes ligand displacement, structural disintegration, and phase shifts in MOFs. Suitable strength is required for a water-stable MOF structure to withstand over water molecule invasions; along with the ensuing loss of crystallinity and overall porosity, water-stable MOF building blocks should be suitably strong. These structures with high stability typically include strong coordination bonds (thermodynamic stability) to prevent the metal-ligand connections from being broken during the hydrolysis reaction. Therefore, it is crucial to accurately determine how water molecules affect the fundamental MOF characteristics (such as structural stability and metal-ligand coordination), especially for the synthesis of water-stable MOFs (WMOFs) [18]. In accordance with Pearson’s HSBA (hard-soft acid-base) principle, the ligands based on carboxylates are known as hard bases, those generate stable MOFs in addition to high-valent metal ions, e.g., Al3+, Ti4+, Fe3+, Cr3+, and Zr4+. Moreover, soft azolate ligands (triazolates, imidazolates, tetrazolates, and pyrazolates) and soft divalent metal ions (Mn2+, Cu2+, Zn2+, Ni2+, and Ag2+) can also form stable MOFs. Zeolitic imidazolate frameworks (ZIFs) are the most well-known examples, composed of Zn2+ and imidazolate linkers [19]. Chemical structures of some carboxylate- and azolate-based linkers are given in Figure 1 and Figure 2 [12][13][14][15][16], respectively.
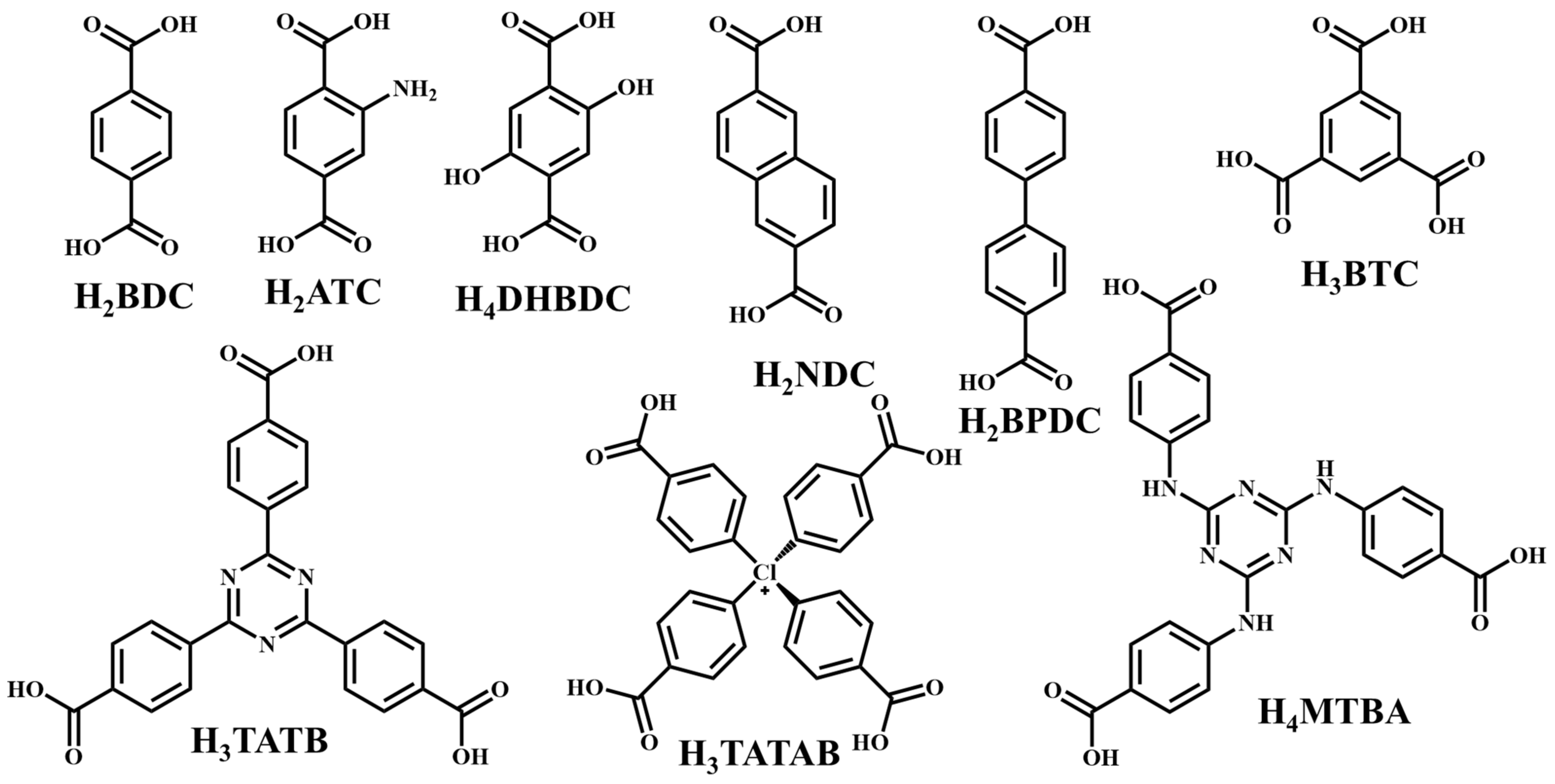
Figure 1. Chemical structures of some carboxylate linkers; H2BDC = 1,4-benzenedicarboxylic acid, H2ATC = 2-aminoterephthalic acid, H2NDC = 2,6-naphthalenedicarboxylic acid, H2BPDC = 4,4′-biphenyldicarboxylic acid, H4DHBDC= 2,5-Dihydroxy-1,4-benzenedicarboxylic acid, H3TATAB = 4,4′, 4″-s-triazine-1,3,5-triyltri-p-aminobenzoic acid, H2TATB = 4,4′,4″-s-triazine-2,4,6-triyl-tribenzoic acid, H4MTBA = methanetetra (4-benzoic acid) [20][21].
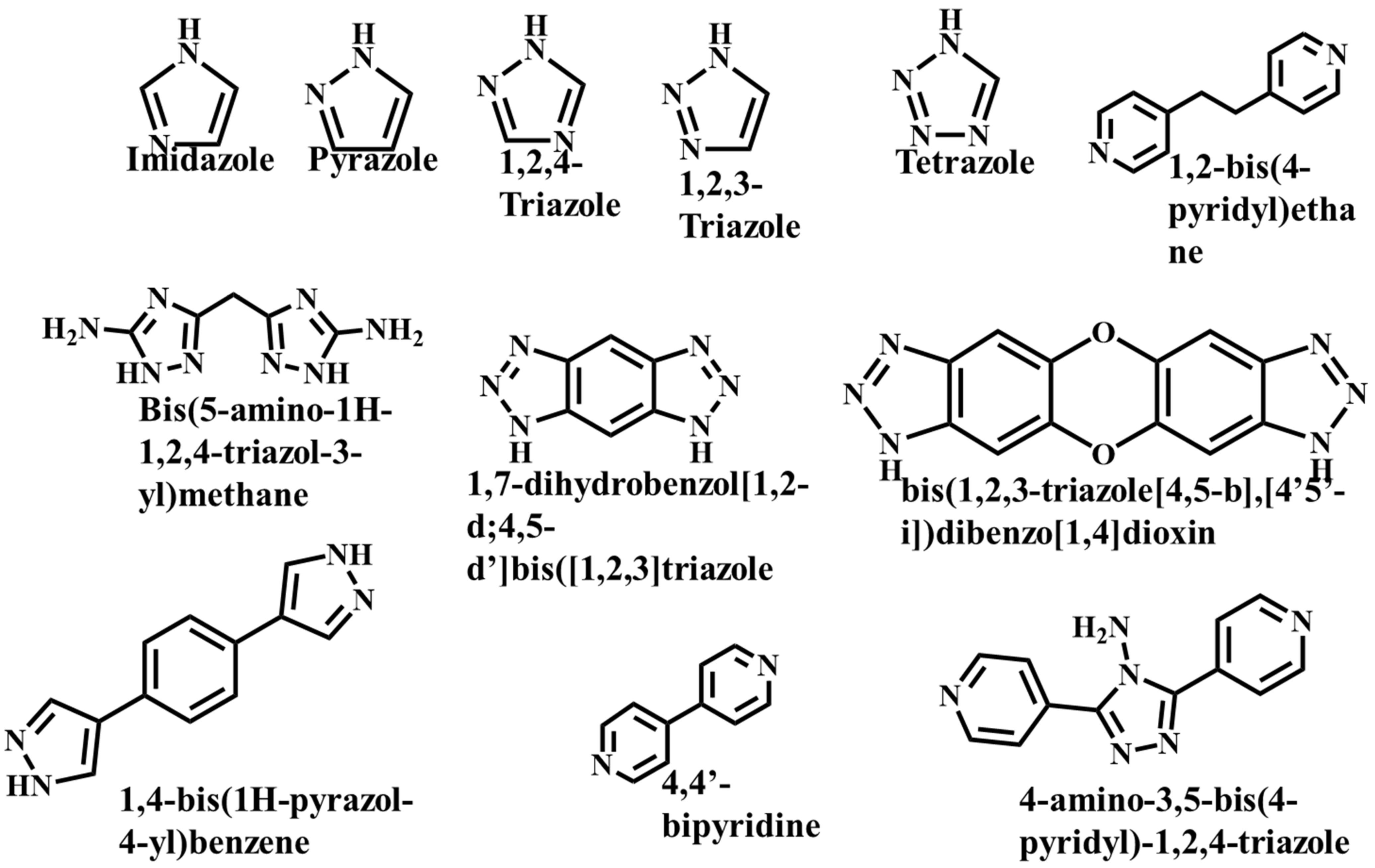
Figure 2. Chemical Structure of some azolate-based and N-doner-based linkers [22].
ZIF is a subfamily of porous MOFs which uses imidazole-based ligand and transition series metal ion (Zn or Co). Their large surface area, adjustable surface characteristics, and chemical stability make them industrially most applicable. Mostly ZIF-based MOFs are water stable in nature. ZIF-8 retained its primary framework in boiling water for one week [18]. It is possible to explain ZIF-8’s exceptional stability via (a) Hydrophobic pores and surface topography which resist water molecules, and (b) Extremely stable covalent interaction between the metal ions (e.g., Zn2+ and Co2+) and imidazolate linker. ZIF-8 was prepared in the shortest time (approx. 5 min.) at room temperature with the highest product yield [23]. They also tuned ZIF-8 crystal size from micrometer (μm) to nanometer (nm), which also changed the morphology of crystals by using different capping agents like cetyltrimethylammonium bromide (CTAB). Some supplementary additives like sorbitan monooleate (Span 80), triethylamine (TEA), and poly (oxyethylene sorbitan monooleate), also known as Tween 80, are added to speed up the crystallization of ZIF in aqueous environments. For example, when TEA is used as a protonation agent, a group of researchers found the synthesis process of ZIF-67 and ZIF-8 in water within ten minutes at ambient temperature and atmospheric pressure [24]. Organic solvents are not environmentally friendly, expensive, and constitute a risk to the environment due to their toxic nature. Therefore, it is essential to create a novel and ecologically friendly synthesis technique that does not call for the use of potentially hazardous organic solvents [25]. Water has driven to be overdrawn this situation, as it is a most environmentally friendly solvent and readily available. As a result, it saves money and is more environmentally friendly than organic solvents. In comparison to organic solvents, water-based MOF synthesis improves material characteristics since organic solvents are locked in pores and difficult to remove compared to water removal. Presently, large numbers of MOFs are synthesized in water, such as ZIF, iso reticular MOFs (IRMOFs), MILs, UiOs, porous coordination network (PCN), and coordination pillared-layer (CPL) [26].
IRMOF series mostly uses dicarboxylic acid and tricarboxylic acid as organic linkers. The most widely studied IRMOF are MOF-5, MOF-177, MOF-74, and MOF-199 (HKUST-1 or Cu-BTC). HKUST-1 can also be synthesized in aqueous conditions. Huo et al. proposed a technique for synthesizing HKUST-1 at room temperature by combining metal salt powder of anhydrous copper (II) acetate (Cu(OAC)2) with excess ligand, i.e., H3BTC or 1,3,5-Benzenetricarboxylic acid only utilizing water as the reaction medium. [27].
MIL series are thermally highly stable classes of MOF. The MIL-53 series is commonly synthesized using a variety of metal ions (e.g., Sc, Fe, Ga, and Al) and linkers based on terephthalic acid, and their derivatives (e.g., fluorine, chlorine, amino, hydroxyl, nitro, and carbamate) as the organic linkers. Cheng and colleagues developed a simple solvothermal method for producing nanocrystals of NH2-MIL-53(Al) by modification of the water content in a DMF-water solvent mixture [28]. Other explored MOFs of the MILs family, including MIL-100, and MIL-53 may be synthesized in an aqueous solution.
UiO series have high thermally and chemically stable morphologies. The solvent DMF is used in the synthesis of the most widely studied UiO sequence, and Zr–MOF, which results in a significant amount of waste and by-products [26].
3. Biological Applications of MOF
MOFs could make a difference in biological applications for their suitable toxicology, acceptable stability, biocompatibility, biodegradability, and low cytotoxic effects.
3.1. MOFs for Biosensing
The biosensing characteristic can be achieved by a biosensor. A biosensor is a sensor that can determine the concentration of a biological analyte and quantify it. MOFs are intended to be a potential contender for electrochemical biosensors due to their high thermal, structural, and chemical stabilities. For biological sensing, biosensors should be extremely selective and sensitive. When compared to inorganic nanomaterials (e.g., gold nanoparticles, graphene oxide, and graphene), MOFs are biodegradable and have a low cytotoxicity, allowing for quicker degradation and the utilization of biocompatible building blocks. MOFs are utilized to detect RNA, DNA, enzyme activity, and small biomolecules. Magnetic resonance imaging (MRI) and computed tomography (CT) are critical clinical diagnostic procedures used to detect various diseases. The biosensors detect the targeting agent based on the various techniques like electrochemical, colorimetric, luminescence, and electroluminescent methods [29].
Electrochemical method-based biosensors contains an electrode often known as a transducer. An electrochemical biosensors for pathogen detection is made up of conducting and semiconducting materials. An electrochemical process involving the electrode and an electrolyte solution of having pathogens converts the chemical energy involved in the binding process between the electrode-immobilized biorecognition components and target pathogens into electrical energy [30]. Energy will be detected by detectors [30][31][32][33].
Colorimetric method-based biosensors are optical sensors, which change color when exposed to different stimuli. A stimulus can be defined as any physical or chemical change that generates optical signals [31].
Luminescence method-based biosensors have been reported for BL and CL detection using chemiluminescence, thermo-chemiluminescence (TCL), and electrogenerated chemiluminescence (ECL) reactions. They can be used for the measurement of images using chemical luminescence-based biosensors [32]. Basically, chemiluminescence is a luminescent signal process produced by an enzyme-labeled antibody, while electroluminescence is a luminescent signal produced by an electron transfer reaction between two luminescent compounds [33].
3.2. Biomedical Imaging of MOFs
MOFs are used in biomedical imaging applications to make it easier to find and identify a number of disorders. MOFs are frequently applied to provide observable signals or to increase the contrast of particular tissues. This is usually achieved by changing the metal nodes of the MOFs. The most frequently used imaging techniques for MOFs are magnetic resonance imaging (MRI), positron emission tomography (PET), and computed tomography (CT). Fluorophores in MOFs have also made it possible for cells to use up conversion for optical imaging [34][35]. Glioma is one of the most common central nervous system tumors with high fatality rates. The spatial resolution, sensitivity, and penetration depth of glioma imaging is improved by integrating CT, MRI, and PAI with a nanocomposite made up of core-shell Au@MIL-88(Fe) [36].
3.3. MOF as a Drug Delivery System (DDS)
DDS is a dynamic biological subject within material science that has a widespread application in human health. When compared to other porous materials, MOFs are a good candidate for drug delivery because of their highly tunable nature (pore size as well as tuning of the metal ion or organic linker), large surface area, and pore size [37]. Nano-MOFs, which were created by scaling down MOF particle size, are effective for drug delivery vectors. In the last decade, they have been a focal point in the area of drug delivery devices for distributing the loaded drug to specified places. Among the reported porous carriers, MOFs gained attention as they had desired characteristics such as having a large cavity size for drug encapsulation, exceptionally high surface area, and a controlled drug-release profile. They exhibited inherent biodegradability and varied functionality for post-synthetic grafting of medicinal molecules because of their metal-ligand interactions, which are relatively labile in nature [38]. A variety of hydrophobic, hydrophilic, and amphiphilic medicinal molecules could be encapsulated in the cavity of MOF and/or attached to the framework structures [39]. Drug loading in MOFs is achieved through covalent interactions or non-covalent interactions [38][39][40]. Drug molecules that are covalently attached to MOFs can release drugs more slowly than drug molecules that are just stuck to their surfaces [39]. Physicochemical properties of MOF materials and the drug molecule (3D arrangement, pore size) are two things that affect how MOFs are used to deliver drugs. It allows the drug molecules to fit within the carrier molecules so that they can easily reach their target. In the case of nanocarriers, burst release of drug molecules were observed. However, release of drug molecules from MOFs is delayed and regulated by matrix breakdown [40]. For example, iron-containing BioMIL-1 MOFs displayed greater nicotinic acid loading (up to 75%) than the native MOF structures and regulated drug delivery [41].
To make its toxicology feasible for MOFs in their biological applications, suitable metal ion and organic linkers with the lowest cytotoxicity level are desired. The best suitable metals are Cu, Mg, Ca, Mn, Fe, Zn, Ti, and Zr (a non-toxic carrier is a prerequisite to every drug). The biodegradability and stability of MOFs is another contentious issue pertaining to their application in drug delivery systems since it facilitates drug diffusion from matrix materials, hence enhancing their drug release efficiency.
MOFs adsorb relevant substances on their exterior surface, channels open, or capture molecules within the frameworks. Additionally, active molecules may possibly be introduced into MOFs via covalent bonding by either a post-synthetic modification or one-pot synthesis [37]. Differences from functionalizing MOFs with therapeutic agents for biological applications are as follows:
-
Surface Adsorption
MOFs are capable of adsorbing functional molecules due to their large surface area and porosity. Most of the time, surface adsorption is conducted by stirring MOFs that have already been made in a solution with functional molecules. Hydrogen bonding, Van der Waals interaction, and π–π* interaction, are the key forces involved in this method. Surface adsorption has been extensively used to immobilize enzymes [42]. In 2006, the Balkus group showed that a microperoxidase-11 (MP-11) catalyst could be physically attached to a MOF during keeping the catalytic performance of the MP-11 catalyst, which contains Cu as metal and nano-crystalline in nature [43].
-
Pore Encapsulation
The pores of MOFs may accommodate a wide variety of functional molecules because of their high porosity and pore adjustable properties, which range from microporous to mesoporous. Anticancer drugs are encapsulated within the host of the MOF for later intracellular uptake and release [37]. For instance, encapsulation of camptothecin was performed using ZIF-8 nanospheres with 70 nm particle size [44].
-
Covalent Binding
The methods described above rely on very weak interactions between molecules and MOFs, which frequently results in delayed leaching difficulties. Functional groups present on the MOF surface, such as carboxyl, amino, and hydroxyl groups, establish covalent interactions with active groups onto the target [37]. Jung et al. revealed the post-synthetic conjugation of Candida Antarctica lipase B (CAL-B) and increased green fluorescent protein (eGFP) on the MOF surface [45].
-
Functional Molecules as the Building Block
Using functional molecules as building blocks is another option. Generally, biomolecules include a number of types of reactive chemicals that are compatible with inorganic metals. Until now, amino acids [46], peptides [47], nucleobases [48], and saccharides [49] might function as organic ligands. These biomolecules are used in the synthesis of bioMOFs. BioMOFs often exhibit superior biocompatibility and unique biological functioning. By mixing zinc acetate dihydrate, adenine, and biphenyl dicarboxylic acid (BPDC), the research team made bioMOF-1, which is crystalline and porous in nature. One of the commonly used chemotherapy agents for ovarian cancer, breast cancer, and lymphoblastic leukemia is doxorubicin hydrochloride (DOX) [50]. As an alternative to whole-body radiation therapy for leukemia, the amphiphilic anticancer agent busulfan (Bu) is frequently utilized in chemotherapy [51]. Topotecan (TPT), which is made up of a camptothecin (CPT), is therapeutically used to treat small cell lung cancer and refractory ovarian cancer [52][53]. ZIF-8 is a MOF which contains zinc as metal node and 2-methylimidazolate as linker. Due to its superior hydrothermal stability, thermal stability, biocompatible qualities and non-toxicity, ZIF-8 is being identified as a possible nanocarrier to use in drug delivery [54]. Notably, in physiological conditions, ZIF-8 is stable, but in acidic environments, it is unstable. Therefore, ZIF-8 is used in pH-sensitive methods related to drug delivery. After its synthesis in 2012, a group of researchers effectively loaded DOX (4.9 wt%) by mixing of ZIF-8, followed by the addition of dry ZIF-8 powder to aqueous medium [55]. This results in being extremely regulated, and after 30 days, 66% drug release were observed. Similarly, ZIF-8 was employed as a pH-responsive drug delivery channel in the case of 5-fluorouracil (5-FU) delivery [56].
The hypoxia-activated prodrug banoxantrone (AQ4N) was loaded onto UiO-66 MOF, and during the manufacture of UiO-66 nanoparticles, p-azido-methyl benzoic acid and monocarboxylate photo color were used as modulators to modify MOF with the photosensitizer, photo color (HPPH), and azide groups (N3). This is used for hypoxia-activated cascade chemotherapy [57]. Figure 3 shows the procedure used for the synthesis of A/UiO-66-HP nanoparticles, photodynamic therapy mechanism involved, and hypoxia-activated cascade chemotherapy.
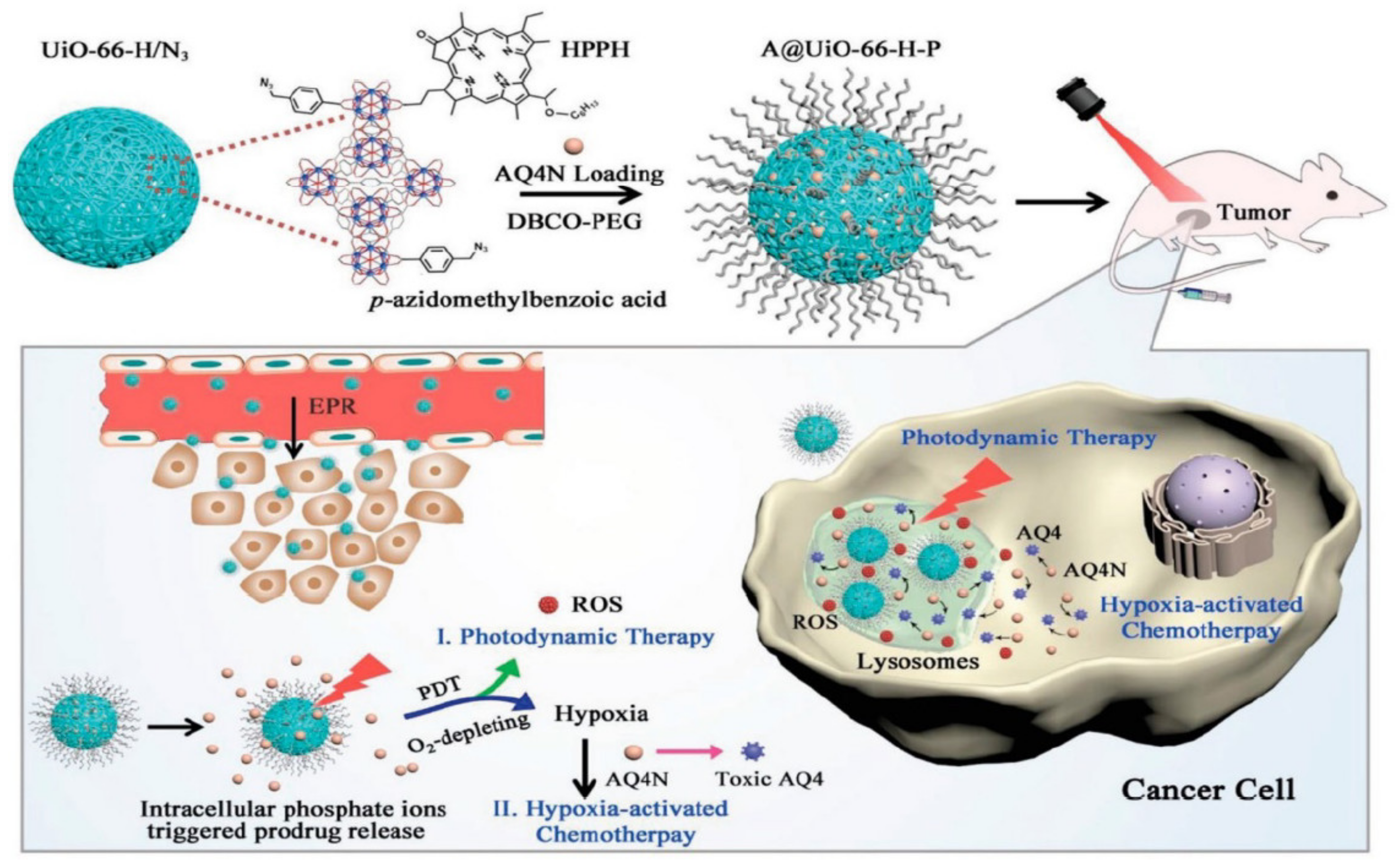
Figure 3. Synthetic procedure of A/UiO-66-HP nanoparticles and mechanism of photodynamic therapy and hypoxia-activated cascade chemotherapy [57].
3.4. Miscellaneous Biomedical Applications of MOFs
Despite the biosensing, bioimaging, and drug delivery, MOFs are also used as the antitumor agent. MOFs are also used in radiotherapy as well as in chemodynamic therapy [58]. In 2018, Kaiyuan Ni and their team reported two hafnium-based MOFs, i.e., Hf6-DBA and Hf12-DBA, for the radiotherapy [59], where DBA stands for 2,5-di(p-ben-zoato)aniline). The SBU unite of these MOFs generates reactive oxygen species (ROS) after the absorbance of X-rays, which results in the higher radioenhancing efficiency. To find out the efficiency of these MOFs, they examined apoptosis and DNA double-strand break (DSB) pathways. After their investigation, they found Hf12-DBA was found more superior than Hf6-DBA for radiotherapy [59]. In 2021, Prajapati and coworkers used CuSO4 as metal and L-cysteine as a linker for the synthesis of a metal organic hybrid CuHARS for the treatment of glioma cells [60]. In an another study, polyallylamine hydrochloride (PAH)-coated CuHARS and cellulose fiber are used for the degradation of S-nitrosothiol for the antimicrobial activity [61]. Healing of wounds is also a major concern for diabetic patients. For this, in 2017, Jisheng Xiao and coworkers reported the wound-healing property of a HKUST-1 after the integration with citrate-based hydrogel. The integration of hydrogel accelerated the wound-healing capacity as well as decreased the toxicity generated by copper metals [62]. Yao et al., in 2020, used ZIF-8 MOF for the wound healing. They loaded omniphobic porous gel with ZIF-8 and used it as a wound healing material. Omniphobic ZIF-8@hydrogel porous wound dressing can prevent bacterial growth and enable the regulated release of the bactericidal, anti-inflammatory, and non-toxic zinc ions for wound healing [63].
This entry is adapted from the peer-reviewed paper 10.3390/polym14214710
References
- Li, B.; Wen, H.M.; Zhou, W.; Chen, B. Porous metal-organic frameworks for gas storage and separation: What, how, and why? J. Phys. Chem. Lett. 2014, 5, 3468–3479.
- Shi, X.; Shan, Y.; Du, M.; Pang, H. Synthesis and application of metal-organic framework films. Coord. Chem. Rev. 2021, 444, 214060.
- Imaz, I.; Rubio-Martínez, M.; An, J.; Solé-Font, I.; Rosi, N.L.; Maspoch, D. Metal-biomolecule frameworks (MBioFs). Chem. Commun. 2011, 47, 7287–7302.
- Seki, K.; Takamizawa, S.; Mori, W. Characterization of microporous copper(II) dicarboxylates (fumarate, terephthalate, and trans-1,4-cyclohexanedicarboxylate) by gas adsorption. Chem. Lett. 2001, 30, 122–123.
- Horcajada, P.; Serre, C.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Metal–Organic Frameworks as Efficient Materials for Drug Delivery. Angew. Chem. 2006, 118, 6120–6124.
- Wu, P.; Wang, J.; He, C.; Zhang, X.; Wang, Y.; Liu, T.; Duan, C. Luminescent metal-organic frameworks for selectively sensing nitric oxide in an aqueous solution and in living cells. Adv. Funct. Mater 2012, 22, 1698–1703.
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 172–178.
- Imaz, I.; Rubio-Martínez, M.; García-Fernández, L.; García, F.; Ruiz-Molina, D.; Hernando, J.; Puntes, V.; Maspoch, D. Coordination polymer particles as potential drug delivery systems. Chem. Commun. 2010, 46, 4737.
- Chen, Y.; Lykourinou, V.; Vetromile, C.; Hoang, T.; Ming, L.J.; Larsen, R.W.; Ma, S. How can proteins enter the interior of a MOF? investigation of cytochrome c translocation into a MOF consisting of mesoporous cages with microporous windows. J. Am. Chem. Soc. 2012, 134, 13188–13191.
- Lykourinou, V.; Chen, Y.; Wang, X.-S.; Meng, L.; Hoang, T.; Ming, L.-J.; Musselman, R.L.; Ma, S. Immobilization of MP-11 into a Mesoporous MetalÀOrganic Framework, : A New Platform for Enzymatic Catalysis. J. Am. Chem. Soc. 2011, 133, 10382–10385.
- Shieh, F.K.; Wang, S.C.; Yen, C.I.; Wu, C.C.; Dutta, S.; Chou, L.Y.; Morabito, J.V.; Hu, P.; Hsu, M.H.; Wu, K.C.W.; et al. Imparting Functionality to Biocatalysts via Embedding Enzymes into Nanoporous Materials by a de Novo Approach: Size-Selective Sheltering of Catalase in Metal-Organic Framework Microcrystals. J. Am. Chem. Soc. 2015, 137, 4276–4279.
- Lyu, F.; Zhang, Y.; Zare, R.N.; Ge, J.; Liu, Z. One-Pot Synthesis of Protein-Embedded Metal−Organic Frameworks with Enhanced Biological Activities. ACS Publ. 2014, 14, 5761–5765.
- Zhang, X.; Wang, B.; Alsalme, A.; Xiang, S.; Zhang, Z.; Chen, B. Design and applications of water-stable metal-organic frameworks: Status and challenges. Coord. Chem. Rev. 2020, 423, 213507.
- Zhang, S.; Liu, Y.; Li, D.; Wang, Q.; Ran, F. Water-soluble MOF nanoparticles modified polyethersulfone membrane for improving flux and molecular retention. Appl. Surf. Sci. 2020, 505, 144553.
- Law, K.Y. Definitions for hydrophilicity, hydrophobicity, and superhydrophobicity: Getting the basics right. J. Phys. Chem. Lett. 2014, 5, 686–688.
- Guo, W.; Errington, J.R. Effect of Surface Hydrophilicity on the Interfacial Properties of a Model Octane-Water-Silica System. J. Phys. Chem. C 2019, 123, 19649–19658.
- Li, R.Y.; Wang, Z.S.; Yuan, Z.Y.; Van Horne, C.; Freger, V.; Lin, M.; Cai, R.K.; Chen, J.P. A comprehensive review on water stable metal-organic frameworks for large-scale preparation and applications in water quality management based on surveys made since 2015. Crit. Rev. Environ. Sci. Technol. 2021, 52, 4038–4071.
- Rowe, M.D.; Tham, D.H.; Kraft, S.L.; Boyes, S.G. Polymer-modified gadolinium metal-organic framework nanoparticles used as multifunctional nanomedicines for the targeted imaging and treatment of cancer. Biomacromolecules 2009, 10, 983–993.
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal–Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, 1704303.
- Banerjee, D.; Hu, Z.; Li, J. Luminescent metal–organic frameworks as explosive sensors. Dalt. Trans. 2014, 43, 10668–10685.
- Lu, W.; Wei, Z.; Gu, Z.Y.; Liu, T.F.; Park, J.; Park, J.; Tian, J.; Zhang, M.; Zhang, Q.; Gentle, T.; et al. Tuning the structure and function of metal–organic frameworks via linker design. Chem. Soc. Rev. 2014, 43, 5561–5593.
- Desai, A.V.; Sharma, S.; Let, S.; Ghosh, S.K. N-donor linker based metal-organic frameworks (MOFs): Advancement and prospects as functional materials. Coord. Chem. Rev. 2019, 395, 146–192.
- Pan, Y.; Liu, Y.; Zeng, G.; Zhao, L.; Lai, Z. Rapid synthesis of zeolitic imidazolate framework-8 (ZIF-8) nanocrystals in an aqueous system. Chem. Commun. 2011, 47, 2071–2073.
- Gross, A.F.; Sherman, E.; Vajo, J.J. Aqueous room temperature synthesis of cobalt and zinc sodalite zeolitic imidizolate frameworks. Dalt. Trans. 2012, 41, 5458–5460.
- Häckl, K.; Kunz, W. Some aspects of green solvents. Comptes Rendus Chim. 2018, 21, 572–580.
- Duan, C.; Yu, Y.; Xiao, J.; Zhang, X.; Li, L.; Yang, P.; Wu, J.; Xi, H. Water-based routes for synthesis of metal-organic frameworks: A review. Sci. China Mater. 2020, 63, 667–685.
- Huo, J.; Brightwell, M.; Scientific, T.F.; El Hankari, S.; Garai, A. A versatile, industrially relevant, aqueous room temperature synthesis of HKUST-1 with high space-time yield Polyaniline gel nanoconposites View project Fluorescent MOFs View project. Artic. J. Mater. Chem. A 2013, 1, 15220–15223.
- Cheng, X.; Zhang, A.; Hou, K.; Liu, M.; Wang, Y.; Song, C.; Zhang, G.; Guo, X. Size- and morphology-controlled NH2-MIL-53(Al) prepared in DMF-water mixed solvents. Dalt. Trans. 2013, 42, 13698–13705.
- Morozova, S.M.; Sharsheeva, A.; Morozov, M.I.; Vinogradov, A.V.; Hey-Hawkins, E. Bioresponsive metal–organic frameworks: Rational design and function. Coord. Chem. Rev. 2021, 431, 213682.
- Cesewski, E.; Johnson, B.N. Electrochemical biosensors for pathogen detection. Biosens. Bioelectron. 2020, 159, 112214.
- Ebralidze, I.I.; Laschuk, N.O.; Poisson, J.; Zenkina, O.V. Colorimetric Sensors and Sensor Arrays. In Nanomaterials Design for Sensing Applications; Zenkina, O., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–39.
- Roda, A.; Mirasoli, M.; Michelini, E.; Di Fusco, M.; Zangheri, M.; Cevenini, L.; Roda, B.; Simoni, P. Progress in chemical luminescence-based biosensors: A critical review. Biosens. Bioelectron. 2016, 76, 164–179.
- Metkar, S.K.; Girigoswami, K. Diagnostic biosensors in medicine—A review. Biocatal. Agric. Biotechnol. 2019, 17, 271–283.
- Wang, H.S. Metal–organic frameworks for biosensing and bioimaging applications. Coord. Chem. Rev. 2017, 349, 139–155.
- Wang, H.S.; Li, J.; Li, J.Y.; Wang, K.; Ding, Y.; Xia, X.H. Lanthanide-based metal-organic framework nanosheets with unique fluorescence quenching properties for two-color intracellular adenosine imaging in living cells. NPG Asia Mater. 2017, 9, e354.
- Zhou, J.; Tian, G.; Zeng, L.; Song, X.; Bian, X.W. Nanoscaled Metal-Organic Frameworks for Biosensing, Imaging, and Cancer Therapy. Adv. Healthc. Mater. 2018, 7, 1800022.
- Sun, Y.; Zheng, L.; Yang, Y.; Qian, X.; Fu, T.; Li, X.; Yang, Z.; Yan, H.; Cui, C.; Tan, W. Metal–Organic Framework Nanocarriers for Drug Delivery in Biomedical Applications. Nano-Micro Lett. 2020, 12, 103.
- Huxford, R.C.; Della Rocca, J.; Lin, W. Metal–organic frameworks as potential drug carriers. Curr. Opin. Chem. Biol. 2010, 14, 262–268.
- Singh, R. Geetanjali Metal organic frameworks for drug delivery. In Applications of Nanocomposite Materials in Drug Delivery; Inamuddin, Asiri, A., Mohammad, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 605–617.
- Beg, S.; Rahman, M.; Jain, A.; Saini, S.; Midoux, P.; Pichon, C.; Jalees Ahmad, F.; Akhter, S. Nanoporous metal organic frameworks as hybrid polymer–metal composites for drug delivery and biomedical applications. Drug Discov. Today 2017, 22, 625–637.
- Miller, S.R.; Heurtaux, D.; Baati, T.; Horcajada, P.; Grenèche, J.M.; Serre, C. Biodegradable therapeutic MOFs for the delivery of bioactive molecules. Chem. Commun. 2010, 46, 4526–4528.
- Zhang, D.H.; Yuwen, L.X.; Peng, L.J. Parameters affecting the performance of immobilized enzyme. J. Chem. 2013, 2013, 946248.
- Pisklak, T.J.; Macías, M.; Coutinho, D.H.; Huang, R.S.; Balkus, K.J. Hybrid materials for immobilization of MP-11 catalyst. Top. Catal. 2006, 38, 269–278.
- Zhuang, J.; Kuo, C.H.; Chou, L.Y.; Liu, D.Y.; Weerapana, E.; Tsung, C.K. Optimized metal-organic-framework nanospheres for drug delivery: Evaluation of small-molecule encapsulation. ACS Nano 2014, 8, 2812–2819.
- Jung, S.; Kim, Y.; Kim, S.J.; Kwon, T.H.; Huh, S.; Park, S. Bio-functionalization of metal–organic frameworks by covalent protein conjugation. Chem. Commun. 2011, 47, 2904–2906.
- Saunders, C.D.L.; Burford, N.; Werner-Zwanziger, U.; McDonald, R. Preparation and comprehensive characterization of ·H2O. Inorg. Chem. 2008, 47, 3693–3699.
- Katsoulidis, A.P.; Park, K.S.; Antypov, D.; Martí-Gastaldo, C.; Miller, G.J.; Warren, J.E.; Robertson, C.M.; Blanc, F.; Darling, G.R.; Berry, N.G.; et al. Guest-adaptable and water-stable peptide-based porous materials by imidazolate side chain control. Angew. Chemie—Int. Ed. 2014, 53, 193–198.
- Anderson, S.L.; Stylianou, K.C. Biologically derived metal organic frameworks. Coord. Chem. Rev. 2017, 349, 102–128.
- Rojas, S.; Devic, T.; Horcajada, P. Metal organic frameworks based on bioactive components. J. Mater. Chem. B 2017, 5, 2560–2573.
- An, J.; Geib, S.J.; Rosi, N.L. Cation-triggered drug release from a porous zinc-adeninate metal-organic framework. J. Am. Chem. Soc. 2009, 131, 8376–8377.
- Vassal, G.; Gouyette, A.; Hartmann, O.; Pico, J.L.; Lemerle, J. Pharmacokinetics of high-dose busulfan in children. Cancer Chemother. Pharmacol. 1989, 24, 386–390.
- Sehouli, J.; Oskay-Özcelik, G. Current role and future aspects of topotecan in relapsed ovarian cancer. Curr. Med. Res. Opin. 2009, 25, 639–651.
- Nicum, S.J.; O’Brien, M.E.R. Topotecan for the treatment of small-cell lung cancer. Expert Rev. Anticancer. Ther. 2007, 7, 795–801.
- Park, K.S.; Ni, Z.; Cô, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 3, 10186–10191.
- Bezerra Vasconcelos, I.; Gonçalves-Silva, T.; Rodrigues, N.M. Cytotoxicity and slow release of the anti-cancer drug doxorubicin from ZIF-8 Potential of the lectin/inhibitor isolated from Crataeva tapia bark (CrataBL) for controlling Callosobruchus maculatus larvae development View project. RSC Adv. 2014, 2, 9437.
- Sun, C.Y.; Qin, C.; Wang, X.L.; Yang, G.S.; Shao, K.Z.; Lan, Y.Q.; Su, Z.M.; Huang, P.; Wang, C.G.; Wang, E.B. Zeolitic imidazolate framework-8 as efficient pH-sensitive drug delivery vehicle. Dalt. Trans. 2012, 41, 6906–6909.
- Giliopoulos, D.; Zamboulis, A.; Giannakoudakis, D.; Bikiaris, D.; Triantafyllidis, K. Polymer/Metal Organic Framework (MOF) Nanocomposites for Biomedical Applications. Molecules 2020, 25, 185.
- Gao, P.; Chen, Y.; Pan, W.; Li, N.; Liu, Z.; Tang, B. Antitumor Agents Based on Metal–Organic Frameworks. Angew. Chemie 2021, 133, 16901–16914.
- Ni, K.; Lan, G.; Chan, C.; Quigley, B.; Lu, K.; Aung, T.; Guo, N.; La Riviere, P.; Weichselbaum, R.R.; Lin, W. Nanoscale metal-organic frameworks enhance radiotherapy to potentiate checkpoint blockade immunotherapy. Nat. Commun. 2018, 9, 2351.
- Prajapati, N.; Karan, A.; Khezerlou, E.; DeCoster, M.A. The Immunomodulatory Potential of Copper and Silver Based Self-Assembled Metal Organic Biohybrids Nanomaterials in Cancer Theranostics. Front. Chem. 2021, 8, 1296.
- Darder, M.; Karan, A.; del Real, G.; DeCoster, M.A. Cellulose-based biomaterials integrated with copper-cystine hybrid structures as catalysts for nitric oxide generation. Mater. Sci. Eng. C 2020, 108, 110369.
- Xiao, J.; Chen, S.; Yi, J.; Zhang, H.F.; Ameer, G.A. A Cooperative Copper Metal–Organic Framework-Hydrogel System Improves Wound Healing in Diabetes. Adv. Funct. Mater. 2017, 27, 1604872.
- Yao, X.; Zhu, G.; Zhu, P.; Ma, J.; Chen, W.; Liu, Z.; Kong, T. Omniphobic Membrane by Microfluidic-Emulsion-Templating Method for Wound Healing. Adv. Funct. Mater. 2020, 30, 1909389.
This entry is offline, you can click here to edit this entry!
