The skeletal muscle is the storage organ for muscle glycogen and the most prominent motor organ of an organism. Consequently, the relationship between the skeletal muscle and energy metabolism cannot be ignored during physical activities, especially during exercise. The Kiss1/GPR54 system is a multifunctional genetic system with an essential role in regulating energy balance and metabolic homeostasis. Expression of Kiss1 and GPR54 mRNAs can be detected in skeletal muscle of some mammals. However, the Kiss1/GPR54 system in skeletal muscles has not been thoroughly studied. Researchers have proposed the speculation on the possible role of the kiss1 /GPRS4 system in skeletal muscle in association with exercise performance.
- Kiss1/GPR54 system
- kisspeptin
- energy metabolism
- skeletal muscle
1. Introduction
In 1996, metastin, encoded by the Kiss1 gene, was found to have the ability to suppress metastatic decrease in melanoma cells [1][2]. Since then, metastin has attracted considerable attention as a metastasis suppressor and was subsequently named kisspeptin. Kiss1, located at the human chromosome 1q32, was identified through subtractive hybridization [3]. The encoded common precursor protein (kisspeptin) contains 145 amino acids, which can be hydrolyzed to generate multiple endogenous mature peptides with a common amidated C-terminal [4], including kisspeptin-54/14/13/10 (Kp-54/14/13/10) (Figure 1) [5]. Kp-10 is the smallest peptide that can activate its receptor and function [6]. Therefore, Kp-10 is considered the predominant form of kisspeptin.
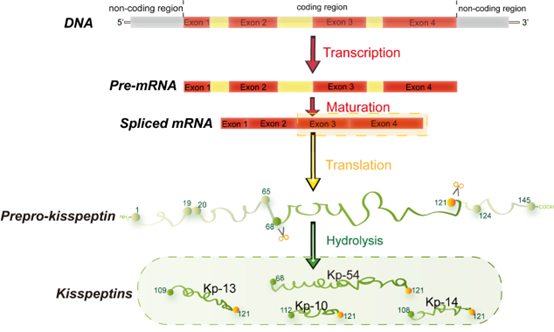
Figure 1. Major structural features of human kisspeptin gene. The human Kiss1 gene consists of four exons, the last two encoding a precursor protein translated into a 145 amino acid. This precursor protein includes a predicted signal peptide of amino acids 1-19, a potential dibasic cleavage site of amino acids 65-68, and a terminal cleavage and amidation site of amino acids 121-124. It can be fragmented into different lengths of kisspeptin with a common amidated C-terminal (KP-54/14/13/10) [7].
In 2001, researchers used three independent experiments to confirm the existence of a unique receptor of kisspeptin and named it hOT7T175 or AXOR12 [8]. As a G protein-coupled receptor, this receptor is found in human chromosome group 19p13.3 and intracellularly coupled to the Gαq/11 subfamily of Gq/11G proteins in cells [9]. Thus, it was eventually renamed GPR54 or Kiss1r. GPR54 consists of five exons and encodes a 398 (395 in mice and 396 in rats) amino acid protein in humans [6]. The activation of GPR54 by kisspeptin causes the activation of Gαq/intracellular Ca2+, mitogen-activated protein kinase, and phosphatidylinositol 3-kinase/AKT pathways [10][11]. In previous studies, the Kiss1/GPRS4 system has been shown to play an important role in mammalian reproductive function and cancer biology.
Kiss1 and GPR54 are widely expressed in the hypothalamus, some peripheral tissues, including adipose tissues [12], and the liver [13], stomach, and pancreas [14][15]. Kisspeptin signaling functions as an anorexia factor, reproductive hormone, behavioral hormone, or transfer inhibitor in different tissues [16][17][18]. Hypothalamic Kiss1 neurons are the regulatory centers of reproductive function and the active molecules of energy balance in the central circuit [19]. Peripheral kisspeptin is mainly secreted by endocrine organs, such as adipose tissues and the liver. They are essential peptides that regulate lipid accumulation and fatty acid metabolism and contribute to glucose homeostasis [20][21]. This review summarizes current evidence showing that kisspeptin plays a role in regulating energy homeostasis by modulating multi-organ function in animals and humans and discusses the controversies within the field of the Kiss1/GPR54 system and the potential physiological implications of the Kiss1/GPR54 system.
2.Possible Role of Kiss1/GPR54 System in Skeletal Muscle
GPR54 exists in human vascular smooth muscles [22], skeletal muscles of frogs [23], and skeletal muscles of mice [24], and Kiss1 is expressed in Rohu’s skeletal muscles [25]. In the cardiovascular smooth muscles of humans, kisspeptin can induce inotropic actions on cardiac function [22]. The Kiss1/GPR54 system in skeletal muscles has not been thoroughly studied. Only partial evidence in the skeletal muscle of some animals is available. The mRNA expression levels of Kiss1 and GPR54 are lower in mouse skeletal muscles than those in adipose tissues and the liver. However, whether a small amount of GPR54 in skeletal muscles can react with kisspeptin in the plasma is still unknown. More studies are needed to confirm the intrinsic link between Kiss1/GPR54 system and skeletal muscles.
2.1 Kiss1/GPR54 System Affects Ca2+ Signaling in Cells
In the skeletal muscle, Ca2+ is a key signaling molecule for proliferation and differentiation [26][27][28]. The proliferation of muscle cells requires the proliferation of mesodermal stem cells, which then gradually specialize into myogenic progenitors and further differentiate into different types of muscle cells [29]. The in vitro differentiation of myoblasts is regulated by an increase in intracellular Ca2+ induced by changes in membrane potential [30][31]. In RyR1 homozygous mutant mice, RyR-mediated Ca2+ release is eliminated, and then perinatal death and severe musculature disorders, including small myotubes and disorganized myofibrils, occur [32]. In addition, the moderate-intensity-exercise-induced adaptive hypertrophy of skeletal muscles is closely related to the recruitment of Ca2+ signaling satellite cells for repairing and regenerating skeletal muscle cells torn and damaged during exercise[29][33].
Kisspeptin activates GPR54 on the cytomembrane, which causes GPR54 and phospholipase C to combine with the G proteins of the Gαq/11 subfamily, eliciting the degradation of phospholipase C (PLC) in cells [34]. The degradation of PLC produces two types of second messengers: diacylglycerol (DAG) and trisphosphate (IP-3) [27]. IP-3 can increase the level of cellular Ca2+ [27][28]. Although these results were obtained from studies on the Kiss1/GPR54 system and cancer, given the existence of tissue interactions, researchers can speculate that Kiss1/GPR54 system is involved in the regulation of skeletal muscle Ca2+ concentration; this hypothesis needs to be confirmed by further studies.
In addition, increased kisspeptin signaling activates another second messenger, DAG, which facilitates the PKC pathway, and PKC activates MAPKs [35]. In mammals, MAPKs are involved in hormonal, neural, and cell division signaling. MAPKs interact with Ca2+ in the musculature and coordinate the regulation of skeletal muscle development [36]. Moreover, MAPKs phosphorylate ERK1/2. In myoblasts, ERK2 promotes myogenic progenitor proliferation by upregulating the expression of cyclin D1 [4]. In addition, the activity of MAPK p38 is induced during the differentiation of L8 myogenic cells, thereby promoting myogenesis [37][38]. The changes in Ca2+ signaling induced by Kiss1 at the subcellular level provide important research ideas to investigate the link between Kiss1 and skeletal muscle. Whether Kiss1 can similarly promote changes in Ca2+ concentrations in skeletal muscle and thus affect the proliferation of skeletal muscle cells may be the next step in research.
2.2 Kiss1/GPR54 System in Mitochondrial Activity
There are two distinct populations of mitochondrial subpopulations in skeletal muscle. They are classified as intermyofibrillar mitochondria (MitoIMF) and subsarcolemmal mitochondria (MitoSS) according to the location of their existence [39]. During long-term aerobic exercise, the proportion of slow-twitch fiber has increased, which subsequently causes an increase in the activity and number of MitoIMF [39][40], but the mechanism is still unclear.
In the human melanoma cell, Kiss1 inhibits the acetyl-CoA carboxylase phosphorylation by AMPK directly or upregulates PGC1α mRNA to activate the AMPK expression [41][42], which enhances mitochondrial β-oxidation and thus reverses the Warburg effect [43][44][45]. Kiss1 can also directly promote mitochondrial biogenesis by regulating the expression of PGC1α [41]. Our previous study has shown that Kiss1 and GPR54 mRNAs can be detected in the skeletal muscles of C57Bl6 mice. The concentration of kisspeptin is stabilized at low-p mol levels in the plasma [46]. Given that the enhancement of mitochondrial activity in the skeletal muscle is reflected by the ability to oxidize fatty acids and substrates [47], which is consistent with the promotion of mitochondrial biogenesis by Kiss1 in human melanoma cells, we speculate that the role of Kiss1 in promoting mitochondrial β-oxidation may be present in the skeletal muscle.
During high-intensity exercise, the phosphagen system provides approximately 50% of the ATP in the first 6 s, and the predominant ATP producer is the glycolysis system in the next 10 s [48]. Increased levels of Ca2+ and inorganic phosphate released from the sarcoplasmic reticulum lead to a high level of pyruvate production through glycogen breakdown. Pyruvate can either be metabolized in the cytoplasm to produce lactate or enter the mitochondria for oxidation [49]. This process requires both the stability of Ca2+ levels and the adequacy of mitochondrial capacity[47][29]. Therefore, combined with the fact that Kiss1 can affect Ca2+ signaling and mitochondrial β-oxidation in tumor biology, we propose that the Kiss1/GPR54 system in the skeletal muscle might have an implication for energy metabolism during exercise (Figure 2)
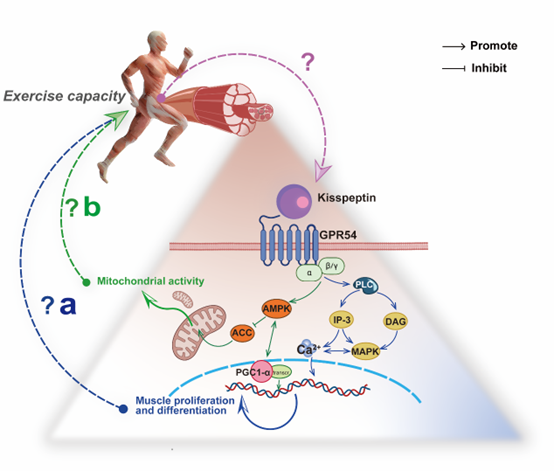
Figure 2. The hypothesis between the Kiss1/GPR54 system in skeletal muscle and exercise capacity. (a) Kisspeptin regulates Ca2+ concentration in skeletal muscle to promote proliferation and differentiation via the PLC-MAPK/IP-3 pathway. (b) Kisspeptin inhibits ACC in skeletal muscle to promote mitochondrial activity by APMK and PGC1α.
This entry is adapted from the peer-reviewed paper 10.3390/cells11193148
References
- Jeong-Hyung Lee; Mary E. Miele; Deana J. Hicks; Karen K. Phillips; Jeffery M. Trent; Bernard E. Weissman; Danny R. Welch; KiSS-1, a Novel Human Malignant Melanoma Metastasis-Suppressor Gene. Journal of the National Cancer Institute 1996, 88, 1731-1737, 10.1093/jnci/88.23.1731.
- Jeong‐Hyung Lee; Danny R. Welch; Identification of highly expressed genes in metastasis‐suppressed chromosome 6/human malignant melanoma hybrid cells using subtractive hybridization and differential display. International Journal of Cancer 1997, 71, 1035-1044, 10.1002/(sici)1097-0215(19970611)71:6<1035::aid-ijc20>3.3.co;2-t.
- Ande West; Patrick J. Vojta; Danny Welch; Bernard E. Weissman; Chromosome Localization and Genomic Structure of the KiSS-1 Metastasis Suppressor Gene (KISS1). Genomics 1998, 54, 145-148, 10.1006/geno.1998.5566.
- Masato Kotani; Michel Detheux; Ann Vandenbogaerde; David Communi; Jean-Marie Vanderwinden; Emmanuel Le Poul; Stéphane Brézillon; Richard Tyldesley; Nathalie Suarez-Huerta; Fabrice Vandeput; et al. The Metastasis Suppressor Gene KiSS-1 Encodes Kisspeptins, the Natural Ligands of the Orphan G Protein-coupled Receptor GPR54. Journal of Biological Chemistry 2001, 276, 34631-34636, 10.1074/jbc.m104847200.
- Camila Martins Trevisan; Erik Montagna; Renato De Oliveira; Denise M. Christofolini; Caio Barbosa; Keith A. Crandall; Bianca Bianco; Kisspeptin/GPR54 System: What Do We Know About Its Role in Human Reproduction?. Cellular Physiology and Biochemistry 2018, 49, 1259-1276, 10.1159/000493406.
- Tetsuya Ohtaki; Yasushi Shintani; Susumu Honda; Hirokazu Matsumoto; Akira Hori; Kimiko Kanehashi; Yasuko Terao; Satoshi Kumano; Yoshihiro Takatsu; Yasushi Masuda; et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 2001, 411, 613-617, 10.1038/35079135.
- Leonor Pinilla; Enrique Aguilar; Carlos Dieguez; Robert P Millar; Manuel Tena-Sempere; Kisspeptins and Reproduction: Physiological Roles and Regulatory Mechanisms. Physiological reviews 2012, 92, 1235-1316, 10.1152/physrev.00037.2010.
- Alison I. Muir; Larissa Chamberlain; Nabil A. Elshourbagy; David Michalovich; Darren J. Moore; Amy Calamari; Philip G. Szekeres; Henry M. Sarau; Jon K. Chambers; Paul Murdock; et al. AXOR12, a Novel Human G Protein-coupled Receptor, Activated by the Peptide KiSS-1. Journal of Biological Chemistry 2001, 276, 28969-28975, 10.1074/jbc.m102743200.
- Nicolas de Roux; Emmanuelle Genin; Jean-Claude Carel; Fumihiko Matsuda; Jean-Louis Chaussain; Edwin Milgrom; Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proceedings of the National Academy of Sciences of the United States of America 2003, 100, 10972-10976, 10.1073/pnas.1834399100.
- Su Zhang; Fangfei Yu; Anran Che; Binghe Tan; Chenshen Huang; Yuxue Chen; Xiaohong Liu; Qi Huang; Wenying Zhang; Chengbin Ma; et al. Neuroendocrine Regulation of Stress‐Induced T Cell Dysfunction during Lung Cancer Immunosurveillance via the Kisspeptin/GPR54 Signaling Pathway. Advanced Science 2022, 9, e2104132, 10.1002/advs.202104132.
- Justo P. Castaño; Antonio J. Martínez-Fuentes; Ester Gutiérrez-Pascual; Hubert Vaudry; Manuel Tena-Sempere; María M. Malagón; Intracellular signaling pathways activated by kisspeptins through GPR54: Do multiple signals underlie function diversity?. Peptides 2009, 30, 10-15, 10.1016/j.peptides.2008.07.025.
- Paweł Kołodziejski; Ewa Pruszyńska-Oszmałek; Tatiana Wojciechowicz; Maciej Sassek; Natalia Leciejewska; Mariami Jasaszwili; Maria Billert; Emilian Małek; Dawid Szczepankiewicz; Magdalena Misiewicz-Mielnik; et al. The Role of Peptide Hormones Discovered in the 21st Century in the Regulation of Adipose Tissue Functions. Genes 2021, 12, 756, 10.3390/genes12050756.
- Stephania Guzman; Magdalena Dragan; Hyokjoon Kwon; Vanessa de Oliveira; Shivani Rao; Vrushank Bhatt; Katarzyna M. Kalemba; Ankit Shah; Vinod K. Rustgi; He Wang; et al. Targeting hepatic kisspeptin receptor ameliorates nonalcoholic fatty liver disease in a mouse model. JCI Insight 2022, 132, 1, 10.1172/jci145889.
- Oline K Rønnekleiv; Jian Qiu; Martin J Kelly; Hypothalamic Kisspeptin Neurons and the Control of Homeostasis. Endocrinology 2021, 163, 1, 10.1210/endocr/bqab253.
- J. E. Bowe; Aileen King; J. S. Kinsey-Jones; V. L. Foot; X. F. Li; K. T. O’Byrne; Shanta Persaud; Peter Jones; Kisspeptin stimulation of insulin secretion: mechanisms of action in mouse islets and rats. Diabetologia 2009, 52, 855-862, 10.1007/s00125-009-1283-1.
- Mehboob A. Hussain; Woo-Jin Song; Andrew Wolfe; There is Kisspeptin – And Then There is Kisspeptin. Trends in Endocrinology & Metabolism 2015, 26, 564-572, 10.1016/j.tem.2015.07.008.
- Kana Ikegami; Teppei Goto; Sho Nakamura; Youki Watanabe; Arisa Sugimoto; Sutisa Majarune; Kei Horihata; Mayuko Nagae; Junko Tomikawa; Takuya Imamura; et al. Conditional kisspeptin neuron-specific Kiss1 knockout with newly generated Kiss1-floxed and Kiss1-Cre mice replicates a hypogonadal phenotype of global Kiss1 knockout mice. The Journal of reproduction and development 2020, 66, 359-367, 10.1262/jrd.2020-026.
- Thuc Ly; Sitaram Harihar; Danny R. Welch; KISS1 in metastatic cancer research and treatment: potential and paradoxes. Cancer and Metastasis Reviews 2020, 39, 739-754, 10.1007/s10555-020-09868-9.
- Víctor M. Navarro; Metabolic regulation of kisspeptin — the link between energy balance and reproduction. Nature Reviews Endocrinology 2020, 16, 407-420, 10.1038/s41574-020-0363-7.
- Víctor M. Navarro; Metabolic regulation of kisspeptin — the link between energy balance and reproduction. Nature Reviews Endocrinology 2020, 16, 407-420, 10.1038/s41574-020-0363-7.
- Tooru M. Mizuno; Fat Mass and Obesity Associated (FTO) Gene and Hepatic Glucose and Lipid Metabolism. Nutrients 2018, 10, 1600, 10.3390/nu10111600.
- Emma J. Mead; Janet Maguire; Rhoda E. Kuc; Anthony Peter Davenport; Kisspeptins Are Novel Potent Vasoconstrictors in Humans, with a Discrete Localization of Their Receptor, G Protein-Coupled Receptor 54, to Atherosclerosis-Prone Vessels. Endocrinology 2007, 148, 140-147, 10.1210/en.2006-0818.
- Rosanna Chianese; Vincenza Ciaramella; Silvia Fasano; Riccardo Pierantoni; Rosaria Meccariello; Kisspeptin Receptor, GPR54, as a Candidate for the Regulation of Testicular Activity in the Frog Rana esculenta1. Biology of Reproduction 2013, 88, 73, 10.1095/biolreprod.112.103515.
- Kristen P. Tolson; Nuha Marooki; Julie‐Ann P. De Bond; Evelyn Walenta; Shannon B.Z. Stephens; Reanna B. Liaw; Rishi Savur; Andrew Wolfe; Da Young Oh; Jeremy T. Smith; et al. Conditional knockout of kisspeptin signaling in brown adipose tissue increases metabolic rate and body temperature and lowers body weight. The FASEB Journal 2019, 34, 107-121, 10.1096/fj.201901600r.
- Ashis Saha; Avinash Pradhan; Sushmita Sengupta; Madhusmita Nayak; Mrinal Samanta; Lakshman Sahoo; Shiba Shankar Giri; Molecular characterization of two kiss genes and their expression in rohu (Labeo rohita) during annual reproductive cycle. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 2016, 191, 135-145, 10.1016/j.cbpb.2015.10.008.
- Stephanie A. Parsons; Douglas P. Millay; Benjamin J. Wilkins; Orlando F. Bueno; Gretchen L. Tsika; Joel R. Neilson; Christine M. Liberatore; Katherine Yutzey; Gerald R. Crabtree; Richard W. Tsika; et al. Genetic Loss of Calcineurin Blocks Mechanical Overload-induced Skeletal Muscle Fiber Type Switching but Not Hypertrophy. Journal of Biological Chemistry 2004, 279, 26192-26200, 10.1074/jbc.m313800200.
- Ke Ji; Lin Ye; Malcolm D. Mason; Wen G. Jiang; The Kiss-1/Kiss-1R complex as a negative regulator of cell motility and cancer metastasis (Review). International journal of molecular medicine 2013, 32, 747-754, 10.3892/ijmm.2013.1472.
- Jean-Marc Navenot; Zixuan Wang; Michael Chopin; Nobutaka Fujii; Stephen C. Peiper; Kisspeptin-10-Induced Signaling of GPR54 Negatively Regulates Chemotactic Responses Mediated by CXCR4: a Potential Mechanism for the Metastasis Suppressor Activity of Kisspeptins. Cancer Research 2005, 65, 10450-10456, 10.1158/0008-5472.can-05-1757.
- Michelle K. Tu; Jacqueline B. Levin; Andrew M. Hamilton; Laura N. Borodinsky; Calcium signaling in skeletal muscle development, maintenance and regeneration. Cell calcium 2016, 59, 91-97, 10.1016/j.ceca.2016.02.005.
- Stéphane Konig; Anne Béguet; Charles R. Bader; Laurent Bernheim; The calcineurin pathway links hyperpolarization (Kir2.1)-induced Ca2+ signals to human myoblast differentiation and fusion. Development 2006, 133, 3107-3114, 10.1242/dev.02479.
- Stéphane Konig; Valérie Hinard; Serge Arnaudeau; Nicolas Holzer; Gaël Potter; Charles R. Bader; Laurent Bernheim; Membrane Hyperpolarization Triggers Myogenin and Myocyte Enhancer Factor-2 Expression during Human Myoblast Differentiation. Journal of Biological Chemistry 2004, 279, 28187-28196, 10.1074/jbc.m313932200.
- Elena Zvaritch; Frederic Depreux; Natasha Kraeva; Ryan E. Loy; Sanjeewa A. Goonasekera; Simona Boncompagni; Alexander Kraev; Anthony O. Gramolini; Robert T. Dirksen; Clara Franzini-Armstrong; et al. An Ryr1 I4895T mutation abolishes Ca 2+ release channel function and delays development in homozygous offspring of a mutant mouse line. Proceedings of the National Academy of Sciences of the United States of America 2007, 104, 18537-18542, 10.1073/pnas.0709312104.
- Sophie Joanisse; Changhyun Lim; James McKendry; Jonathan C. McLeod; Tanner Stokes; Stuart M. Phillips; Recent advances in understanding resistance exercise training-induced skeletal muscle hypertrophy in humans. F1000Research 2020, 9, 141, 10.12688/f1000research.21588.1.
- Jérôme A.J. Becker; Jean-François Mirjolet; Jérôme Bernard; Emmanuel Burgeon; Marie-Jeanne Simons; Gilbert Vassart; Marc Parmentier; Frédérick Libert; Activation of GPR54 promotes cell cycle arrest and apoptosis of human tumor cells through a specific transcriptional program not shared by other Gq-coupled receptors. Biochemical and biophysical research communications 2005, 326, 677-686, 10.1016/j.bbrc.2004.11.094.
- Stephania Guzman; Muriel Brackstone; Sally Radovick; Andy V. Babwah; Moshmi M. Bhattacharya; KISS1/KISS1R in Cancer: Friend or Foe?. Frontiers in Endocrinology 2018, 9, 437, 10.3389/fendo.2018.00437.
- Xiaoning Li; Junhua Xiao; Kai Li; Yuxun Zhou; MiR-199-3p modulates the onset of puberty in rodents probably by regulating the expression of Kiss1 via the p38 MAPK pathway. Molecular and cellular endocrinology 2020, 518, 110994, 10.1016/j.mce.2020.110994.
- Anna Zetser; Dale Frank; Eyal Bengal; MAP Kinase Converts MyoD into an Instructive Muscle Differentiation Factor in Xenopus. Developmental biology 2001, 240, 168-181, 10.1006/dbio.2001.0465.
- Zetser, A.; Gredinger, E.; Bengal, E.; p38 mitogen-activated protein kinase pathway promotes skeletal muscle differentiation. Participation of the Mef2c transcription factor. . J Biol Chem 1999, 274,, 5193-5200,, 10.1074/jbc.274.8.5193..
- Carsten Lundby; Robert A Jacobs; Adaptations of skeletal muscle mitochondria to exercise training. null 1970, 101, 17-22, 10.5167/uzh-122925.
- David A. Hood; Jonathan M. Memme; Ashley N. Oliveira; Matthew Triolo; Maintenance of Skeletal Muscle Mitochondria in Health, Exercise, and Aging. Annual Review of Physiology 2019, 81, 19-41, 10.1146/annurev-physiol-020518-114310.
- Sharon J. Manley; Wen Liu; Danny R. Welch; The KISS1 metastasis suppressor appears to reverse the Warburg effect by shifting from glycolysis to mitochondrial beta-oxidation. Journal of Molecular Medicine 2017, 95, 951-963, 10.1007/s00109-017-1552-2.
- Wen Liu; Benjamin H. Beck; Kedar S. Vaidya; Kevin T. Nash; Kyle P. Feeley; Scott W. Ballinger; Keke M. Pounds; Warren L. Denning; Anne R. Diers; Aimee Landar; et al. Metastasis Suppressor KISS1 Seems to Reverse the Warburg Effect by Enhancing Mitochondrial Biogenesis. Cancer Research 2014, 74, 954-963, 10.1158/0008-5472.can-13-1183.
- L. Tong; Acetyl-coenzyme A carboxylase: crucial metabolic enzyme and attractive target for drug discovery. Cellular and Molecular Life Sciences 2005, 62, 1784-1803, 10.1007/s00018-005-5121-4.
- Tae-Suk Kim; Patrick Leahy; Hedley C. Freake; Promoter Usage Determines Tissue Specific Responsiveness of the Rat Acetyl-CoA Carboxylase Gene. Biochemical and biophysical research communications 1996, 225, 647-653, 10.1006/bbrc.1996.1224.
- Maria V. Liberti; Jason W. Locasale; The Warburg Effect: How Does it Benefit Cancer Cells?. Trends in biochemical sciences 2016, 41, 211-218, 10.1016/j.tibs.2015.12.001.
- Jimena Pita; Vicente Barrios; Teresa Gavela-Pérez; Gabriel Á. Martos-Moreno; María T. Muñoz-Calvo; Jesús Pozo; Adela Rovira; Jesús Argente; Leandro Soriano-Guillén; Circulating kisspeptin levels exhibit sexual dimorphism in adults, are increased in obese prepubertal girls and do not suffer modifications in girls with idiopathic central precocious puberty. Peptides 2011, 32, 1781-1786, 10.1016/j.peptides.2011.07.016.
- Jesus R. Huertas; Rafael A. Casuso; Pablo Hernansanz Agustín; Sara Cogliati; Stay Fit, Stay Young: Mitochondria in Movement: The Role of Exercise in the New Mitochondrial Paradigm. Oxidative Medicine and Cellular Longevity 2019, 2019, 1-18, 10.1155/2019/7058350.
- Michelle L. Parolin; Alan Chesley; Mark P. Matsos; Lawrence L. Spriet; Norman L. Jones; George J. F. Heigenhauser; Regulation of skeletal muscle glycogen phosphorylase and PDH during maximal intermittent exercise. American Journal of Physiology-Endocrinology and Metabolism 1999, 277, E890-E900, 10.1152/ajpendo.1999.277.5.e890.
- George A. Brooks; The Science and Translation of Lactate Shuttle Theory. Cell metabolism 2018, 27, 757-785, 10.1016/j.cmet.2018.03.008.
- Michelle L. Parolin; Alan Chesley; Mark P. Matsos; Lawrence L. Spriet; Norman L. Jones; George J. F. Heigenhauser; Regulation of skeletal muscle glycogen phosphorylase and PDH during maximal intermittent exercise. American Journal of Physiology-Endocrinology and Metabolism 1999, 277, E890-E900, 10.1152/ajpendo.1999.277.5.e890.
- George A. Brooks; The Science and Translation of Lactate Shuttle Theory. Cell metabolism 2018, 27, 757-785, 10.1016/j.cmet.2018.03.008.
