Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Solid-state NMR is a nondestructive and noninvasive technique used to study the chemical structure and dynamics of starch-based materials and to bridge the gap between structure–function relationships and industrial applications.
- solid-state NMR spectroscopy
- starch
- food science
1. Introduction
The world population is expected to increase to 10 billion by 2050 [1], resulting in growing concerns over global food security. Food production is one of the largest industries globally [2]. Estimates by the United Nations Food and Agriculture Organization (FAO) suggest that a 50% production increase may be needed to meet future demands [1]. A key concern is whether the high demand for food may lead to instability in value-chains, and furthermore, if production targets can be met considering increased water and land scarcity. The future of sustainable food systems may include a shift towards a plant-based diet, as well as significant reductions in food waste [1]. To achieve this, the efficiency of production for existing plant-based foods may need to be improved. Thus, it is critical to understand the connection between the form, function, and properties of the food constituents [3].
Starch is a highly abundant, biodegradable, and hydrophilic carbohydrate typically found in staple crops such as corn, potatoes, wheat, rice, and green fruits [4][5][6][7]. It is an important raw material within a wide range of industries from packaging (e.g., coatings, films adhesives) to biomedical and pharmaceuticals (e.g., tissue and drug carriers) [8]. It is used most frequently in the food industry, where it is estimated that up to 60% [3] of the starch produced is used either as a food product, or a food-based additive for preservative, thickening, texturizing, emulsion stabilization, aroma and flavor encapsulation, or quality enhancement [8][9]. Starch can be used to produce biodegradable packaging films, used to extend the shelf life of foods [10][11], or for encapsulation of food compounds which can improve food quality via protecting bioactive food ingredients from oxidation, or degradation due to UV or acidic conditions [12].
2. Study of Dynamics in Starch in the Presence of Plasticizers and Structural Modifications
Many industrial and food applications of starch are directly related to its physical-chemical properties, such as gelatinization, crystallinity, adhesion, solubility, and viscosity. Starch plasticization, via the addition of water and glycerol in different proportions, can tune the thermoplastic properties and change the phase transition of starch. This, in turn, produces a physically modified starch in a homogeneous polymeric state [13][14][15][16][17].
Carr–Purcell–Meiboom–Gill (CPMG) echo decay train [18][19] is an essential component in NMR pulse sequences used for measuring the dynamic properties of starch [20]. Train pulses refocus the inhomogeneous broadening of the nuclear spins. This makes it possible to obtain spin–spin T2 relaxation decays that possess crucial information regarding the dynamics and composition of native and plasticized starch. The CPMG decay curves presented in Figure 1a show a slight difference for native starch (10.8% water) and starch with addition of water (24.2% water). However, upon the addition of glycerol, a significant difference was revealed. Three separated peaks appeared for native and water mixed starch, as represented in Figure 1b. In contrast, four peaks were observed in the case of glycerol addition (with and without water).
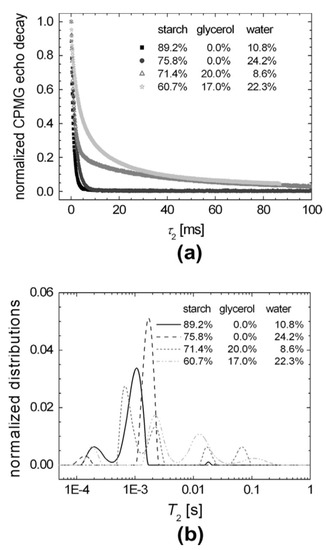
Figure 1. T2 relaxation time NMR measurements: (a) CPMG-based NMR experiments for starch with variable glycerol and water content; and (b) T2 time distributions obtained from the CPMG decay curves via Laplace inversion. Adapted with permission from Ref. [20]. Copyright 2013, Elsevier.
From the three peaks observed for native starch, two could be related to the rigid backbone chain. The first peak at 0.2 msec could be associated with the polymeric chain segments with the lowest mobility located close to the branched backbone. The second peak at 1 msec could be associated with a more mobile segment located away from the core branched region. The third peak at 20 msec represents the mobile branches of the amylopectin microstructure and free amylose end chains.
Upon the addition of water, a shift in the mobile and semi-mobile peaks to higher T2 values was observed. The peak corresponding to the rigid component shifted in the opposite direction, thus indicating the formation of soft matter structures. The addition of glycerol lead to the formation of a semi-mobile region. This semi-mobile region consists of two peaks at 0.6 and 2.5 msec and a mobile one at around 20 and 70 msec. This is related to an increase in the amylose free end chains and amylopectin lateral branches. This suggests an increase in the total mobility of the starch polymeric chain [20].
Starch modification and blending with active compounds have been considered a wide sectional area in the food and product industry. However little information, on a molecular level, is known about the dominant interactions and binding sites between the starch and the integrated active compounds [21][22][23][24][25][26][27][28][29]. The interaction between potato starch and cuminaldehyde was analyzed via ssNMR [30], and found to be based on hydrogen bonding, with primary starch binding sites on the oxygen atoms of the hydroxyl-2, 3 functional groups [30].
Conventional solid-state NMR experiments, including the 1H single pulse depicted in Figure 2a and the 13C CP MAS experiments presented in Figure 2b, were performed on porous starch (PS) and a blend of starch and cuminaldehyde (C/PS). For the purposes of comparison, a 13C solution state NMR experiment was conducted on cuminaldehyde (C).
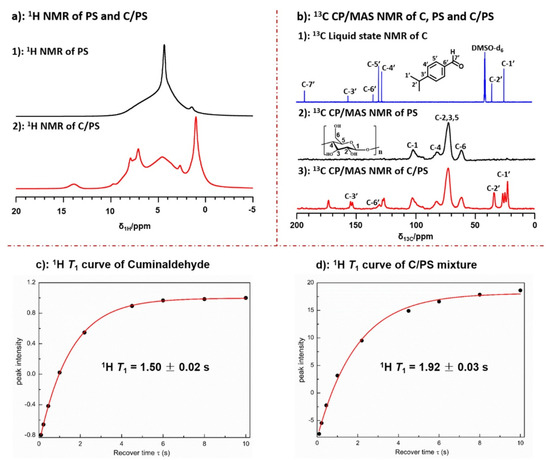
Figure 2. ssNMR techniques for measuring the structure and relaxation properties of PS (black) and C/PS (red): (a) single pulse 1H MAS NMR spectra for PS and C/PS; (b) 13C CP MAS NMR spectra for PS and C/PS compared to solution state spectrum of cuminaldehyde; and (c) 1H T1 relaxation curve for cuminaldehyde; and (d) 1H T1 relaxation curve for C/PS. Adapted with permission from Ref. [30]. Copyright 2022, Elsevier.
The results obtained from proton and carbon solid-state NMR spectra were consistent with starch loaded with significant amounts of cuminaldehyde. Upon comparing the solution state spectra of cuminaldehyde to the solid-state one for C/PS, it was found that only a single methyl peak appeared in the solution state spectrum, while three peaks between 20 and 30 ppm were detected in the solid-state spectrum. This indicates interactions between cuminaldehyde methyl groups and starch. It also indicates that three different environments of free and adsorbed cuminaldehyde were present. The same phenomena appeared in C3 of the phenyl group, where two different peaks appeared in the solid-state spectrum compared to one single peak in solution state.
Molecular mobility was investigated by measuring the relaxation properties for cuminaldehyde represented in Figure 2c and C/PS represented in Figure 2d. The 1H T1 measurements show a significant difference in the molecular dynamics between cuminaldehyde in its pure form (1H T1 = 1.5 sec) and when adsorbed in the starch structure (1H T1 = 1.92 sec). The increase in the 1H T1 values for cuminaldehyde was attributed to the restricted mobility of the cuminaldehyde molecules in the starch structure, thus resulting in an increase in the longitudinal relaxation [30].
This entry is adapted from the peer-reviewed paper 10.3390/polym14214686
References
- García-Oliveira, P.; Fraga-Corral, M.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Solutions for the Sustainability of the Food Production and Consumption System. Crit. Rev. Food Sci. Nutr. 2022, 62, 1765–1781.
- Blazek, J.; Gilbert, E.P. Application of Small-Angle X-ray and Neutron Scattering Techniques to the Characterisation of Starch Structure: A Review. Carbohydr. Polym. 2011, 85, 281–293.
- Copeland, L.; Blazek, J.; Salman, H.; Tang, M.C. Form and Functionality of Starch. Food Hydrocoll. 2009, 23, 1527–1534.
- Bangar, S.P.; Ashogbon, A.O.; Singh, A.; Chaudhary, V.; Whiteside, W.S. Enzymatic Modification of Starch: A Green Approach for Starch Applications. Carbohydr. Polym. 2022, 287, 119265.
- Chakraborty, I.; Pooja, N.; Mal, S.S.; Paul, U.C.; Rahman, M.H.; Mazumder, N. An Insight into the Gelatinization Properties Influencing the Modified Starches Used in Food Industry: A Review. Food Bioprocess Technol. 2022, 15, 1195–1223.
- Cui, C.; Jia, Y.; Sun, Q.; Yu, M.; Ji, N.; Dai, L.; Wang, Y.; Qin, Y.; Xiong, L.; Sun, Q. Recent Advances in the Preparation, Characterization, and Food Application of Starch-Based Hydrogels. Carbohydr. Polym. 2022, 291, 119624.
- Obadi, M.; Xu, B. Review on the Physicochemical Properties, Modifications, and Applications of Starches and Its Common Modified Forms Used in Noodle Products. Food Hydrocoll. 2021, 112, 106286.
- Fan, Y.; Picchioni, F. Modification of Starch: A Review on the Application of “Green” Solvents and Controlled Functionalization. Carbohydr. Polym. 2020, 241, 116350.
- Boutboul, A.; Giampaoli, P.; Feigenbaum, A.; Ducruet, V. Influence of the Nature and Treatment of Starch on Aroma Retention. Carbohydr. Polym. 2002, 47, 73–82.
- Baysal, G.; Doğan, F. Investigation and Preparation of Biodegradable Starch-Based Nanofilms for Potential Use of Curcumin and Garlic in Food Packaging Applications. J. Biomater. Sci. 2020, 31, 1127–1143.
- Baysal, G.; Çelik, B.Y. Synthesis and Characterization of Antibacterial Bio-Nano Films for Food Packaging. J. Environ. Sci. Health Part B 2019, 54, 79–88.
- Guo, Y.; Qiao, D.; Zhao, S.; Zhang, B.; Xie, F. Starch-Based Materials Encapsulating Food Ingredients: Recent Advances in Fabrication Methods and Applications. Carbohydr. Polym. 2021, 270, 118358.
- Nessi, V.; Rolland-Sabaté, A.; Lourdin, D.; Jamme, F.; Chevigny, C.; Kansou, K. Multi-Scale Characterization of Thermoplastic Starch Structure Using Second Harmonic Generation Imaging and NMR. Carbohydr. Polym. 2018, 194, 80–88.
- Pushpadass, H.A.; Kumar, A.; Jackson, D.S.; Wehling, R.L.; Dumais, J.J.; Hanna, M.A. Macromolecular Changes in Extruded Starch-Films Plasticized with Glycerol, Water and Stearic Acid. Starch 2009, 61, 256–266.
- Smits, A.L.M.; Kruiskamp, P.H.; van Soest, J.J.G.; Vliegenthart, J.F.G. Interaction between Dry Starch and Plasticisers Glycerol or Ethylene Glycol, Measured by Differential Scanning Calorimetry and Solid State NMR Spectroscopy. Carbohydr. Polym. 2003, 53, 409–416.
- Šoltýs, A.; Hronský, V.; Šmídová, N.; Olčák, D.; Ivanič, F.; Chodák, I. Solid-State 1H and 13C NMR of Corn Starch Plasticized with Glycerol and Urea. Eur. Polym. J. 2019, 117, 19–27.
- Vrábel, P.; Baran, A.; Kovaľaková, M.; Fričová, O.; Hutníková, M.; Olčák, D. Characterization of Native and Plasticized Starch Using Solid State NMR. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2018; Volume 1996, p. 20049.
- van Duynhoven, J.; Voda, A.; Witek, M.; van As, H. Chapter 3—Time-Domain NMR Applied to Food Products. In Annual Reports on NMR Spectroscopy; Academic Press: Cambridge, MA, USA, 2010; Volume 69, pp. 145–197. ISBN 0066-4103.
- Vallurupalli, P.; Hansen, D.F.; Lundström, P.; Kay, L.E. CPMG Relaxation Dispersion NMR Experiments Measuring Glycine 1Hα and 13Cα Chemical Shifts in the ‘Invisible’ Excited States of Proteins. J. Biomol. NMR 2009, 45, 45–55.
- Cioica, N.; Fechete, R.; Cota, C.; Nagy, E.M.; David, L.; Cozar, O. NMR Relaxation Investigation of the Native Corn Starch Structure with Plasticizers. J. Mol. Struct. 2013, 1044, 128–133.
- Brito, L.M.; Sebastião, P.J.O.; Bruno Tavares, M.I. NMR Relaxometry Evaluation of Nanostructured Starch-PLA Blends. Polym. Test. 2015, 45, 161–167.
- Cheng, W.; Luo, Z.; Li, L.; Fu, X. Preparation and Characterization of Debranched-Starch/Phosphatidylcholine Inclusion Complexes. J. Agric. Food Chem. 2015, 63, 634–641.
- Fričová, O.; Hutníková, M.; Kovaľaková, M.; Baran, A. Influence of Aging on Molecular Motion in PBAT-Thermoplastic Starch Blends Studied Using Solid-State NMR. Int. J. Polym. Anal. Charact. 2020, 25, 275–282.
- Martini, F.; Hughes, D.J.; Badolato Bönisch, G.; Zwick, T.; Schäfer, C.; Geppi, M.; Alam, M.A.; Ubbink, J. Antiplasticization and Phase Behavior in Phase-Separated Modified Starch-Sucrose Blends: A Positron Lifetime and Solid-State NMR Study. Carbohydr. Polym. 2020, 250, 116931.
- Spěváček, J.; Brus, J.; Divers, T.; Grohens, Y. Solid-State NMR Study of Biodegradable Starch/Polycaprolactone Blends. Eur. Polym. J. 2007, 43, 1866–1875.
- Zhang, Q.; Xu, K.; Wang, P. Study on Structure and Molecular Dynamics of Starch/Poly(Sodium Acrylate)-Grafted Superabsorbent by 13C Solid State NMR. Fibers Polym. 2008, 9, 271–275.
- Zhang, X.; Dean, K.; Burgar, I.M. A High-Resolution Solid-State NMR Study on Starch–Clay Nanocomposites and the Effect of Aging on Clay Dispersion. Polym. J. 2010, 42, 689–695.
- Hughes, D.; Tedeschi, C.; Leuenberger, B.; Roussenova, M.; Coveney, A.; Richardson, R.; Bönisch, G.B.; Alam, M.A.; Ubbink, J. Amorphous-Amorphous Phase Separation in Hydrophobically-Modified Starch–Sucrose Blends II. Crystallinity and Local Free Volume Investigation Using Wide-Angle X-Ray Scattering and Positron Annihilation Lifetime Spectroscopy. Food Hydrocoll. 2016, 58, 316–323.
- Hughes, D.J.; Bönisch, G.B.; Zwick, T.; Schäfer, C.; Tedeschi, C.; Leuenberger, B.; Martini, F.; Mencarini, G.; Geppi, M.; Alam, M.A.; et al. Phase Separation in Amorphous Hydrophobically Modified Starch–Sucrose Blends: Glass Transition, Matrix Dynamics and Phase Behavior. Carbohydr. Polym. 2018, 199, 1–10.
- Ma, Y.; Wang, Z.; Wang, Y.; Zhang, S. Molecular Insight into the Interactions between Starch and Cuminaldehyde Using Relaxation and 2D Solid-State NMR Spectroscopy. Carbohydr. Polym. 2022, 278, 118932.
This entry is offline, you can click here to edit this entry!
