Cancer is a predominant cause of mortality all over the world. Lung, prostate, and colorectal cancer are the more frequent in men while breast and colorectal have a high incidence in women. Major progress aside, some cancers are still frequent and one major issue is improvements in detection methods. Imaging techniques have a major role, but inflammatory, tumoral markers and calculated scores may contribute to the assessment of prognosis. The erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and carcinoembryonic antigen cell adhesion molecule (CEACAM) have been used for decades and do not have a clear use for diagnosis or prognosis yet. The CEACAM family includes 12 human members, and some of them have a cluster differentiation (CD). CD66 may be an interesting indicator of disease severity. Beside interleukin-6 (IL-6), the high level of which is observed in patients with a high mortality rate, other cytokines IL-17A, IL-22, and transforming growth factor -β (TGF-β) are expressed at the tumor level. The detection of circulating tumor cells has been improved but is still of undetermined value. Circulating tumor DNA (ctDNA) was recently studied in CRC stage II patients and may be helpful for chemotherapy management.
1. Inflammatory Markers in CRC
1.1. Erythrocyte Sedimentation Rate (ESR) and Hemogram
Historically, erythrocyte sedimentation rate (ESR) was routinely used as a marker of inflammation, infection, and cancer. The highest values were observed in different types of cancer and tuberculosis. Blood leukocyte count, neutrophil lymphocyte ratio, and ESR have been investigated as possible predictive indicators of prognosis in cancer patients. Complete blood cell count is routinely prescribed in patients over 50 years old. CRC may provoke gut bleeding and, consequently, reductions in the red blood cell count and hemoglobin may occur. In other cases, when an inflammatory process is induced by CRC, an increase in white blood cell count and platelet count may be observed and more specialized tests and investigation are necessary. In cases with early hepatic metastasis, modification of liver enzymes may be a first signal. Increased ESR was associated with reduced overall survival in patients with soft tissue sarcoma [
13]. A poor oncologic outcome has been reported to be associated with elevated ESR in multiple myeloma and Hodgkin’s disease [
14,
15].
1.2. C-Reactive Protein (CRP)
C-reactive protein is routinely measured in patients with possible infectious or inflammatory disease. CRP is one of the major indicators of acute phase inflammatory response including cancer. CRP exists in multiple isoforms and may act as a mediator of defense response against cancer [
16]. In its monomeric isoform, CRP reacts with the cell membrane and activated blood cells such as platelets and leukocytes. The pentameric isoform is the form with which blood level is measured. High levels (>10 µg/mL) were found to be associated with cancer development. CRP is of potential value for a prognosis; however, CRP values can change rapidly and a direct relationship to the stage of the extent of the disease cannot be established.
Inflammatory cytokine interleukin 6 (IL-6) and CRP blood levels are frequently correlated in pathological conditions [
17]. CRP may stimulate production of interleukin 8 (IL-8). The increased level of interleukin 1 β (IL-1β) and IL-6, frequently observed in cancer, may amplify CRP production. Evaluation of CRP in cancer appears as a tool which may indicate disease severity and progression which can be useful for the therapeutic management of patients.
1.3. Systemic Inflammatory Score or Index
Several scores or indexes have been proposed to evaluate the prognosis of patients with colorectal cancer. In several cohorts of patients undergoing surgery, systemic inflammation reaction was evaluated by the modified Glasgow Prognostic Score (mGPS) [
18,
19], platelet-lymphocyte ratio (PLR), neutrophil–lymphocyte ratio (NLR) [
20,
21], lymphocyte–monocyte ratio (LMR) [
22], and prognostic nutritional index (PNI) [
23].
The modified Glasgow Prognostic Score (mGPS), an inflammation-based prognostic score, uses thresholds of C-reactive protein (>10 mg/L) and albumin (<35 g/L). The addition of neutrophil and platelet counts increased the prognostic value of the mGPS [
18].
A statistical analysis using univariable analysis showed a poor overall survival in patients with preoperative NLR ≥ 5 and greater recurrence when NLR is between 1.25–5.19. According to this study NLR has a prognostic value in patients undergoing elective surgery for mismatch repair-deficient colorectal cancer [
24].
According to the authors, lymphocyte–C-reactive protein ratio (LCR) is a predictor of postoperative anastomotic leakage [
25]. PLR, according to a meta-analysis, may be used as an additional predictor of overall survival [
26]. A meta-analysis of LMR suggests that a high ratio may be a significant predictor of better overall survival; however, it is only based on the results of retrospective studies [
27]. The preoperative and postoperative low PNI were observed in the group of patients with poor survival rates [
23].
Some points remained to be underlined, which limited the value of these indexes or scores. There is no quality control of the values which are issued from different laboratories, from different countries, and included a very large number of patients. The statistical analysis is sophisticated, but it is well established that we may find a correlation when the number of individuals is large enough and there are multiple parameters. If, as underlined by Rossi S. et al. [
28], common factors may be responsible for the CRP, albumin synthesis, and bone marrow stimulation, the interest of multiplying the score is probably limited. Gene expression analysis or cytokine measurement may be more performant.
1.4. Cytokines
Inflammatory conditions are considered to favor cancer development. Several clinical or experimental studies pointed out a relationship between interleukin-6 (IL-6) and inflammation in patients with colorectal cancer. Chronic inflammation is considered an important risk factor for cancer development in particular inflammatory bowel diseases [
29]. IL-6 expression is correlated with CRC prognosis. Increased IL-6 is observed in patients with advanced CRC stage and a reduced life expectancy. According to the potential role of IL-6 through Janus kinases (JAKs) and signal transducer activator of transcription 3 (STAT3), several therapeutic approaches targeting IL-6/STAT3 have been explored [
30]. The production of interferon-γ (IFN-γ) by lymphocytes has been demonstrated to limit tumor growth [
31,
32,
33]. On the other hand, Th17 CD4, producing interleukin-17A (IL-17A) present in CRC, facilitates tumor progression in human and in experimental models [
34,
35]. IL-17A, IL-22, and IL-6 induce the activation of NF-κB and participate in CRC cell proliferation [
36]. In 1994 Ueda T. et al. wrote that IL6 blood level was significantly elevated in different ways depending on the stage of differentiation of the adenocarcinoma [
37]. A case control study and meta-analysis [
38] described that IL8 may be a promising marker and found that there is heterogeneity in the European subgroup of patients with CRC. IL8 appears to have a promoting effect on cell migration through upregulation of integrin αvβ6 which is involved in CRC cell migration [
39]. In addition, a meta-analysis [
40] showed that higher levels of IL8 were correlated with lymphatic and liver metastasis. Interleukin10 has immunomodulatory properties. Low levels of IL10 and IL18 are found in patients with a better prognosis and a longer life expectancy [
41].
Transforming growth factor-β (TGF-β) has a dual role in tumor development, it may limit cancer cell proliferation at an early stage. On tumor progress, it facilitates tumor angiogenesis and metastasis [
42]. Components involved (reactive oxygen species, proteolytic enzymes, cytokines, and growth factors) in inflammatory reaction participate in the tissue reaction in the vicinity of tumors. Cancer cell dedifferentiation facilitates tumor invasion [
43]. Cancer metastasis is facilitated by the enhancement of vascular permeability. In the microvasculature, circulating tumor cells tend to invade into stromal tissue. Interactions between endothelial cells (EC) and tumors are important steps mediated by different receptors or chemical structures. Monocytes may release several factors, platelet derived growth factor, vascular endothelial growth factor, and fibroblast growth factor, which may interfere with tumor growth and may enhance the expression of ICAM, VCAM-1, E Selectin, P selectin, and matrix metalloproteinases (MMPs) [
44]. Tumor cell extravasation is dependent upon vascular permeability [
45]. Two mechanisms may be involved in transendothelial permeability: vesicle transport and migration through endothelial cell junctions. A derived collagen tripeptide (proline–glycine–proline) promotes VE-cadherin phosphorylation and enhanced vascular permeability. Tumor cells can bind to endothelial cells and induce EC necrosis via a tumor necrosis factor (TNF) receptor family mechanism. Glycocalyx molecules, hyaluronic acid, heparan sulfate, and chondroitin sulfate limit the access of circulating tumor cells to adhesion receptors, such as intercellular cell adhesion molecule-1 (ICAM-1) or P selectin [
46].
2. Tumor Markers
2.1. Carcinoembryonic Antigen (CEA)
In CRC the introduction of the measurement of CEA is common but the variability limits the use as a formal diagnosis tool. The carcinoembryonic antigen (CEA), first isolated from human CRC is probably one of the major phenotypic change detectable in human cancer cells [
47]. It is a glycoprotein which belongs to the immunoglobulin superfamily of proteins. The genotypic basis is incompletely known and still the goal of research [
48]. Antibodies directed against CEA react with numerous proteins which were initially termed non-specific cross-reacting antigen (NCA) [
49]. Glycosylated modified CEA is present in CRC. Tumor specific glycosylated CEA (Lewis X and Lewis Y) can interact with dendritic cell-specific intercellular adhesion molecule3-grabbing non integrin (DC-SIGN) [
50].
2.2. CEA Structure
The human CEA gene family contains 29 genes/pseudogene. The CEA family of proteins has been proposed to be divided into three groups: CEA cell adhesion molecule (CEACAM), pregnancy-specific glycoprotein (PSG), and pseudogene group [
51]. The carcinoembryonic antigen (CEA) family nomenclature has been redefined [
52]. The members of the family are now named CEACAM with a number and some of them have a cluster of differentiation (CD).
2.3. CEACAM Family
The CEACAM joints the group of cell adhesion receptors. Attention was paid to the involvement of selectins first in inflammation and hemostasis, but also most recently in cancer metastasis [
53,
54]. The proteins possess an amino terminal Ig variable (IgV)-like domain. CEACAM family members (CEACAM1, CEACAM3, and CEACAM4) are connected to the intracellular domain by transmembrane helices [
55]. The CEACAM are also known as CD66a to CD66e [
56].
CEACAM1 and CEACAM3 encode transmembrane proteins while CEACAM8, CEACAM6 and CEA anchored in the cell membrane. Anoikis-mediated cell death occurred simultaneously with loss of integrins. CEACAM6 protects many cell lines from death [
57,
58]. CEACAM16 is a secreted form found in cochlear cells. A mutation of CEACAM 16 leads to autosomal dominant hearing loss [
59].
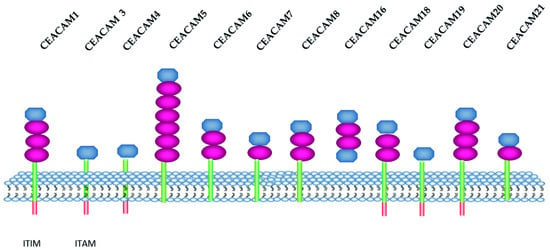
In humans, the CEACAM listed group consists of 12 members [
60] (
Figure 1), while a larger number was described in mice. In
Table 1 CEACAM and CD nomenclature are listed.
Figure 1. Schematic representation of human CEACAM isoforms. CEACAM belong to the immunoglobulin super family. They have at least one immunoglobulin-like domain Ig variable (IgV) and Ig constant (IgC). IgV and IgC are colored in blue and red, respectively, the cytoplasmic domain in orange and the GP-anchor in green. ITIM: immunoreceptor tyrosine-based inhibition motif. ITAM: immunoreceptor tyrosine-based activation motif (
uniprot.org;
proteinatlas.org) (accessed date 18 November 2021).
2.4. CEACAM and Leukocytes
CEACAM1, CEACAM8, CEACAM6, and CEACAM3 (either CD66a, CD66b, CD66c, and CD66d) are expressed on human neutrophils. Anti-CD66 antibodies increased leukocyte adhesion to human endothelial cells [
61]. CEACAM1 may be expressed by activated T lymphocytes. Mucin domain 3 (TIM3) is expressed on Th1 and Th17 cells [
62]. TIM 3 can ligate CEACAM1 [
60]. CEACAM1 interaction with TIM3 increased the inhibitory properties mediated by these receptors [
63]. The interaction between CEACAM1 and TIM3 can result in T cell suppression and may play a role in regulating autoimmunity [
64,
65].
3. Micro-RNA and Circulating Tumor DNA (ctDNA) in CRC Patients
A new approach using micro-RNA (miRNA) as a biomarker for CRC was investigated suggesting that miRNA-106a expression was increased and miRNA-30a-3p, miRNA 145, miRNA-125a, and miRNA-133a expression was decreased in CRC tissues. miRNAs may become candidates to develop biomarkers [
93].
DNA with tumor specific modification can be found in blood. ctDNA comes from dead tumor cells and are small fragments, including specific alteration in tumor oncogene microsatellites [
94,
95]. In patients with CRC treated by surgery and chemotherapy, the presence of ctDNA may represent a risk factor, but in several studies the survival time was similar with positive or negative ctDNA [
96]. A meta-analysis reveals that detection panels contained genetic or epigenetic alterations. The most frequent mutations were KRAS, but other mutations can be associated. ctDNA appears to be an independent factor of prognosis [
97]. Stage II CRC patients with TP53, APC, and KRAS mutations and postoperative ctDNA positivity have a reduced disease-free survival. One of the major problems is the absence of a standardized definition of ctDNA. Another difficulty is the low level of ctDNA and the difference in techniques used for the detection. In most of the studies ctDNA-positive patients have a poorer prognosis. Beside the Dukes’ staging and tumor node and metastasis (TNM) staging [
98], the carcinoembryonic antigen blood level provided prognostic information (
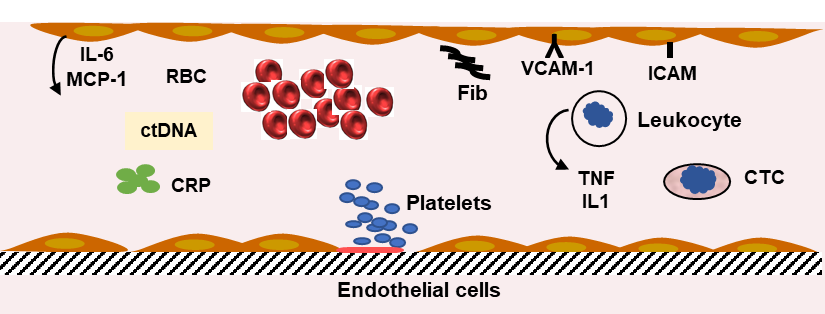
Figure 3).
Figure 3. Inflammation and inflammatory markers. The defense mechanism involves leukocytes, platelets, and liver synthesis of proteins. The increase in fibrinogen (Fib) synthesis participates in the abnormal red blood cell (RBC) rheology and the increase in erythrocyte sedimentation rate. C reactive protein (CRP) blood level (pentamer) is increased. Several cytokine levels are elevated. Interleukin-6 (IL-6) is produced by endothelial cells (EC) and liver cells. Macrophage chemoattractant protein-1 (MCP1) is secreted by EC, tumor necrosis factor α (TNF), and interleukin-1β (IL1) by lymphocytes. The blood levels of cytokines are indicators of the inflammatory reaction induced by colorectal cancer. Circulating tumor cell (CTC) and circulating tumor DNA (ctDNA) can be measured in blood and used as an index of tumor progression.
This entry is adapted from the peer-reviewed paper 10.3390/ijms232112968