Several reports have shown that the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has the potential to also be neurotropic. Four Adverse Outcome Pathways (AOPs) leading to neurological adverse outcomes (AO), anosmia, encephalitis, stroke, and seizure, were developed. Biological key events (KEs) identified to induce these AOs included binding to ACE2, blood–brain barrier (BBB) disruption, hypoxia, neuroinflammation, and oxidative stress. The modularity of AOPs allows the construction of AOP networks to visualize core pathways and recognize neuroinflammation and BBB disruption as shared mechanisms.
- AOP
- SARS-CoV-2
- neuropathology
- anosmia
1. ACE2 Receptor Interaction
2. Blood–Brain Barrier (BBB) Disruption
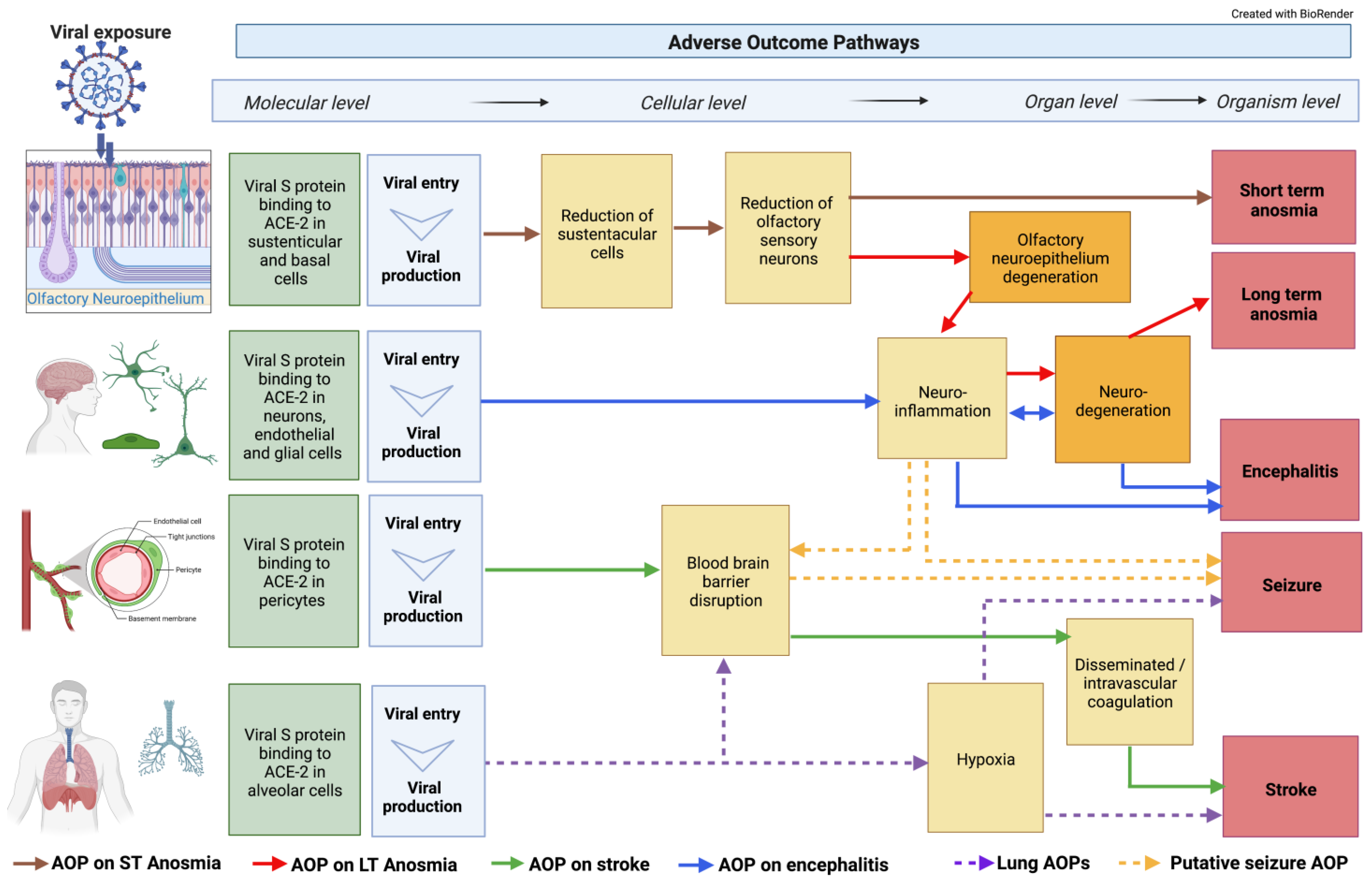
3. Hypoxia and Hypoxemia
4. Neuroinflammation and Oxidative Stress
5. Neurodegeneration
6. Anosmia
7. Encephalitis
8. Stroke
9. Seizure and Epilepsy
10. Integration of Classical AOPs
This entry is adapted from the peer-reviewed paper 10.3390/cells11213411
References
- Xu, X.; Chen, P.; Wang, J.; Feng, J.; Zhou, H.; Li, X.; Zhong, W.; Hao, P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020, 63, 457–460.
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280 e278.
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273.
- Benton, D.J.; Wrobel, A.G.; Xu, P.; Roustan, C.; Martin, S.R.; Rosenthal, P.B.; Skehel, J.J.; Gamblin, S.J. Receptor binding and priming of the spike protein of SARS-CoV-2 for membrane fusion. Nature 2020, 588, 327–330.
- Pezzini, A.; Padovani, A. Lifting the mask on neurological manifestations of COVID-19. Nat. Rev. Neurol. 2020, 16, 636–644.
- Cui, H.; Su, S.; Cao, Y.; Ma, C.; Qiu, W. The Altered Anatomical Distribution of ACE2 in the Brain With Alzheimer’s Disease Pathology. Front. Cell Dev. Biol. 2021, 9, 684874.
- Sardu, C.; Gambardella, J.; Morelli, M.B.; Wang, X.; Marfella, R.; Santulli, G. Hypertension, Thrombosis, Kidney Failure, and Diabetes: Is COVID-19 an Endothelial Disease? A Comprehensive Evaluation of Clinical and Basic Evidence. J. Clin. Med. 2020, 9, 1417.
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M.; et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020, 370, 856–860.
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brunink, S.; Greuel, S.; et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175.
- Butowt, R.; Meunier, N.; Bryche, B.; von Bartheld, C.S. The olfactory nerve is not a likely route to brain infection in COVID-19: A critical review of data from humans and animal models. Acta Neuropathol. 2021, 141, 809–822.
- Buzhdygan, T.P.; DeOre, B.J.; Baldwin-Leclair, A.; Bullock, T.A.; McGary, H.M.; Khan, J.A.; Razmpour, R.; Hale, J.F.; Galie, P.A.; Potula, R.; et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier. Neurobiol. Dis. 2020, 146, 105131.
- Khaddaj-Mallat, R.; Aldib, N.; Bernard, M.; Paquette, A.S.; Ferreira, A.; Lecordier, S.; Saghatelyan, A.; Flamand, L.; ElAli, A. SARS-CoV-2 deregulates the vascular and immune functions of brain pericytes via Spike protein. Neurobiol. Dis. 2021, 161, 105561.
- Matschke, J.; Lutgehetmann, M.; Hagel, C.; Sperhake, J.P.; Schroder, A.S.; Edler, C.; Mushumba, H.; Fitzek, A.; Allweiss, L.; Dandri, M.; et al. Neuropathology of patients with COVID-19 in Germany: A post-mortem case series. Lancet Neurol. 2020, 19, 919–929.
- Lee, M.H.; Perl, D.P.; Nair, G.; Li, W.; Maric, D.; Murray, H.; Dodd, S.J.; Koretsky, A.P.; Watts, J.A.; Cheung, V.; et al. Microvascular Injury in the Brains of Patients with COVID-19. N. Engl. J. Med. 2021, 384, 481–483.
- Spudich, S.; Nath, A. Nervous system consequences of COVID-19. Science 2022, 375, 267–269.
- Ye, Q.; Zhou, J.; He, Q.; Li, R.T.; Yang, G.; Zhang, Y.; Wu, S.J.; Chen, Q.; Shi, J.H.; Zhang, R.R.; et al. SARS-CoV-2 infection in the mouse olfactory system. Cell Discov. 2021, 7, 49.
- Zazhytska, M.; Kodra, A.; Hoagland, D.A.; Fullard, J.F.; Shayya, H.; Omer, A.; Firestein, S.; Gong, Q.; Canoll, P.D.; Goldman, J.E.; et al. Disruption of nuclear architecture as a cause of COVID-19 induced anosmia. bioRxiv 2021, 9, 430314.
- Junqueira, C.; Crespo, A.; Ranjbar, S.; de Lacerda, L.B.; Lewandrowski, M.; Ingber, J.; Parry, B.; Ravid, S.; Clark, S.; Schrimpf, M.R.; et al. FcgammaR-mediated SARS-CoV-2 infection of monocytes activates inflammation. Nature 2022, 606, 576–584.
- Chen, Z.; Li, G. Immune response and blood-brain barrier dysfunction during viral neuroinvasion. Innate Immun. 2021, 27, 109–117.
- Jaunmuktane, Z.; Mahadeva, U.; Green, A.; Sekhawat, V.; Barrett, N.A.; Childs, L.; Shankar-Hari, M.; Thom, M.; Jager, H.R.; Brandner, S. Microvascular injury and hypoxic damage: Emerging neuropathological signatures in COVID-19. Acta Neuropathol. 2020, 140, 397–400.
- Achar, A.; Ghosh, C. COVID-19-Associated Neurological Disorders: The Potential Route of CNS Invasion and Blood-Brain Relevance. Cells 2020, 9, 2360.
- Krasemann, S.; Haferkamp, U.; Pfefferle, S.; Woo, M.S.; Heinrich, F.; Schweizer, M.; Appelt-Menzel, A.; Cubukova, A.; Barenberg, J.; Leu, J.; et al. The blood-brain barrier is dysregulated in COVID-19 and serves as a CNS entry route for SARS-CoV-2. Stem. Cell Rep. 2022, 17, 307–320.
- Paniz-Mondolfi, A.; Bryce, C.; Grimes, Z.; Gordon, R.E.; Reidy, J.; Lednicky, J.; Sordillo, E.M.; Fowkes, M. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J. Med. Virol. 2020, 92, 699–702.
- Erickson, M.A.; Rhea, E.M.; Knopp, R.C.; Banks, W.A. Interactions of SARS-CoV-2 with the Blood-Brain Barrier. Int. J. Mol. Sci. 2021, 22, 2681.
- Solomon, I.H.; Normandin, E.; Bhattacharyya, S.; Mukerji, S.S.; Keller, K.; Ali, A.S.; Adams, G.; Hornick, J.L.; Padera, R.F., Jr.; Sabeti, P. Neuropathological Features of COVID-19. N. Engl. J. Med. 2020, 383, 989–992.
- Thakur, K.T.; Miller, E.H.; Glendinning, M.D.; Al-Dalahmah, O.; Banu, M.A.; Boehme, A.K.; Boubour, A.L.; Bruce, S.S.; Chong, A.M.; Claassen, J.; et al. COVID-19 neuropathology at Columbia University Irving Medical Center/New York Presbyterian Hospital. Brain 2021, 144, 2696–2708.
- Frontera, J.A.; Melmed, K.; Fang, T.; Granger, A.; Lin, J.; Yaghi, S.; Zhou, T.; Lewis, A.; Kurz, S.; Kahn, D.E.; et al. Toxic Metabolic Encephalopathy in Hospitalized Patients with COVID-19. Neurocrit. Care 2021, 35, 693–706.
- Taylor, C.T.; Doherty, G.; Fallon, P.G.; Cummins, E.P. Hypoxia-dependent regulation of inflammatory pathways in immune cells. J. Clin. Investig. 2016, 126, 3716–3724.
- Cioffi, M.; Kuffer, J.; Strobel, S.; Dubini, G.; Martin, I.; Wendt, D. Computational evaluation of oxygen and shear stress distributions in 3D perfusion culture systems: Macro-scale and micro-structured models. J. Biomech. 2008, 41, 2918–2925.
- Hayley, S.; Sun, H. Neuroimmune multi-hit perspective of coronaviral infection. J. Neuroinflamm. 2021, 18, 231.
- Amruta, N.; Chastain, W.H.; Paz, M.; Solch, R.J.; Murray-Brown, I.C.; Befeler, J.B.; Gressett, T.E.; Longo, M.T.; Engler-Chiurazzi, E.B.; Bix, G. SARS-CoV-2 mediated neuroinflammation and the impact of COVID-19 in neurological disorders. Cytokine Growth Factor Rev. 2021, 58, 1–15.
- Kanberg, N.; Ashton, N.J.; Andersson, L.M.; Yilmaz, A.; Lindh, M.; Nilsson, S.; Price, R.W.; Blennow, K.; Zetterberg, H.; Gisslen, M. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19. Neurology 2020, 95, e1754–e1759.
- Aschner, M. Immune and inflammatory responses in the CNS: Modulation by astrocytes. Toxicol. Lett. 1998, 102-103, 283–287.
- Graeber, M.B.; Streit, W.J. Microglia: Immune network in the CNS. Brain Pathol. 1990, 1, 2–5.
- Monnet-Tschudi, F.; Zurich, M.G.; Honegger, P. Neurotoxicant-induced inflammatory response in three-dimensional brain cell cultures. Hum. Exp. Toxicol. 2007, 26, 339–346.
- Streit, W.J.; Walter, S.A.; Pennell, N.A. Reactive microgliosis. Prog. Neurobiol. 1999, 57, 563–581.
- Kraft, A.D.; Harry, G.J. Features of microglia and neuroinflammation relevant to environmental exposure and neurotoxicity. Int. J. Environ. Res. Public Health 2011, 8, 2980–3018.
- Claycomb, K.I.; Johnson, K.M.; Winokur, P.N.; Sacino, A.V.; Crocker, S.J. Astrocyte regulation of CNS inflammation and remyelination. Brain Sci. 2013, 3, 1109–1127.
- Dong, Y.; Benveniste, E.N. Immune function of astrocytes. Glia 2001, 36, 180–190.
- Brown, G.C.; Bal-Price, A. Inflammatory neurodegeneration mediated by nitric oxide, glutamate, and mitochondria. Mol. Neurobiol. 2003, 27, 325–355.
- Nakajima, K.; Kohsaka, S. Microglia: Neuroprotective and neurotrophic cells in the central nervous system. Curr Drug Targets Cardiovasc. Haematol. Disord 2004, 4, 65–84.
- Murta, V.; Villarreal, A.; Ramos, A.J. Severe Acute Respiratory Syndrome Coronavirus 2 Impact on the Central Nervous System: Are Astrocytes and Microglia Main Players or Merely Bystanders? ASN Neuro 2020, 12, 1759091420954960.
- Song, E.; Bartley, C.M.; Chow, R.D.; Ngo, T.T.; Jiang, R.; Zamecnik, C.R.; Dandekar, R.; Loudermilk, R.P.; Dai, Y.; Liu, F.; et al. Divergent and self-reactive immune responses in the CNS of COVID-19 patients with neurological symptoms. Cell Rep. Med. 2021, 2, 100288.
- Franke, C.; Ferse, C.; Kreye, J.; Reincke, S.M.; Sanchez-Sendin, E.; Rocco, A.; Steinbrenner, M.; Angermair, S.; Treskatsch, S.; Zickler, D.; et al. High frequency of cerebrospinal fluid autoantibodies in COVID-19 patients with neurological symptoms. Brain Behav. Immun. 2021, 93, 415–419.
- Eden, A.; Kanberg, N.; Gostner, J.; Fuchs, D.; Hagberg, L.; Andersson, L.M.; Lindh, M.; Price, R.W.; Zetterberg, H.; Gisslen, M. CSF Biomarkers in Patients With COVID-19 and Neurologic Symptoms: A Case Series. Neurology 2021, 96, e294–e300.
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405.
- Mohammadi, S.; Moosaie, F.; Aarabi, M.H. Understanding the Immunologic Characteristics of Neurologic Manifestations of SARS-CoV-2 and Potential Immunological Mechanisms. Mol. Neurobiol. 2020, 57, 5263–5275.
- Pereira, A. Long-Term Neurological Threats of COVID-19: A Call to Update the Thinking About the Outcomes of the Coronavirus Pandemic. Front. Neurol. 2020, 11, 308.
- Lu, Y.; Li, X.; Geng, D.; Mei, N.; Wu, P.Y.; Huang, C.C.; Jia, T.; Zhao, Y.; Wang, D.; Xiao, A.; et al. Cerebral Micro-Structural Changes in COVID-19 Patients - An MRI-based 3-month Follow-up Study. EClinicalMedicine 2020, 25, 100484.
- Arachchillage, D.R.J.; Laffan, M. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 1233–1234.
- Lee, S.; Viqar, F.; Zimmerman, M.E.; Narkhede, A.; Tosto, G.; Benzinger, T.L.; Marcus, D.S.; Fagan, A.M.; Goate, A.; Fox, N.C.; et al. White matter hyperintensities are a core feature of Alzheimer’s disease: Evidence from the dominantly inherited Alzheimer network. Ann. Neurol. 2016, 79, 929–939.
- Wen, Y.; Yang, S.; Liu, R.; Brun-Zinkernagel, A.M.; Koulen, P.; Simpkins, J.W. Transient cerebral ischemia induces aberrant neuronal cell cycle re-entry and Alzheimer’s disease-like tauopathy in female rats. J. Biol. Chem. 2004, 279, 22684–22692.
- Gerges Harb, J.; Noureldine, H.A.; Chedid, G.; Eldine, M.N.; Abdallah, D.A.; Chedid, N.F.; Nour-Eldine, W. SARS, MERS and COVID-19: Clinical manifestations and organ-system complications: A mini review. Pathog. Dis. 2020, 78, ftaa033.
- Qi, F.; Qian, S.; Zhang, S.; Zhang, Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem. Biophys. Res Commun. 2020, 526, 135–140.
- Sungnak, W.; Huang, N.; Becavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-Lopez, C.; Maatz, H.; Reichart, D.; Sampaziotis, F.; et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020, 26, 681–687.
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020, 80, 607–613.
- Mahalakshmi, A.M.; Ray, B.; Tuladhar, S.; Bhat, A.; Paneyala, S.; Patteswari, D.; Sakharkar, M.K.; Hamdan, H.; Ojcius, D.M.; Bolla, S.R.; et al. Does COVID-19 contribute to development of neurological disease? Immun. Inflamm. Dis. 2021, 9, 48–58.
- Meng, X.; Deng, Y.; Dai, Z.; Meng, Z. COVID-19 and anosmia: A review based on up-to-date knowledge. Am. J. Otolaryngol. 2020, 41, 102581.
- Saniasiaya, J.; Islam, M.A.; Abdullah, B. Prevalence of Olfactory Dysfunction in Coronavirus Disease 2019 (COVID-19): A Meta-analysis of 27,492 Patients. Laryngoscope 2021, 131, 865–878.
- AbdelHamid, S.G.; Refaat, A.A.; Benjamin, A.M.; Elmawardy, L.A.; Elgendy, L.A.; Manolly, M.M.; Elmaksoud, N.A.; Sherif, N.; Hamdy, N.M. Deciphering epigenetic(s) role in modulating susceptibility to and severity of COVID-19 infection and/or outcome: A systematic rapid review. Environ. Sci. Pollut. Res. Int. 2021, 28, 54209–54221.
- Coecke, S.; Munoz, A.; D’Alessandro, V.; De Bernardi, F.; Romeo, P.; Torres, F.; Harris, G.; Parvatam, S. Knowledge from human relevant cell, tissue and mathematics-based methods as key tools for understanding COVID-19. In The Coronavirus Pandemic and the Future: Virology, Epidemiology, Translational Toxicology and Therapeutics; Waters, M.D., Dhawan, A., Marrs, T., Anderson, D., Warren, S., Hughes, C.L., Eds.; Royal Society of Chemistry: London, UK, 2021; pp. 452–455. Available online: https://www.chemistryworld.com/the-coronavirus-pandemic-and-the-future/knowledge-from-human-relevant-cell-tissue-and-mathematics-based-methods-as-key-tools-for-understanding-covid-19/4013732.article (accessed on 13 September 2022).
- Bilinska, K.; Jakubowska, P.; Von Bartheld, C.S.; Butowt, R. Expression of the SARS-CoV-2 Entry Proteins, ACE2 and TMPRSS2, in Cells of the Olfactory Epithelium: Identification of Cell Types and Trends with Age. ACS Chem. Neurosci. 2020, 11, 1555–1562.
- Zazhytska, M.; Kodra, A.; Hoagland, D.A.; Frere, J.; Fullard, J.F.; Shayya, H.; McArthur, N.G.; Moeller, R.; Uhl, S.; Omer, A.D.; et al. Non-cell-autonomous disruption of nuclear architecture as a potential cause of COVID-19-induced anosmia. Cell 2022, 185, 1052–1064.e12.
- Prem, B.; Liu, D.T.; Besser, G.; Sharma, G.; Dultinger, L.E.; Hofer, S.V.; Matiasczyk, M.M.; Renner, B.; Mueller, C.A. Long-lasting olfactory dysfunction in COVID-19 patients. Eur. Arch. Otorhinolaryngol. 2022, 279, 3485–3492.
- SARS-CoV-2 Infection of Olfactory Epithelium Leading to Impaired Olfactory Function (Short-Term Anosmia). Available online: https://aopwiki.org/aops/394 (accessed on 13 September 2022).
- Shahbaz, M.A.; De Bernardi, F.; Alatalo, A.; Sachana, M.; Clerbaux, L.-A.; Muñoz, A.; Parvatam, S.; Landesmann, B.; Kanninen, K.M.; Coecke, S. Mechanistic Understanding of the Olfactory Neuroepithelium Involvement Leading to Short-Term Anosmia in COVID-19 Using the Adverse Outcome Pathway Framework. Cells 2022, 11, 3027.
- Wu, Y.; Xu, X.; Chen, Z.; Duan, J.; Hashimoto, K.; Yang, L.; Liu, C.; Yang, C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020, 87, 18–22.
- Iaconetta, G.; De Luca, P.; Scarpa, A.; Cassandro, C.; Cassandro, E. Meningoencephalitis Associated with SARS-Coronavirus-2. Transl. Med. UniSa 2020, 23, 42–47.
- Moriguchi, T.; Harii, N.; Goto, J.; Harada, D.; Sugawara, H.; Takamino, J.; Ueno, M.; Sakata, H.; Kondo, K.; Myose, N.; et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020, 94, 55–58.
- Xiang, Y.T.; Zhao, Y.J.; Liu, Z.H.; Li, X.H.; Zhao, N.; Cheung, T.; Ng, C.H. The COVID-19 outbreak and psychiatric hospitals in China: Managing challenges through mental health service reform. Int. J. Biol. Sci. 2020, 16, 1741–1744.
- Vargas, G.; Medeiros Geraldo, L.H.; Gedeao Salomao, N.; Viana Paes, M.; Regina Souza Lima, F.; Carvalho Alcantara Gomes, F. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and glial cells: Insights and perspectives. Brain Behav. Immun. Health 2020, 7, 100127.
- Ransohoff, R.M.; Perry, V.H. Microglial physiology: Unique stimuli, specialized responses. Annu. Rev. Immunol. 2009, 27, 119–145.
- Chatterjee, D.; Biswas, K.; Nag, S.; Ramachandra, S.G.; Das Sarma, J. Microglia play a major role in direct viral-induced demyelination. Clin. Dev. Immunol. 2013, 2013, 510396.
- Wheeler, D.L.; Sariol, A.; Meyerholz, D.K.; Perlman, S. Microglia are required for protection against lethal coronavirus encephalitis in mice. J. Clin. Investig. 2018, 128, 931–943.
- Binding of Sars-CoV-2 Spike Protein to ACE 2 Receptors Expressed on Brain Cells (Neuronal and Non-Neuronal) Leads to Neuroinflammation Resulting in Encephalitis. Available online: https://aopwiki.org/aops/374 (accessed on 13 September 2022).
- Montalvan, V.; Lee, J.; Bueso, T.; De Toledo, J.; Rivas, K. Neurological manifestations of COVID-19 and other coronavirus infections: A systematic review. Clin. Neurol. Neurosurg. 2020, 194, 105921.
- Aghayari Sheikh Neshin, S.; Shahjouei, S.; Koza, E.; Friedenberg, I.; Khodadadi, F.; Sabra, M.; Kobeissy, F.; Ansari, S.; Tsivgoulis, G.; Li, J.; et al. Stroke in SARS-CoV-2 Infection: A Pictorial Overview of the Pathoetiology. Front. Cardiovasc. Med. 2021, 8, 649922.
- Divani, A.A.; Andalib, S.; Di Napoli, M.; Lattanzi, S.; Hussain, M.S.; Biller, J.; McCullough, L.D.; Azarpazhooh, M.R.; Seletska, A.; Mayer, S.A.; et al. Coronavirus Disease 2019 and Stroke: Clinical Manifestations and Pathophysiological Insights. J. Stroke Cerebrovasc. Dis. 2020, 29, 104941.
- Zhou, Y.L.; Lu, J.; Cheng, Y.B.; Xin, N. Nervous system complications of COVID-19 with a focus on stroke. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 13044–13048.
- Binding of Sars-CoV-2 Spike Protein to ACE 2 Receptors Expressed on Pericytes Leads to Disseminated Intravascular Coagulation Resulting in Cerebrovascular Disease (Stroke). Available online: https://aopwiki.org/aops/395 (accessed on 13 September 2022).
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J., Jr.; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE official report: A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482.
- Vitalakumar, D.; Sharma, A.; Kumar, A.; Flora, S.J.S. Neurological Manifestations in COVID-19 Patients: A Meta-Analysis. ACS Chem. Neurosci. 2021, 12, 2776–2797.
- Nikbakht, F.; Mohammadkhanizadeh, A.; Mohammadi, E. How does the COVID-19 cause seizure and epilepsy in patients? The potential mechanisms. Mult. Scler. Relat. Disord. 2020, 46, 102535.
- Kwon, A.; Kwak, B.O.; Kim, K.; Ha, J.; Kim, S.J.; Bae, S.H.; Son, J.S.; Kim, S.N.; Lee, R. Cytokine levels in febrile seizure patients: A systematic review and meta-analysis. Seizure 2018, 59, 5–10.
- Swissa, E.; Serlin, Y.; Vazana, U.; Prager, O.; Friedman, A. Blood-brain barrier dysfunction in status epileptics: Mechanisms and role in epileptogenesis. Epilepsy Behav. 2019, 101, 106285.
- de Vries, E.E.; van den Munckhof, B.; Braun, K.P.; van Royen-Kerkhof, A.; de Jager, W.; Jansen, F.E. Inflammatory mediators in human epilepsy: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2016, 63, 177–190.
- Kovacs, R.; Heinemann, U.; Steinhauser, C. Mechanisms underlying blood-brain barrier dysfunction in brain pathology and epileptogenesis: Role of astroglia. Epilepsia 2012, 53 (Suppl. S6), 53–59.
- Asadi-Pooya, A.A.; Simani, L.; Shahisavandi, M.; Barzegar, Z. COVID-19, de novo seizures, and epilepsy: A systematic review. Neurol. Sci. 2021, 42, 415–431.
- Lau, K.K.; Yu, W.C.; Chu, C.M.; Lau, S.T.; Sheng, B.; Yuen, K.Y. Possible central nervous system infection by SARS coronavirus. Emerg. Infect. Dis. 2004, 10, 342–344.
- Hung, E.C.; Chim, S.S.; Chan, P.K.; Tong, Y.K.; Ng, E.K.; Chiu, R.W.; Leung, C.B.; Sung, J.J.; Tam, J.S.; Lo, Y.M. Detection of SARS coronavirus RNA in the cerebrospinal fluid of a patient with severe acute respiratory syndrome. Clin. Chem. 2003, 49, 2108–2109.
- Villeneuve, D.L.; Crump, D.; Garcia-Reyero, N.; Hecker, M.; Hutchinson, T.H.; LaLone, C.A.; Landesmann, B.; Lettieri, T.; Munn, S.; Nepelska, M.; et al. Adverse outcome pathway (AOP) development I: Strategies and principles. Toxicol. Sci. 2014, 142, 312–320.
- Villeneuve, D.L.; Angrish, M.M.; Fortin, M.C.; Katsiadaki, I.; Leonard, M.; Margiotta-Casaluci, L.; Munn, S.; O’Brien, J.M.; Pollesch, N.L.; Smith, L.C.; et al. Adverse outcome pathway networks II: Network analytics. Environ. Toxicol. Chem. 2018, 37, 1734–1748.
