Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biology
African American (AA) men have 2.4 times higher mortality rate due to prostate cancer than White men in the United States. Evidence implicates circadian rhythm disruption (CRD) as a potential driver of prostate cancer risk and progression. AA men are particularly vulnerable to CRDs due to greater exposure to night shift work, artificial light at night, noise pollution, racial discrimination, and socioeconomic disadvantages.
- prostate cancer
- circadian genes
- night shift work
- artificial light at night
- jet lag
1. Introduction
Prostate cancer (PCa) is the second leading cause of cancer-related mortality for men in the United States [1,2]. Significant racial disparities exist at all stages of PCa treatment, including diagnosis, management, and follow-up care [3]. African American (AA) men are 1.6 times as likely to be diagnosed with PCa and at a 2.4 times higher risk to die of the disease compared to White men [4]. The racial disparities in PCa may be due to lower rates of health literacy, comorbidities such as diabetes and obesity, and behavioral factors such as smoking; in addition, racial bias and barriers to healthcare access impact PCa outcomes of AA men [3]. While the current risk factors for PCa are age, race, and family history, increased epidemiological evidence points to the role of circadian rhythm disorder (CRD) in cancer progression [5]. In addition to cancer metastasis, CRD is a risk factor for various other conditions, such as chronic sleep deprivation, obesity, metabolic syndrome, cardiovascular diseases, and psychiatric diseases [6]. CRD is characterized by the lack of synchrony between the endogenous master circadian clock and the external light–dark cycles [7].
AA men and women may be at heightened vulnerability for developing CRD due to a greater prevalence of night shift work, environmental factors (e.g., living in neighborhoods characterized by high noise pollution, exposure to low daytime light levels or too much nighttime light), and chronic conditions (e.g., diabetes, obesity, long-term stress, cardiovascular disease) [8,9,10,11,12,13]. The underdiagnosis of sleep disorders, including obstructive sleep apnea (OSA), within predominately AA communities contributes to abnormal sleep architecture, which puts OSA patients at high risk for CRD. Further research is required to understand the exact relationship between CRD on OSA [14,15,16,17]. Epidemiological evidence so far points to the connections between PCa and CRD. Meta-analyses on the health outcomes of airplane pilots [18,19] and studies on female night shift nurses [20] have demonstrated the contribution of CRD (due to jetlag or night shift work) to both prostate and breast cancer [21]. Sleep disruption and light-induced melatonin suppression, both related outcomes of CRD, are associated with an increased risk for advanced PCa [22,23]. Lastly, the consequences of CRD—including circadian gene polymorphisms and conditions such as diabetes, obesity, and depression—contribute to an increased PCa risk [24,25,26,27,28].
Taken together, the unique impact CRD has on the AA population and its role in PCa, elucidating the biological mechanisms through which CRD contributes to PCa, may be critical to mitigating the racial disparities in PCa outcomes. Compelling evidence suggests that CRD may contribute to PCa progression through (a) circadian-gene variants [29,30,31] (b) stress and obesity-related biological pathways [32,33,34], and (c) melatonin inhibition [35]. As a result of CRDs, circadian clock genes no longer function as tumor suppressors, contributing to worse PCa outcomes. The complex interplay between stress, obesity, and circadian disruption may have detrimental effects on the tumor microenvironment and could enhance the stress-related PCa growth pathway, otherwise known as the glucocorticoid-mediated androgen receptor signaling pathway. Considering these findings, targeting the melatonin pathway and the glucocorticoid receptor, both of which are implicated in CRD, may provide new opportunities to impair PCa growth and overcome therapeutic resistance, respectively.
2. Regulation of the Circadian Clock System
Driven by the 24 h rotation of the planet, almost all organisms have adapted on earth by developing an internal biological clock system known as the circadian system, which rhythmically synchronizes sleep, metabolic, and dietary behavior to light/dark cycles [36]. In any given tissue, around 10–20% of the genome is expressed in a circadian manner [37]. Circadian rhythms play a significant role in the sleep/wake cycle, metabolic function, and gene expression [38]. Disruption of circadian rhythms has major consequences on the body’s ability to regulate metabolic homeostasis [39].
The central circadian clock, located in the suprachiasmatic nuclei (SCN) of the anterior hypothalamus, regulates the timing of activities of the peripheral clocks [40]. Light–dark patterns synchronize the SCN with the external environment, assuring that the body does the “right thing at the right time” [41]. In diurnal species, the light phase is associated with an increase in body temperature, heart rate, and blood pressure. During the dark phase, melatonin production increases, body temperature declines, heart rate slows down, and blood pressure lowers [42]. Within individual cells, the circadian clock is self-regulated by transcriptional–translational feedback loops (TTFLs) [43]. TTFLs are comprised of a positive arm with a heterodimeric complex at its core that behaves as the activator of the system, promoting the transcription of one or more components of the negative arm, which, when translated, inhibits the activity of the positive arm. Transcription activator factors CLOCK and BMAL1 make up one arm of the feedback loop, and repressor proteins PER and CRY, made from Per1/2/3 and Cry1/2 genes, make up the other arm. Accessory TTFLs regulate the primary TTFL. The first accessory loop is made up of RORs and nuclear REV-ERB receptors, while the second accessory loop is composed of D-box-related genes and transcription factors, including albumin D-binding protein (DBP), thyrotroph embryonic factor (TEF), and hepatic leukemia factor (HLF) [7,44].
In humans, during the light phase (morning), transcription activators BMAL1 and CLOCK form heterodimers, bind to the E-box (5′-CACGTG-3′), and signal the transcription of target genes Period (Per 1/2/3) and Cryptochrome (Cry 1/2) [45]. These target genes encode transcriptional repressors, PER and CRY proteins. During the light phase, PER and CRY transcription is high, and PER and CRY proteins accumulate in the cytoplasm. During the dark phase (evening), PER and CRY proteins dimerize to form PER–CRY complexes, with subsequent nuclear translocation and inactivation of the CLOCK/BMAL1-mediated transcription, reprising their own transcription and closing the loop. As the dark phase progresses, PER and CRY complexes are gradually phosphorylated by casein kinase I (CkIδ and CkIε) and 5′ AMP-activated protein kinase (AMPK), and subsequently realize their degradation through the proteasome pathway [46]. Degradation of the PER and CRY repressor proteins allows for CLOCK-BMAL1 transcription to resume, thus initiating a new transcriptional cycle [47]. In addition to the primary feedback loop, accessory loops are formed from nuclear orphan receptors, retinoid-related orphan receptors (RORs), and REV-ERBα/β, which target BMAL1 production through binding to ROR- binding elements (ROREs) [7]. REV-ERBα/β inhibits BMAL1 transcription upon binding, while ROR, acting as a positive regulator, initiates BMAL1 transcription [45] (Figure 1).
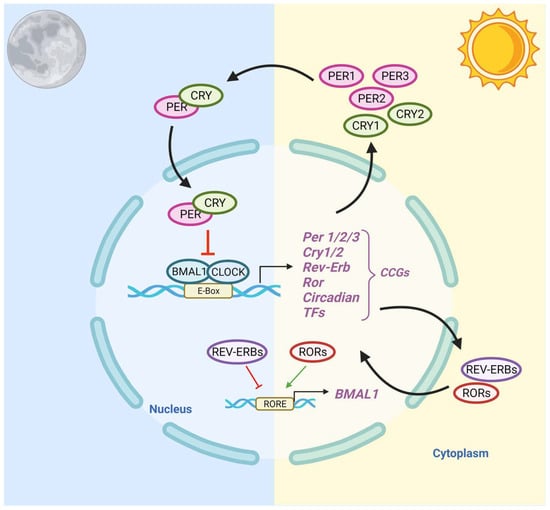
Figure 1. Schematic Representation of the Circadian Clock Transcriptional–Translational Feedback Loops. The main feedback loop is comprised of activator proteins Brain and Muscle ARNT-Like 1 (BMAL1) and Circadian Locomotor Output Cycles Kaput (CLOCK), and two repressor proteins, Period (PER) and Cryptochrome (CRY). BMAL1 and CLOCK heterodimerize and bind to the E-box, which activates the transcription of CRY (1-2), PER (1-3), RORα, REV-ERBα, and Clock transcription factors (TFs). The primary negative feedback occurs when CRY and PER accumulate and dimerize in the cytoplasm and translocate to the nucleus to inhibit the BMAL1: CLOCK. In the secondary feedback loop, RORα and REV-ERBα activate and inhibit the transcription of BMAL1.
While the SCN mainly relies on cues from light–dark cycles to entrain the biological clock, peripheral clocks, such as the ones in the reproductive, endocrine, and immune systems, receive timing signals from the SCN as well as from feeding patterns, temperature, and hormones [48,49,50]. The feed/fasting cycle dictates nutrient intake within specific periods during the day: the periodic phosphorylation of energy sensors such as AMP-activated protein kinase (AMPK) promotes ATP production in response to exercise/fasting and encourages the breakdown of fatty acids, glucose, and triglycerides after eating a meal. AMPK destabilizes CRY1 in peripheral proteins and interacts with SIRTUIN 1(SIRT1), which modulates transcription factors including PER2 [51]. In addition to nutrient-sensing molecules, ligand-activated transcription factors can have several effects on clock genes; REV-ERB regulates gluconeogenesis and the lipid metabolism while repressing Bmal1 transcription, while transcription factor ROR, its competitive inhibitor, induces Bmal1 expression upon binding. PPAR is activated by fatty acids and plays a role in lipid homeostasis through a positive feedback loop with BMAL1 protein [52]. Thus, peripheral clocks are involved in several important metabolic functions, including digestion, hormone secretion, lipid homeostasis, and the immune system response [53].
3. Epidemiological Evidence—The Link between CRDs and Prostate Cancer Risk
Disruption of circadian rhythms is thought to be caused by environmental noise pollution [54,55], jet lag [56,57], night shift work [58], and artificial light at night (ALAN) [59]. CRD is associated with various health consequences, including premature death, metabolic syndrome, obesity, immune dysregulation, reproductive problems, stress, and depression [6]. Compelling evidence demonstrates that PCa is linked with both the causes and consequences of CRDs [60].
3.1. Causes of CRD
Noise pollution: Nocturnal environmental noise, such as noise from transportation or industrial plants, can induce disturbances in sleep quality, metabolic and psychiatric changes, and alterations in sleep architecture [61]. As a consequence of noise exposure, the redistribution of time spent in different sleep stages—increasing wakefulness and stage one sleep, and decreasing slow-wave sleep and REM sleep—negatively impacts cognitive performance, mood, and energy restoration while increasing daytime sleepiness [62]. Epidemiological studies have found that exposure to traffic noise at night increases the risk of hypertension, heart disease, and stroke. Nocturnal noise contributes to an increased risk for cardiovascular comorbidity through the greater secretion of endocrine hormones, including cortisol, noradrenaline, and adrenaline [61]. Nocturnal ambient noise exposure is associated with dysregulation of the central circadian clock, and may be involved in alternations in peripheral clock genes [63].
Jetlag: CRD may contribute to PCa through jetlag. Studies involving US pilots and astronauts have determined there is an increased risk of developing PCa, yet not PCa mortality [64,65,66]. Longer air hours, number of employment years, and radiation exposure positively correlate with increased PCa risk [19,67]. Male pilots are at least twice as likely to develop PCa than men in the general population, and several subgroups, including AA pilots and military pilots, were found to have an increased risk of PCa [18,68].
Night shift work: In addition to jetlag, numerous studies have established the impact of night shift work on cancer risk. In 2017, the International Agency for Research on Cancer identified rotating shift work, in association with circadian disruption, as a probable human carcinogen, placing it in the same risk category as ultraviolet radiation, benzo(a)pyrene, and acrylamide [69]. Co-exposures within night shift workplaces, including noise levels and light at night, may additionally be linked with CRD [70]. A longitudinal study found that female nurses who engaged in nightshift work for over thirty years displayed a 36% increase in the relative risk of breast cancer [71]. The established evidence for the effect of night shift work on the prevalence of breast cancer has incited the investigation of night shift work in relation to PCa [72]. Several meta-analyses, including cohort-based studies in Japan, Canada, and Spain, found an association between night shift work and PCa [21,72,73,74,75]. In the Spanish cohort, workers with a longer duration of work hours were more likely to have tumors with a worse prognosis, while the Canadian population study determined that night-workers were at increased risk for developing PCa, regardless of work duration [74,75]. Additionally, a fixed vs. rotating night shift work has a differential effect on PCa risk, with rotating shift workers having a 20% higher risk for developing PCa than fixed schedule night shift workers [76]. Greater PCa risk has been reported in firefighters, health practitioners, and police, all of which typically require some degree of night shift work [77]. AA firefighters, machinery maintenance workers, and railroad workers are particularly more at risk for developing PCa [65]. Despite evidence implicating a positive correlation between night shift work and PCa, some reports found no such association [78,79]. These diverse findings may be related to the size of the cohort, differences in fixed schedules vs. rotating schedules, and duration of occupation.
Artificial light at night: While the current literature on the effects of ALAN as an environmental risk factor is limited, there is evidence for the association between ALAN and PCa incidence [80,81]. The established literature on the link between ALAN exposure and the risk of breast cancer has promoted further exploration of the effects on other hormone-dependent cancers, including PCa [80,82,83,84,85]. Several cross-geographic studies have found a significant positive association between population exposure to ALAN and the incidence of prostate, breast, colorectal, and lung cancers individually, after adjusting for population size, electricity consumption, air pollution, and total area of land covered by forest [86,87]. A case–control study in Spain found that night shift workers had a slightly higher prostate cancer risk compared to non-night shift workers; the risk increased with longer light exposure and was more pronounced for high-risk prostate tumors [75]. Exposure to ALAN affects melatonin levels, a potential mechanism linking shift work with increased PCa risk [88]. Lower melatonin serum levels have been associated with exposure to ALAN [89]. Thus, the lack of melatonin, a circadian hormone with potential anti-cancer effects, may be correlated with enhanced tumor development. A direct link between ALAN, melatonin suppression, and increased risk for cancer, however, has not been established, mainly due to a lack of measurements of ALAN using calibrated personal light-measuring devices.
3.2. Consequences of CRD
Behavioral stress: Behavioral stress and psychosocial factors, which has a bidirectional relationship with CRD, have an impact on PCa outcomes [90]. PCa patients report the highest levels of stress and anxiety on average compared to other cancer patients. Findings from studies on patient anxiety, stress, and prostate specific antigen (PSA) levels are heterogeneous [91,92,93]. Clinical studies have found that participants with high cortisol levels had a positive correlation with high prostate specific antigen (PSA) values, indicating high PCa risk [94]. Among a cohort of World Trade Center responders during the 9/11 terrorist attacks, re-experiencing a traumatic event was correlated with increased PCa incidence [95]. Greater perceived stress is associated with increased PCa-specific mortality, grieving and sleep loss, and a lack of adequate social support [96]. Patients utilizing high-effort coping (a coping mechanism used for race-based discrimination and mistreatment) had a slightly greater PCa risk relative to men who had decreased levels of high-effort coping, demonstrating how race-based discrimination may be a contributing factor to the stress-related PCa pathway [97]. Lastly, glucocorticoid (GR) signaling, which is overstimulated in chronic stress conditions, contributes to the progression of metastatic-castration-resistant prostate cancer (mCRPC), by promoting AR-target genes in the absence of androgens [98].
Obesity: Obesity is associated with multiple chronic problems as well as hormonal changes, which have an impact on the progression of PCa. Studies investigating the potential role of obesity in PCa risk, progression, and mortality yield heterogeneous results. Measures of obesity, such as body mass index (BMI) > 30, waist–hip ratio, and waist circumference (WC), are positively correlated with risk for advanced PCa and mortality due to PCa [99,100,101,102]. Obesity has a stronger association with PCa risk among AA men compared to White men [103]. Among a racially diverse cohort, AA men had the greatest obesity rates (BMI > 35), and obese AA men were more likely to have positive surgical margins, higher-grade tumors, and higher rates of biochemical failure after a radical prostatectomy [104]. A higher BMI has been associated with increased progression towards mCRPC, a 3-fold risk of developing metastases, and PC-specific mortality [105]. These findings provide new insights into the role of CRD and the consequences of CRD (e.g., obesity and stress) for PCa progression.
This entry is adapted from the peer-reviewed paper 10.3390/cancers14205116
This entry is offline, you can click here to edit this entry!
