Vitamin D is a micronutrient that plays a role in the homeostasis of various body organs, including skeletal muscle. Skeletal muscle growth and regeneration are critically affected by satellite cells, skeletal muscle stem cells. The discovery of vitamin D receptors on satellite cells supports the role of vitamin D in regulating satellite cell function. In vivo studies have shown the effect of vitamin D on skeletal muscle growth in early life, muscle homeostasis in aging, and skeletal muscle regeneration in conditions of muscle injury or chronic disease.
- vitamin D
- satellite cells
- skeletal muscle
- in vivo
1. Introduction
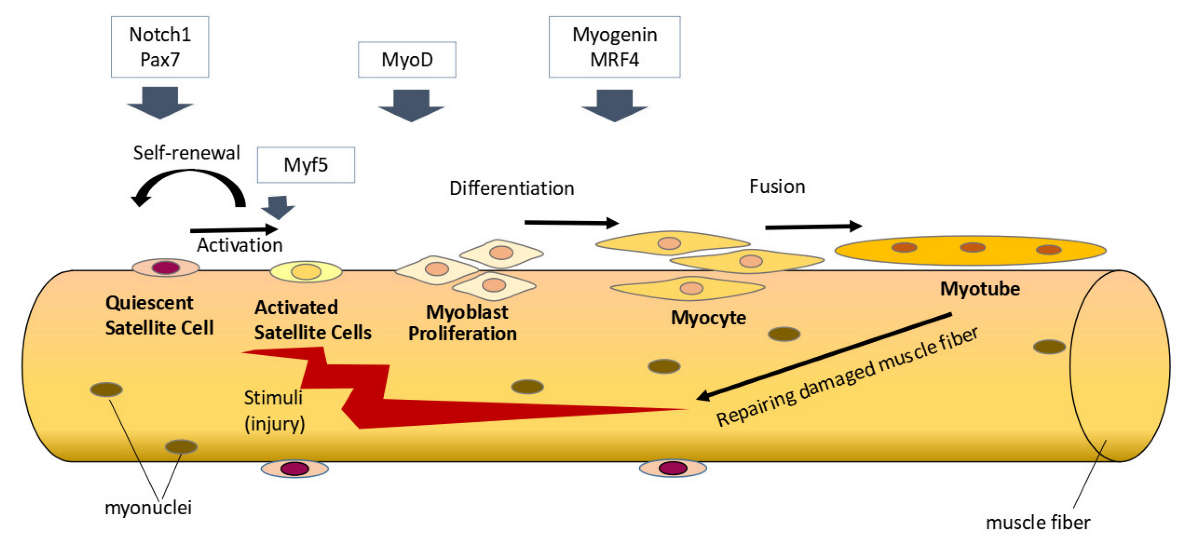
2. Effects of Vitamin D on Satellite Cells
3. Conclusion and Future Perspectives
In vivo studies support a direct role of vitamin D on satellite cells’ function during muscle growth, injury, aging, or chronic disease. Vitamin D appears to increase satellite cell proliferation in the early life period during rapid muscle growth. Adequate vitamin D status is required to support the satellite cells’ function in skeletal muscle regeneration during acute injury. However, the administration of high doses of vitamin D decreases satellite cell differentiation and delays new muscle fiber formation. Vitamin D deficiency in aging was associated with the decrease in Notch signaling resulting in satellite cells losing their quiescent and differentiating prematurely. Vitamin D supplementation ameliorates the impairment of satellite cell function in chronic disease. Thus, to provide optimal effects on satellite cells’ function, it is necessary to administer vitamin D at a dose according to the physiological needs of each individual. Further research is needed to determine the most appropriate dose and duration of vitamin D supplementation in the various age groups and specific conditions such as in early life, injury, aging, or chronic disease.
This entry is adapted from the peer-reviewed paper 10.3390/nu14214558
References
- Ran Zhang; Declan P Naughton; Vitamin D in health and disease: Current perspectives. Nutrition Journal 2010, 9, 65-65, 10.1186/1475-2891-9-65.
- Shelby E. Bollen; Philip J. Atherton; Myogenic, genomic and non‐genomic influences of the vitamin D axis in skeletal muscle. Cell Biochemistry and Function 2020, 39, 48-59, 10.1002/cbf.3595.
- Joan M. Lappe; The Role of Vitamin D in Human Health: A Paradigm Shift. Journal of Evidence-Based Complementary & Alternative Medicine 2011, 16, 58-72, 10.1177/1533210110392952.
- Christine M. Latham; Camille R. Brightwell; Alexander R. Keeble; Brooke D. Munson; Nicholas T. Thomas; Alyaa M. Zagzoog; Christopher S. Fry; Jean L. Fry; Vitamin D Promotes Skeletal Muscle Regeneration and Mitochondrial Health. Frontiers in physiology 2021, 12, 660498, 10.3389/fphys.2021.660498.
- Michael F. Holick; Vitamin D Deficiency. The New England Journal of Medicine 2007, 357, 266-281, 10.1056/nejmra070553.
- Adriana S. Dusso; Alex J. Brown; Eduardo Slatopolsky; Vitamin D. American Journal of Physiology-Renal Physiology 2005, 289, F8-F28, 10.1152/ajprenal.00336.2004.
- Hector F. DeLuca; The Metabolism and Functions of Vitamin D. null 1986, 196, 361-375, 10.1007/978-1-4684-5101-6_24.
- Armin Zittermann; Jan F. Gummert; Nonclassical Vitamin D Actions. Nutrients 2010, 2, 408-425, 10.3390/nu2040408.
- Lisa Ceglia; Vitamin D and its role in skeletal muscle. Current Opinion in Clinical Nutrition and Metabolic Care 2009, 12, 628-633, 10.1097/mco.0b013e328331c707.
- Yu Xin Wang; Michael A. Rudnicki; Satellite cells, the engines of muscle repair. Nature Reviews Molecular Cell Biology 2011, 13, 127-133, 10.1038/nrm3265.
- William Chen; David Datzkiw; Michael A. Rudnicki; Satellite cells in ageing: use it or lose it. Open biology 2020, 10, 200048, 10.1098/rsob.200048.
- Norio Motohashi; Molecular Regulation of Muscle Satellite Cell Self-Renewal. Journal of stem cell research & therapy 2012, 01, e002, 10.4172/2157-7633.s11-e002.
- Nicolas A. Dumont; Yu Xin Wang; Michael A. Rudnicki; Intrinsic and extrinsic mechanisms regulating satellite cell function. Development 2015, 142, 1572-1581, 10.1242/dev.114223.
- Andreas Marg; Helena Escobar; Sina Gloy; Markus Kufeld; Joseph Zacher; Andreas Spuler; Carmen Birchmeier; Zsuzsanna Izsvak; Simone Spuler; Human satellite cells have regenerative capacity and are genetically manipulable. JCI Insight 2014, 124, 4257-4265, 10.1172/jci63992.
- Camila F. Almeida; Stephanie A. Fernandes; Antonio F. Ribeiro Junior; Oswaldo Keith Okamoto; Mariz Vainzof; Muscle Satellite Cells: Exploring the Basic Biology to Rule Them. Stem Cells International 2016, 2016, 1-14, 10.1155/2016/1078686.
- Karl Olsson; Amarjit Saini; Anna Strömberg; Seher Alam; Mats Lilja; Eric Rullman; Thomas Gustafsson; Evidence for Vitamin D Receptor Expression and Direct Effects of 1α,25(OH)2D3 in Human Skeletal Muscle Precursor Cells. Endocrinology 2016, 157, 98-111, 10.1210/en.2015-1685.
- Melissa Braga; Zena Simmons; Keith C Norris; Monica G Ferrini; Jorge N Artaza; Vitamin D induces myogenic differentiation in skeletal muscle derived stem cells. Endocrine connections 2017, 6, 139-150, 10.1530/ec-17-0008.
- Ratchakrit Srikuea; Muthita Hirunsai; Narattaphol Charoenphandhu; Regulation of vitamin D system in skeletal muscle and resident myogenic stem cell during development, maturation, and ageing. Scientific Reports 2020, 10, 1-17, 10.1038/s41598-020-65067-0.
- Kathryn H. Alliband; Sofia V. Kozhevnikova; Tim Parr; Preeti H. Jethwa; John M. Brameld; In vitro Effects of Biologically Active Vitamin D on Myogenesis: A Systematic Review. Frontiers in physiology 2021, 12, 736708, 10.3389/fphys.2021.736708.
- D.D.W. Cornelison; Context matters: In vivo and in vitro influences on muscle satellite cell activity. Journal of Cellular Biochemistry 2008, 105, 663-669, 10.1002/jcb.21892.
- Hang Yin; Feodor Price; Michael Rudnicki; Satellite Cells and the Muscle Stem Cell Niche. Physiological reviews 2013, 93, 23-67, 10.1152/physrev.00043.2011.
- Shihuan Kuang; Mark A. Gillespie; Michael A. Rudnicki; Niche Regulation of Muscle Satellite Cell Self-Renewal and Differentiation. Cell stem cell 2008, 2, 22-31, 10.1016/j.stem.2007.12.012.
- Benjamin D. Cosgrove; Alessandra Sacco; Penney M. Gilbert; Helen M. Blau; A home away from home: Challenges and opportunities in engineering in vitro muscle satellite cell niches. Differentiation 2009, 78, 185-194, 10.1016/j.diff.2009.08.004.
- Teresa A Davis; Marta L Fiorotto; Regulation of muscle growth in neonates. Current Opinion in Clinical Nutrition and Metabolic Care 2009, 12, 78-85, 10.1097/mco.0b013e32831cef9f.
- Fuminori Kawano; Yoshiaki Takeno; Naoya Nakai; Yoko Higo; Masahiro Terada; Takashi Ohira; Ikuya Nonaka; Yoshinobu Ohira; Essential role of satellite cells in the growth of rat soleus muscle fibers. American Journal of Physiology-Cell Physiology 2008, 295, C458-C467, 10.1152/ajpcell.00497.2007.
- Igor Bendik; Angelika Friedel; Franz F. Roos; Peter Weber; Manfred Eggersdorfer; Vitamin D: a critical and essential micronutrient for human health. Frontiers in physiology 2014, 5, 248, 10.3389/fphys.2014.00248.
- K. C. Hutton; M. A. Vaughn; Gilberto Litta; B. J. Turner; J. D. Starkey; Effect of vitamin D status improvement with 25-hydroxycholecalciferol on skeletal muscle growth characteristics and satellite cell activity in broiler chickens1,2. Journal of Animal Science 2014, 92, 3291-3299, 10.2527/jas.2013-7193.
- Hui Zhou; Yuling Chen; Gang Lv; Yong Zhuo; Yan Lin; Bin Feng; Zhengfeng Fang; Lianqiang Che; Jian Li; Shengyu Xu; et al. Improving maternal vitamin D status promotes prenatal and postnatal skeletal muscle development of pig offspring. Nutrition 2016, 32, 1144-1152, 10.1016/j.nut.2016.03.004.
- M. T Thayer; J. L. Nelssen; A. J. Langemeier; J. Morton; J. M. Gonzales; S. R. Kruger; Z. Ou; A. J. Makowski; J. R. Bergstrom; The Effects of Maternal Dietary Supplementation of Cholecalciferol (Vitamin D3) and 25(OH)D3 on Sow and Progeny Performance. Kansas Agricultural Experiment Station Research Reports 2018, 4, 2, 10.4148/2378-5977.7650.
- Gordon Warren; Mukesh Summan; Xin Gao; Rebecca Chapman; Tracy Hulderman; Petia P. Simeonova; Mechanisms of skeletal muscle injury and repair revealed by gene expression studies in mouse models. The Journal of physiology 2007, 582, 825-841, 10.1113/jphysiol.2007.132373.
- Yu, S., Ren, B., Chen, H., Goltzman, D., Yan, J., & Miao, D.; 1,25-Dihydroxyvitamin D deficiency induces sarcopenia by inducing skeletal muscle cell senescence. Am J Transl Res 2021, 13, 12638–12649, .
- Ratchakrit Srikuea; Muthita Hirunsai; Effects of intramuscular administration of 1α,25(OH)2D3 during skeletal muscle regeneration on regenerative capacity, muscular fibrosis, and angiogenesis. Journal of Applied Physiology 2016, 120, 1381-1393, 10.1152/japplphysiol.01018.2015.
- Tohru Hosoyama; Hiroki Iida; Minako Kawai-Takaishi; Ken Watanabe; Vitamin D Inhibits Myogenic Cell Fusion and Expression of Fusogenic Genes. Nutrients 2020, 12, 2192, 10.3390/nu12082192.
- Kevin J P Ryan; Zoe C T R Daniel; Lucinda J L Craggs; Tim Parr; John M Brameld; Dose-dependent effects of vitamin D on transdifferentiation of skeletal muscle cells to adipose cells. Journal of Endocrinology 2013, 217, 45-58, 10.1530/joe-12-0234.
- Daniel J. Owens; Adam P. Sharples; Ioanna Polydorou; Nura Alwan; Timothy Donovan; Jonathan Tang; William D. Fraser; Robert G. Cooper; James P. Morton; Claire Stewart; et al. A systems-based investigation into vitamin D and skeletal muscle repair, regeneration, and hypertrophy. American Journal of Physiology-Endocrinology and Metabolism 2015, 309, E1019-E1031, 10.1152/ajpendo.00375.2015.
- Maura H. Parker; The altered fate of aging satellite cells is determined by signaling and epigenetic changes. Frontiers in genetics 2015, 6, 59-59, 10.3389/fgene.2015.00059.
- Irina M. Conboy; Michael J. Conboy; Amy J. Wagers; Eric R. Girma; Irving L. Weissman; Thomas A. Rando; Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005, 433, 760-764, 10.1038/nature03260.
- Carla Domingues-Faria; Audrey Chanet; Jérôme Salles; Alexandre Berry; Christophe Giraudet; Véronique Patrac; Philippe Denis; Katia Bouton; Nicolas Goncalves-Mendes; Marie-Paule Vasson; et al. Vitamin D deficiency down-regulates Notch pathway contributing to skeletal muscle atrophy in old wistar rats. Nutrition & Metabolism 2014, 11, 47-47, 10.1186/1743-7075-11-47.
- Karl Olsson; Amarjit Saini; Anna Strömberg; Seher Alam; Mats Lilja; Eric Rullman; Thomas Gustafsson; Evidence for Vitamin D Receptor Expression and Direct Effects of 1α,25(OH)2D3 in Human Skeletal Muscle Precursor Cells. Endocrinology 2016, 157, 98-111, 10.1210/en.2015-1685.
- Suchitra D. Gopinath; Ashley E. Webb; Anne Brunet; Thomas A. Rando; FOXO3 Promotes Quiescence in Adult Muscle Stem Cells during the Process of Self-Renewal. Stem cell reports 2014, 2, 414-426, 10.1016/j.stemcr.2014.02.002.
- Y Ono; F Calhabeu; J E Morgan; T Katagiri; H Amthor; P S Zammit; BMP signalling permits population expansion by preventing premature myogenic differentiation in muscle satellite cells. Cell Death & Differentiation 2010, 18, 222-234, 10.1038/cdd.2010.95.
- Amalia Stantzou; Elija Schirwis; Sandra Swist; Sonia Alonso-Martin; Ioanna Polydorou; Faouzi Zarrouki; Etienne Mouisel; Cyriaque Beley; Anaïs Julien; Fabien Le Grand; et al. BMP signaling regulates satellite cell dependent postnatal muscle growth. Development 2017, 144, 2737-2747, 10.1242/dev.144089.
- Zipora Yablonka-Reuveni; Rony Seger; Anthony J. Rivera; Fibroblast Growth Factor Promotes Recruitment of Skeletal Muscle Satellite Cells in Young and Old Rats. Journal of Histochemistry & Cytochemistry 1999, 47, 23-42, 10.1177/002215549904700104.
- Joe V. Chakkalakal; Kieran M. Jones; M. Albert Basson; Andrew S. Brack; The aged niche disrupts muscle stem cell quiescence. Nature 2012, 490, 355-360, 10.1038/nature11438.
- Josiane Joseph; Jason D. Doles; Disease-associated metabolic alterations that impact satellite cells and muscle regeneration: perspectives and therapeutic outlook. Nutrition & Metabolism 2021, 18, 1-8, 10.1186/s12986-021-00565-0.
- Colleen F. McKenna; Christopher S. Fry; Altered satellite cell dynamics accompany skeletal muscle atrophy during chronic illness, disuse, and aging. Current Opinion in Clinical Nutrition and Metabolic Care 2017, 20, 447-452, 10.1097/mco.0000000000000409.
- Lifang Han; Gang Wang; Shaopu Zhou; Chenghao Situ; Zhiming He; Yuying Li; Yudan Qiu; Yu Huang; Aimin Xu; Michael Tim Yun Ong; et al. Muscle satellite cells are impaired in type 2 diabetic mice by elevated extracellular adenosine. Cell reports 2022, 39, 110884, 10.1016/j.celrep.2022.110884.
- Yasuro Furuichi; Yuki Kawabata; Miho Aoki; Yoshitaka Mita; Nobuharu L. Fujii; Yasuko Manabe; Excess Glucose Impedes the Proliferation of Skeletal Muscle Satellite Cells Under Adherent Culture Conditions. Frontiers in Cell and Developmental Biology 2021, 9, 640399, 10.3389/fcell.2021.640399.
- Yasuro Furuichi; Yuki Kawabata; Miho Aoki; Yoshitaka Mita; Nobuharu L. Fujii; Yasuko Manabe; Excess Glucose Impedes the Proliferation of Skeletal Muscle Satellite Cells Under Adherent Culture Conditions. Frontiers in Cell and Developmental Biology 2021, 9, 640399, 10.3389/fcell.2021.640399.
- Yasuro Furuichi; Yuki Kawabata; Miho Aoki; Yoshitaka Mita; Nobuharu L. Fujii; Yasuko Manabe; Excess Glucose Impedes the Proliferation of Skeletal Muscle Satellite Cells Under Adherent Culture Conditions. Frontiers in Cell and Developmental Biology 2021, 9, 640399, 10.3389/fcell.2021.640399.
