Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Hydrogen-based energy can play a vital role in this aspect. This energy is green, clean, and renewable. Electrochemical hydrogen devices have been used extensively in nuclear power plants to manage hydrogen-based renewable fuel. Doped zirconate materials are commonly used as an electrolyte in these electrochemical devices. These materials have excellent physical stability and high proton transport numbers, which make them suitable for multiple applications. Doping enhances the physical and electronic properties of zirconate materials and makes them ideal for practical applications.
- perovskite oxide
- proton-conducting oxide
- zirconate
1. Introduction
As a result of the Industrial Revolution and technological advancements, the globe requires alternative energy sources to supply the ever-increasing demand for energy [1][2][3]. In addition, With the rapid depletion of fossil fuel resources and the negative impact of fossil fuel combustion on the environments [4][5][6][7], scientists have turned their attention to other renewable sources, such as electrochemical hydrogen devices based on proton-conducting materials [8][9][10][11]. Proton conductors typically have positively charged protonic species, such as H+, H3O+, and NH4+ [12][13]. Proton-conducting materials provide higher conductivity at lower temperatures with longer lifetimes and less expense than traditional oxide ionic electrolyte conductors [14][15]. In addition, these conductors lose conductivity at higher temperatures due to reversible or irreversible loss of carriers [16]. These characteristics enable these materials to operate at narrow ranges of temperature.
Proton conductors can be used in various electrochemical energy devices, such as batteries, fuel-cell electrolytes, water electrolyzers’ membrane, hydrogen pumps, hydrogen sensors, and hydrogen gas separation systems [17][18][19][20]. Organic polymer, inorganic oxides, and lattice defect oxides are examples of the different types of proton conductors. Compared to the other proton conductors, lattice defect-type oxides, i.e., perovskite-type proton-conducting oxides, are the promising proton conductors due to having the highest proton conductivity and chemical stability within desired temperatures [12][21][22]. A typical chemical formula of a perovskite proton conductor is ABO3 (A = Ba, Ca, Sr, etc.; B = Zr, Ce, Tb, Th, etc.) [23][24]. In addition, perovskite materials have higher conversion efficiency and are less expensive than other proton conductors [25][26]. These unique properties of perovskite materials have increased their utility in renewable energy applications, especially in solar cells [27]. Among different types of perovskite proton-conducting materials, zirconate materials are the most widely studied/used due to their high chemical stability and excellent proton conductivity [16][28][29][30].
Zirconate materials such as BaZrO3-based materials are considered promising proton-conducting materials and are widely used in chemical and electrical sectors. However, many studies have shown that cerate-based proton conductors such as BaCeO3 have high proton conductivity among perovskite-based materials [12]. The drawback of BaCeO3-based materials is that they are unstable in CO2 and water vapor atmospheres, making them unsuitable for applications [31][32]. In contrast, BaZrO3-based proton conductors are stable in CO2 and water vapor environments which are attractive properties for electrochemical device application in harsh atmospheres [25]. Moreover, BaZrO3-based materials have better physical properties, including chemical stability and higher mechanical hardness than BaCeO3-based proton-conducting material [33]. Ken Kurosaki et al. reported that BaZrO3 exhibits high thermal conductivity due to the high strength between Zr and O [34]. However, the BaZrO3-based proton conductor’s proton conductivity is lower than the BaCeO3-based proton conductor, which can be improved by doping with trivalent cations such as Gd3+, Y3+, In3+, Yb3+ [35][36]. Pergolesi et al. have reported that Y3+ doped in BaZrO3 enhances chemical stability, but the poor sinterability increases grain-boundary resistance, which is responsible for reducing proton conductivity [37]. Therefore, the sintering temperature must be increased with decreased grain-boundary resistance to improve electrical properties in zirconate-based proton conductors [38]. Recent research has shown that In-doped zirconate-based perovskite proton conductors exhibit better sintering activities with excellent chemical stability [39]. Consequently, experiments with different doping concentrations and synthesis methods are used to develop high-performing doped BaZrO3 material.
Zirconate materials have low thermal conductivity, low dielectric loss, and very low thermal expansion coefficient [16][40][41], making them more favorable for electrochemical devices than other proton-conducting oxide materials. Furthermore, compared to other proton-conducting materials in hydrogen sensors, zirconate-based hydrogen sensors have been demonstrated to be affordable, portable, and temporally correct due to their high chemical stability, smaller dimensions, and cheapness [16][42]. Hydrogen can be separated in zirconate-based proton conductors in a controlled way simply by changing the applied current in the electrochemical cell; thus, they can be utilized as hydrogen pumps [16]. Zirconate proton conductors can be used as membrane separators at high temperatures, enabling them to act as a sensitive tritium monitor system [43]. Such a device is helpful in removing inference from radionucleotides and concentrating tritium, since it can operate like an electrochemical hydrogen isotope pump [43]. In addition, tritium release has been reported in zirconate proton-conducting material spheres as far back as 30 years ago, and scientists are making more advancements in that technology [44][45][46][47][48][49].
2. Proton-Conducting Zirconates
Perovskite proton-conductor oxides, i.e., zirconates and cerate-based materials, are well-known proton conductors for electrochemical device applications due to their excellent physical properties [14][50]. BaZrO3 is a promising zirconate proton conductor widely used in refractory and electrical sectors. This material has excellent stability in a harsh environment, low proton migration, high melting temperature, high thermal expansion coefficient, excellent structure, and mechanical properties at high temperatures [51][52]. Furthermore, BaZrO3 does not show any phase transition between low and high temperatures, making it suitable for electrochemical devices, including tritium monitoring systems, tritium recovery systems, hydrogen sensors, and hydrogen pumps [46][53][54].
Although cerate-based proton conductor like BaCeO3, has the highest proton conductivity among other proton-conductor materials, it is unstable in water vapor and CO2 atmosphere, whereas BaZrO3 materials show stability in harsh weather (water vapor and CO2) [16]. Alkaline earth zirconates, such as those found in CaZrO3, BaZrO3, and SrZrO3, are typically more chemically stable and have more mechanical strength than alkaline earth cerate ceramics [55][56]. Many studies have shown that doping with BaZrO3 can enhance proton conductivity and high chemical stability. The general formula of doping zirconate is AZr1-xDxO3−δ, where trivalent dopant D is used to replace the tetravalent Zr to create oxygen vacancy, which is crucial for proton-conduction perovskite (ABO3) lattice structure [57]. The proton conductivity of the BaZrO3 is greatly affected by the type and amount of the dopant used in the barium zirconate. With increasing Zr materials, the electrolyte sintering temperature is also increased, and as a result, the ionic conductivity is decreased [58]. Moreover, BaZrO3 has high grain-boundary resistance which hinders electrochemical applications. Therefore, to improve the proton conductivity, it is essential to maintain a minimum grain-boundary resistance and high sintering temperature [59][60].
Studies have shown that Y-doped BaZrO3 (BaZr1−xYxO3−δ) exhibits excellent chemical stability with high proton conductivity [61]. For example, Liu et al. investigated BaZr1−xYxO3−δ electrolyte by partially replacing Zr4+ with neodymium (Nd3+) to enhance the sinterability and conductivity of the electrolyte [62]. The results showed that BaZr0.7 Nd0.1YxO3−δ had higher proton conductivity than BaZr1−xYxO3−δ electrolyte and that Nd3+ doping increased the chemical stability. However, neodymium (Nb) is a rare-earth element and expensive, which is not feasible for commercial application. On the other hand, mixed BaCeO3-BaZrO3 with dopant shows higher chemical stability but enriched Zr, restricting applications due to poor sintering and high grain-boundary resistance [63][64]. Therefore, further modification is required in zirconate to improve its proton conductivity with suitable stability for electrochemical application.
3. Electrochemical Hydrogen Device
Electrochemical devices are an essential scientific innovation enabling the development of an electric vehicle for the future. The principles of electrochemistry have materialized in hydrogen storage [65], hydrogen sensor [66], and hydrogen compressor [67] applications, as well as different chemical sensor applications. The basic electrochemical hydrogen devices have the following components: anode (electrode), electrolyte (proton-conducting solid), and cathode (electrode) (Figure 1) [68]. Electrochemical hydrogen devices use two fundamental principles: electromotive force (EMF) and the hydrogen transport phenomenon of the electrolyte. Recently, electrochemical devices have extensively used proton-conducting zirconates [16]. The small radius of protons enables the ions to fit into the interlayer structure of the cathode.
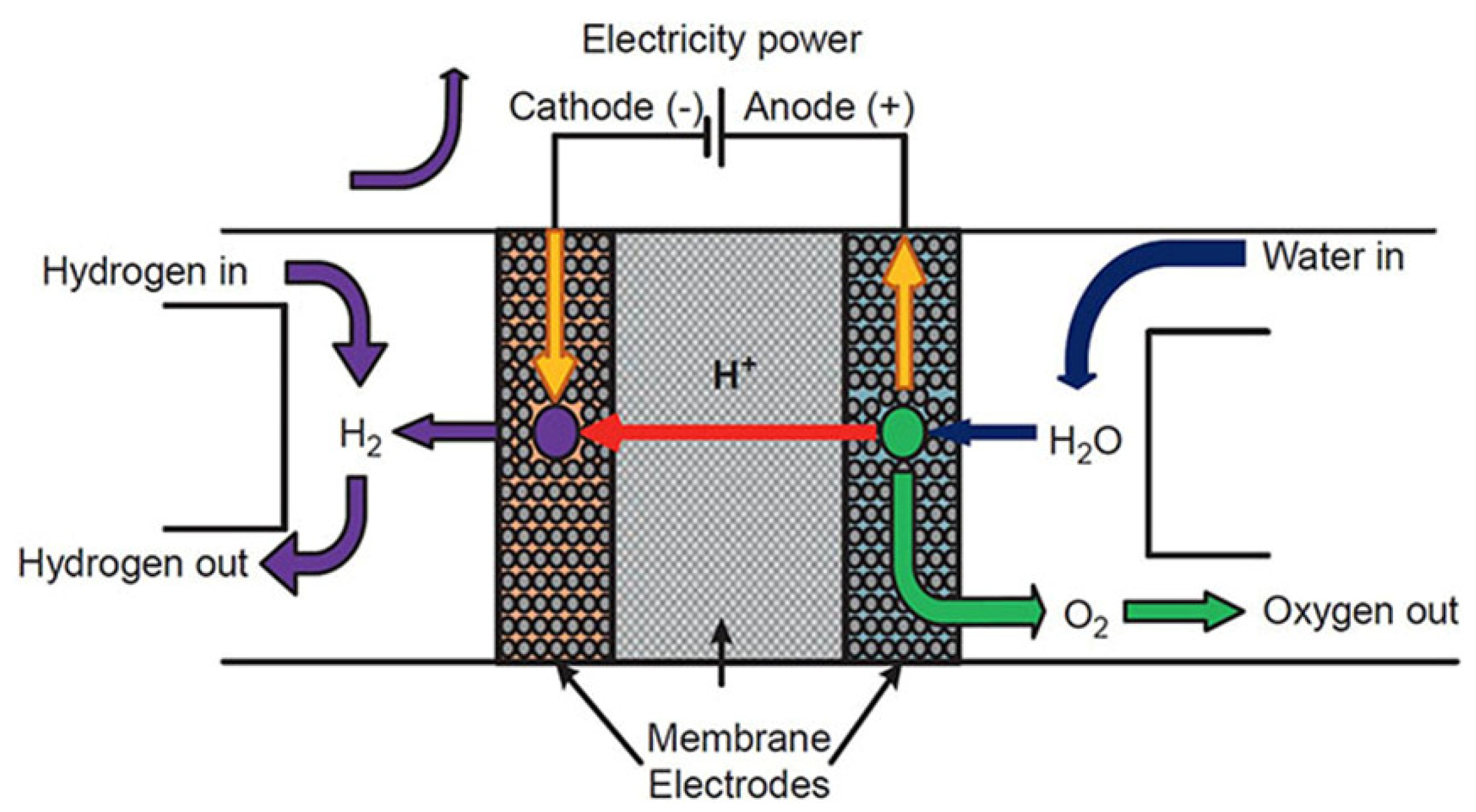
Figure 1. The fundamental design and operation of a proton-exchange membrane (PEM)-based electrochemical hydrogen device. Reprinted with permission from Ref. [68]. Copyright 2019 Elsevier.
These electrochemical devices utilize EMF the same as the principle of galvanic cells. The device is called a hydrogen sensor when EMF is used to produce signals. On the other hand, if the EMF force of the electrochemical cell is used to separate hydrogen, it is called a hydrogen pump. Radioactive isotopes like tritium (3H) can be separated using the same principles. An electrochemical reactor is necessary to convert water vapor and methane to tritium. Similarly, tritium can be monitored as a function of applied current, thus making this electrochemical device a platform for tritium monitoring. Moreover, separating radioactive molecules like Rn and enrichment of tritium can be effective for lower levels of tritium detection [69].
This entry is adapted from the peer-reviewed paper 10.3390/nano12203581
References
- Sharma, P.; Said, Z.; Kumar, A.; Nižetić, S.; Pandey, A.; Hoang, A.T.; Huang, Z.; Afzal, A.; Li, C.; Le, A.T.; et al. Recent Advances in Machine Learning Research for Nanofluid-Based Heat Transfer in Renewable Energy System. Energy Fuels 2022, 36, 6626–6658.
- Siram, O.; Sahoo, N.; Saha, U.K. Changing Landscape of India’s Renewable Energy and the Contribution of Wind Energy. Clean. Eng. Technol. 2022, 8, 100506.
- Sircar, A.; Tripathi, G.; Bist, N.; Shakil, K.A.; Sathiyanarayanan, M. Emerging Technologies for Sustainable and Smart Energy; CRC Press: Boca Raton, FL, USA, 2022; ISBN 9781003307402.
- Chia, S.R.; Nomanbhay, S.; Ong, M.Y.; Shamsuddin, A.H.B.; Chew, K.W.; Show, P.L. Renewable Diesel as Fossil Fuel Substitution in Malaysia: A Review. Fuel 2022, 314, 123137.
- Karakoc, T.H.; Colpan, C.O.; Ekici, S.; Yetik, O. Promising Fuels and Green Energy Technologies for Aviation. Int. J. Green Energy 2022.
- Gogoi, P.; Tudu, B.; Saikia, P. Hydrogen Fuel: Clean Energy Production Technologies. In Status and Future Challenges for Non-conventional Energy Sources Volume 1; Springer: Berlin/Heidelberg, Germany, 2022; pp. 133–154.
- Khan, M.; Das, R.C.; Casey, J.; Reese, B.L.; Akintunde, B.; Pathak, A.K. Near Room Temperature Magnetocaloric Properties in Ni Deficient (Mn0.525Fe0.5)Ni0.975Si0.95Al0.05. AIP Adv. 2022, 12, 035227.
- Hossain, M.K.; Raihan, G.A.; Akbar, M.A.; Kabir Rubel, M.H.; Ahmed, M.H.; Khan, M.I.; Hossain, S.; Sen, S.K.; Jalal, M.I.E.; El-Denglawey, A. Current Applications and Future Potential of Rare Earth Oxides in Sustainable Nuclear, Radiation, and Energy Devices: A Review. ACS Appl. Electron. Mater. 2022, 4, 3327–3353.
- Andrews, J.; Rezaei Niya, S.M.; Ojha, R. Electrochemical Hydrogen Storage in Porous Carbons with Acidic Electrolytes: Uncovering the Potential. Curr. Opin. Electrochem. 2022, 31, 100850.
- Sedighi, F.; Ghiyasiyan-Arani, M.; Behpour, M. Ternary Nanocomposites of Ce2W2O9/CoWO4/Porous Carbon; Design, Structural Study and Electrochemical Hydrogen Storage Application. Fuel 2022, 310, 122218.
- Hossain, M.K.; Rubel, M.H.K.; Akbar, M.A.; Ahmed, M.H.; Haque, N.; Rahman, M.F.; Hossain, J.; Hossain, K.M. A Review on Recent Applications and Future Prospects of Rare Earth Oxides in Corrosion and Thermal Barrier Coatings, Catalysts, Tribological, and Environmental Sectors. Ceram. Int. 2022, 48, 32588–32612.
- Kreuer, K.D. Proton-Conducting Oxides. Annu. Rev. Mater. Res. 2003, 33, 333–359.
- Colomban, P. Proton Conductors and Their Applications: A Tentative Historical Overview of the Early Researches. Solid State Ion. 2019, 334, 125–144.
- Duan, C.; Huang, J.; Sullivan, N.; O’Hayre, R. Proton-Conducting Oxides for Energy Conversion and Storage. Appl. Phys. Rev. 2020, 7, 011314.
- Leonard, K.; Druce, J.; Thoreton, V.; Kilner, J.A.; Matsumoto, H. Exploring Mixed Proton/Electron Conducting Air Electrode Materials in Protonic Electrolysis Cell. Solid State Ion. 2018, 319, 218–222.
- Hossain, M.K.; Chanda, R.; El-Denglawey, A.; Emrose, T.; Rahman, M.T.; Biswas, M.C.; Hashizume, K. Recent Progress in Barium Zirconate Proton Conductors for Electrochemical Hydrogen Device Applications: A Review. Ceram. Int. 2021, 47, 23725–23748.
- Hussain, S.; Li, Y. Review of Solid Oxide Fuel Cell Materials: Cathode, Anode, and Electrolyte. Energy Transit. 2020, 4, 113–126.
- Harada, K.; Tanii, R.; Matsushima, H.; Ueda, M.; Sato, K.; Haneda, T. Effects of Water Transport on Deuterium Isotope Separation during Polymer Electrolyte Membrane Water Electrolysis. Int. J. Hydrog. Energy 2020, 45, 31389–31395.
- Adhikari, S.; Fernando, S. Hydrogen Membrane Separation Techniques. Ind. Eng. Chem. Res. 2006, 45, 875–881.
- Li, Y.; Kappis, K.; Papavasiliou, J.; Fu, Z.; Chen, L.; Li, H.; Vlachos, D.E.; Avgouropoulos, G. Insights on the Electrochemical Performance of a Molten Proton Conductor Fuel Cell with Internal Methanol Reformer. J. Power Sources 2022, 542, 231813.
- Bonanos, N. Perovskite Proton Conductor. In Encyclopedia of Applied Electrochemistry; Springer: New York, NY, USA, 2014; pp. 1514–1520.
- Pei, K.; Zhou, Y.; Xu, K.; Zhang, H.; Ding, Y.; Zhao, B.; Yuan, W.; Sasaki, K.; Choi, Y.; Chen, Y.; et al. Surface Restructuring of a Perovskite-Type Air Electrode for Reversible Protonic Ceramic Electrochemical Cells. Nat. Commun. 2022, 13, 2207.
- Rubel, M.H.K.; Mitro, S.K.; Hossain, M.K.; Hossain, K.M.; Rahaman, M.M.; Hossain, J.; Mondal, B.K.; Akter, A.; Rahman, M.F.; Ahmed, I.; et al. First-Principles Calculations to Investigate Physical Properties of Single-Cubic (Ba0.82K0.18)(Bi0.53Pb0.47)O3 Novel Perovskite Superconductor. Mater. Today Commun. 2022, 33, 104302.
- Rubel, M.H.K.; Hossain, M.A.; Hossain, M.K.; Hossain, K.M.; Khatun, A.A.; Rahaman, M.M.; Ferdous Rahman, M.; Hossain, M.M.; Hossain, J. First-Principles Calculations to Investigate Structural, Elastic, Electronic, Thermodynamic, and Thermoelectric Properties of CaPd3B4O12 (B = Ti, V) Perovskites. Results Phys. 2022, 42, 105977.
- De Souza, E.C.C.; Muccillo, R. Properties and Applications of Perovskite Proton Conductors. Mater. Res. 2010, 13, 385–394.
- Zhou, M.; Liu, Z.; Chen, M.; Zhu, Z.; Cao, D.; Liu, J. Electrochemical Performance and Chemical Stability of Proton-conducting BaZr0.8−xCexY0.2O3−δ Electrolytes. J. Am. Ceram. Soc. 2022, 105, 5711–5724.
- Zhou, D.; Zhou, T.; Tian, Y.; Zhu, X.; Tu, Y. Perovskite-Based Solar Cells: Materials, Methods, and Future Perspectives. J. Nanomater. 2018, 2018, 8148072.
- Yang, Y.; Ling, X.; Qiu, W.; Bian, J.; Zhang, X.; Chen, Q. Surface-Enhanced Raman Scattering Spectroscopy Reveals the Phonon Softening of Yttrium-Doped Barium Zirconate Thin Films. J. Phys. Chem. C 2022, 126, 10722–10728.
- Nur Syafkeena, M.A.; Zainor, M.L.; Hassan, O.H.; Baharuddin, N.A.; Othman, M.H.D.; Tseng, C.-J.; Osman, N. Review on the Preparation of Electrolyte Thin Films Based on Cerate-Zirconate Oxides for Electrochemical Analysis of Anode-Supported Proton Ceramic Fuel Cells. J. Alloys Compd. 2022, 918, 165434.
- Peltzer, D.; Múnera, J.; Cornaglia, L. Study of the Sorption Properties of Alkali Zirconate-Based Sorbents at High Temperature in the Presence of Water and Low CO2 Concentration. J. Alloys Compd. 2022, 895, 162419.
- Rashid, N.L.R.M.; Samat, A.A.; Jais, A.A.; Somalu, M.R.; Muchtar, A.; Baharuddin, N.A.; Wan Isahak, W.N.R. Review on Zirconate-Cerate-Based Electrolytes for Proton-Conducting Solid Oxide Fuel Cell. Ceram. Int. 2019, 45, 6605–6615.
- Chen, M.; Zhou, M.; Liu, Z.; Liu, J. A Comparative Investigation on Protonic Ceramic Fuel Cell Electrolytes BaZr0.8Y0.2O3−δ and BaZr0.1Ce0.7Y0.2O3−δ with NiO as Sintering Aid. Ceram. Int. 2022, 48, 17208–17216.
- Yamanaka, S.; Fujikane, M.; Hamaguchi, T.; Muta, H.; Oyama, T.; Matsuda, T.; Kobayashi, S.; Kurosaki, K. Thermophysical Properties of BaZrO3 and BaCeO3. J. Alloys Compd. 2003, 359, 109–113.
- Kurosaki, K.; Adachi, J.; Maekawa, T.; Yamanaka, S. Thermal Conductivity Analysis of BaUO3 and BaZrO3 by Semiempirical Molecular Dynamics Simulation. J. Alloys Compd. 2006, 407, 49–52.
- Borland, H.; Llivina, L.; Colominas, S.; Abellà, J. Proton Conducting Ceramics for Potentiometric Hydrogen Sensors for Molten Metals. Fusion Eng. Des. 2013, 88, 2431–2435.
- Tanaka, M.; Ohshima, T. Recovery of Hydrogen from Gas Mixture by an Intermediate-Temperature Type Proton Conductor. Fusion Eng. Des. 2010, 85, 1038–1043.
- Pergolesi, D.; Fabbri, E.; D’Epifanio, A.; Di Bartolomeo, E.; Tebano, A.; Sanna, S.; Licoccia, S.; Balestrino, G.; Traversa, E. High Proton Conduction in Grain-Boundary-Free Yttrium-Doped Barium Zirconate Films Grown by Pulsed Laser Deposition. Nat. Mater. 2010, 9, 846–852.
- Assabumrungrat, S.; Sangtongkitcharoen, W.; Laosiripojana, N.; Arpornwichanop, A.; Charojrochkul, S.; Praserthdam, P. Effects of Electrolyte Type and Flow Pattern on Performance of Methanol-Fuelled Solid Oxide Fuel Cells. J. Power Sources 2005, 148, 18–23.
- Sun, W.; Liu, M.; Liu, W. Chemically Stable Yttrium and Tin Co-Doped Barium Zirconate Electrolyte for Next Generation High Performance Proton-Conducting Solid Oxide Fuel Cells. Adv. Energy Mater. 2013, 3, 1041–1050.
- Hossain, M.K.; Biswas, M.C.; Chanda, R.K.; Rubel, M.H.K.; Khan, M.I.; Hashizume, K. A Review on Experimental and Theoretical Studies of Perovskite Barium Zirconate Proton Conductors. Emerg. Mater. 2021, 4, 999–1027.
- Zhang, W.; Hu, Y.H. Progress in Proton-conducting Oxides as Electrolytes for Low-temperature Solid Oxide Fuel Cells: From Materials to Devices. Energy Sci. Eng. 2021, 9, 984–1011.
- Schwandt, C.; Fray, D.J. The Titanium/Hydrogen System as the Solid-State Reference in High-Temperature Proton Conductor-Based Hydrogen Sensors. J. Appl. Electrochem. 2006, 36, 557–565.
- Tanaka, M.; Sugiyama, T.; Ohshima, T.; Yamamoto, I. Extraction of Hydrogen and Tritium Using High-Temperature Proton Conductor for Tritium Monitoring. Fusion Sci. Technol. 2011, 60, 1391–1394.
- Miller, J.M.; Bokwa, S.R.; Macdonald, D.S.; Verrall, R.A. Tritium Recovery from Lithium Zirconate Spheres. Fusion Technol. 1991, 19, 996–999.
- Hossain, M.K.; Tamura, H.; Hashizume, K. Visualization of Hydrogen Isotope Distribution in Yttrium and Cobalt Doped Barium Zirconates. J. Nucl. Mater. 2020, 538, 152207.
- Hossain, M.K.; Hashizume, K.; Hatano, Y. Evaluation of the Hydrogen Solubility and Diffusivity in Proton-Conducting Oxides by Converting the PSL Values of a Tritium Imaging Plate. Nucl. Mater. Energy 2020, 25, 100875.
- Hossain, M.K.; Iwasa, T.; Hashizume, K. Hydrogen Isotope Dissolution and Release Behavior in Y-doped BaCeO3. J. Am. Ceram. Soc. 2021, 104, 6508–6520.
- Hossain, M.K.; Yamamoto, T.; Hashizume, K. Effect of Sintering Conditions on Structural and Morphological Properties of Y- and Co-Doped BaZrO3 Proton Conductors. Ceram. Int. 2021, 47, 27177–27187.
- Hossain, M.K.; Yamamoto, T.; Hashizume, K. Isotopic Effect of Proton Conductivity in Barium Zirconates for Various Hydrogen-Containing Atmospheres. J. Alloys Compd. 2022, 903, 163957.
- Han, D.; Liu, X.; Bjørheim, T.S.; Uda, T. Yttrium-Doped Barium Zirconate-Cerate Solid Solution as Proton Conducting Electrolyte: Why Higher Cerium Concentration Leads to Better Performance for Fuel Cells and Electrolysis Cells. Adv. Energy Mater. 2021, 11, 2003149.
- Liu, Y.; Zhang, W.; Wang, B.; Sun, L.; Li, F.; Xue, Z.; Zhou, G.; Liu, B.; Nian, H. Theoretical and Experimental Investigations on High Temperature Mechanical and Thermal Properties of BaZrO3. Ceram. Int. 2018, 44, 16475–16482.
- Draber, F.M.; Ader, C.; Arnold, J.P.; Eisele, S.; Grieshammer, S.; Yamaguchi, S.; Martin, M. Publisher Correction: Nanoscale Percolation in Doped BaZrO3 for High Proton Mobility. Nat. Mater. 2020, 19, 577.
- Hossain, M.K.; Hashizume, K. Dissolution and Release Behavior of Hydrogen Isotopes from Barium-Zirconates. Proc. Int. Exch. Innov. Conf. Eng. Sci. 2020, 6, 34–39.
- Perrichon, A.; Jedvik Granhed, E.; Romanelli, G.; Piovano, A.; Lindman, A.; Hyldgaard, P.; Wahnström, G.; Karlsson, M. Unraveling the Ground-State Structure of BaZrO3 by Neutron Scattering Experiments and First-Principles Calculations. Chem. Mater. 2020, 32, 2824–2835.
- Abdalla, A.M.; Hossain, S.; Radenahmad, N.; Petra, P.M.I.; Somalu, M.R.; Rahman, S.M.H.; Eriksson, S.G.; Azad, A.K. Synthesis and Characterization of Sm1-xZrxFe1-yMgyO3 (x, y = 0.5, 0.7, 0.9) as Possible Electrolytes for SOFCs. Key Eng. Mater. 2018, 765, 49–53.
- Dai, H.; Kou, H.; Wang, H.; Bi, L. Electrochemical Performance of Protonic Ceramic Fuel Cells with Stable BaZrO3-Based Electrolyte: A Mini-Review. Electrochem. Commun. 2018, 96, 11–15.
- Park, K.-Y.; Seo, Y.; Kim, K.B.; Song, S.-J.; Park, B.; Park, J.-Y. Enhanced Proton Conductivity of Yttrium-Doped Barium Zirconate with Sinterability in Protonic Ceramic Fuel Cells. J. Alloys Compd. 2015, 639, 435–444.
- Yoo, Y.; Lim, N. Performance and Stability of Proton Conducting Solid Oxide Fuel Cells Based on Yttrium-Doped Barium Cerate-Zirconate Thin-Film Electrolyte. J. Power Sources 2013, 229, 48–57.
- Shafi, S.P.; Bi, L.; Boulfrad, S.; Traversa, E. Yttrium and Nickel Co-Doped BaZrO3 as a Proton-Conducting Electrolyte for Intermediate Temperature Solid Oxide Fuel Cells. ECS Trans. 2015, 68, 503–508.
- Bi, L.; Traversa, E. Synthesis Strategies for Improving the Performance of Doped-BaZrO3 Materials in Solid Oxide Fuel Cell Applications. J. Mater. Res. 2014, 29, 1–15.
- Dai, H. Proton Conducting Solid Oxide Fuel Cells with Chemically Stable BaZr0.75Y0.2Pr0.05O3−δ Electrolyte. Ceram. Int. 2017, 43, 7362–7365.
- Liu, Y.; Ran, R.; Tade, M.O.; Shao, Z. Structure, Sinterability, Chemical Stability and Conductivity of Proton-Conducting BaZr0.6M0.2Y0.2O3−δ Electrolyte Membranes: The Effect of the M Dopant. J. Memb. Sci. 2014, 467, 100–108.
- Loureiro, F.J.A.; Nasani, N.; Reddy, G.S.; Munirathnam, N.R.; Fagg, D.P. A Review on Sintering Technology of Proton Conducting BaCeO3-BaZrO3 Perovskite Oxide Materials for Protonic Ceramic Fuel Cells. J. Power Sources 2019, 438, 226991.
- Rajendran, S.; Thangavel, N.K.; Ding, H.; Ding, Y.; Ding, D.; Reddy Arava, L.M. Tri-Doped BaCeO3–BaZrO3 as a Chemically Stable Electrolyte with High Proton-Conductivity for Intermediate Temperature Solid Oxide Electrolysis Cells (SOECs). ACS Appl. Mater. Interfaces 2020, 12, 38275–38284.
- Jurewicz, K.; Frackowiak, E.; Béguin, F. Towards the Mechanism of Electrochemical Hydrogen Storage in Nanostructured Carbon Materials. Appl. Phys. A 2004, 78, 981–987.
- Korotcenkov, G.; Han, S.D.; Stetter, J.R. Review of Electrochemical Hydrogen Sensors. Chem. Rev. 2009, 109, 1402–1433.
- Bouwman, P. Electrochemical Hydrogen Compression (EHC) Solutions for Hydrogen Infrastructure. Fuel Cells Bull. 2014, 2014, 12–16.
- Keçebaş, A.; Kayfeci, M.; Bayat, M. Electrochemical Hydrogen Generation. In Solar Hydrogen Production; Elsevier: Amsterdam, The Netherlands, 2019; pp. 299–317.
- Tanaka, M.; Sugiyama, T. Development of a Tritium Monitor Combined with an Electrochemical Tritium Pump Using a Proton Conducting Oxide. Fusion Sci. Technol. 2015, 67, 600–603.
This entry is offline, you can click here to edit this entry!
