Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
In pSS, chronic antigenic stimulation gradually drives the evolution from polyclonal B-cell expansion to oligoclonal/monoclonal B-cell predominance to malignant B-cell transformation. Thus, most pSS-related lymphomas are B-cell non-Hodgkin lymphomas (NHLs), with mucosa-associated lymphoid tissue (MALT) lymphomas predominating, followed by diffuse large B-cell lymphomas (DLBCLs) and nodal marginal zone lymphomas (NMZLs).
- Sjögren’s syndrome
- lymphoma
- biomarkers
1. Introduction
Primary Sjögren’s syndrome (pSS) is a systemic autoimmune disease characterized by B-cell hyperactivity [1]. Lymphomagenesis in the setting of pSS is considered to be a multi-step process arising from persistent polyclonal B-cell activation due to chronic antigenic stimulation in the salivary glands (SGs) of pSS patients, which in turn leads to oligoclonal/monoclonal B-cell expansion followed by the selection of premalignant B-cell clones, which will eventually progress to lymphoma [1][2]. Lymphoepithelial sialadenitis, the histologic hallmark of pSS, is characterized by a different lymphocytic composition based on lesion severity, with the B-cell component predominating over T-cells in the more severe lesions [3]. One could argue that this is not merely a histological finding since an overt B-cell hyperactive state defines a phenotypically unique pSS patient subset with features that have been recognized as predisposing factors for lymphomagenesis [1][4]. The stepwise transition to lymphoma is also reflected by distinct serological findings, with polyclonal B-cell proliferation leading to autoantibody positivity and hypergammaglobulinemia, and oligoclonal/monoclonal pre-malignant B-cell expansion resulting in cryoglobulinemia.
B-cell non-Hodgkin lymphomas (NHL) predominate among other hematological malignancies reported in pSS patients [5][6], while not so often, the development of other lymphoid malignancies, such as T-cell lymphomas or multiple myeloma, may occur [6][7]. While in the general population, the three most frequent B-cell NHL subtypes are diffuse large B-cell lymphoma (DLBCL) (40%), followed by follicular lymphoma (FL) (20%) and marginal zone lymphoma (MZL) (7%) [8], the distribution changes in pSS patients, with marginal zone mucosa-associated lymphoid tissue (MALT) lymphomas constituting the most frequent B-cell NHL subtype (48.5–76%), followed by DLBCL (9–17%) and nodal MZL (NMZL) (7–15%) [5][9][10][11][12][13][14][15][16]. There is, though, a subset of studies demonstrating DLBCLs to be the most frequent lymphoma developing in the setting of pSS [17], while others report a similar frequency of MALT lymphomas and DLBCLs [18]. This difference could be attributed to the different nature of the studies, with cohort studies from Rheumatology or other tertiary centers demonstrating MALT lymphomas as the predominant pSS-associated lymphoma subtype, and epidemiological studies rendering similar frequencies of MALT lymphomas and DLBCLs in the setting of pSS.
It has been demonstrated that pSS-related B-cell NHLs are not associated with viruses [i.e., Epstein–Barr virus (EBV), Hepatitis C virus (HCV), human T-cell lymphotropic virus 1(HTLV-1)] known to be present in lymphomas arising in non-pSS patients [16]. Some of the cytogenetic abnormalities [i.e., translocations t(14;18) or trisomies 18, 3, and 12), and mutations of oncogenes (i.e., p53) described in non-pSS-related lymphomas have also been described in lymphomas complicating pSS [16][19][20][21].
Overall, pSS seems to have a slight impact on patients’ survival compared to the general population, with standardized mortality rates (SMR) ranging from 1.02 [95% confidence interval (CI) 0.4–2.0] to 4.66 (95% CI 3.85–5.6) in different studies [15][22][23][24][25][26]. Researchers should notice, though, that pSS patients who develop lymphoma tend to have higher mortality rates compared to those without lymphoma. Namely, Theander et al. reported that for pSS patients developing lymphoproliferative disorders, the SMR was as high as 7.89 (95% CI 2.89–17.18) compared to the general population of pSS patients with an estimated SMR of 1.17 (95% CI 0.81–1.63) [26], while in the study by Voulgarelis et al., the SMR for pSS patients with lymphoma was 3.25 (95% CI 1.32–6.76) compared to 1.08 (95% CI 0.79–1.45) for those without lymphoma [15].
Despite the increase in mortality rates attributed to lymphoma, the prognosis of pSS patients with B-cell NHL is generally favorable, and the overall survival (OS) rates are rather high [5][11][12][15][17].
2. Predisposing Factors
2.1. Demographic Features
It has been demonstrated that gender and age at pSS diagnosis may act as predisposing factors for lymphoma development. In 1998, Ramos-Casals reported a higher incidence of lymphoma for pSS patients with disease onset at a young age (20 ± 34 years, mean 28 years) [27]. The majority of other published studies, though, do not support the notion that young age at pSS diagnosis is a predisposing feature for lymphoma development [6][18][28][29][30]. A recent multicenter study, including 1997 pSS patients, demonstrated that pSS patients with disease onset at an age of ≤35 years, as well as pSS patients with disease onset at an age of ≥65 years, are at increased risk for lymphoma development compared to patients with disease onset at an age of 35–65 years. Of note, the authors demonstrated two incidence peaks of lymphoma for the early-onset pSS patients (≤35 years old), within 3 years of onset and after 10 years of onset, while for late-onset pSS patients (≥65 years old), lymphoma was diagnosed within the first 6 years [31].
The majority of published research supports an increased incidence of lymphoma for male pSS patients compared to females [10][32][33][34], while a subset of studies reports no such difference [30][35]. It should be noted, though, that in the general population as well, NHL is slightly more common in men [36]. The male gender has been identified as an independent predisposing factor for pSS-related lymphoma development in one study [33], while Gondran et al. described that for male pSS patients who develop lymphoma, the time interval between pSS and lymphoma diagnosis is shorter compared to females [37]. These findings prompt researchers to consider whether gender itself acts as a lymphoma-predisposing feature or whether male patients simply accumulate other characteristics that have been validated as lymphoma risk factors. However, it has been demonstrated that there are no differences in the frequencies of classical lymphoma predictors between males and females [33]; further research is required.
2.2. Clinical Predisposing Factors
The most common clinical predictor for lymphoma development in the setting of pSS is persistent salivary gland enlargement (SGE). The first report of SGE conferring an increased risk for pSS-associated lymphoma development came in 1978 by Kasan et al. [Relative Risk (RR) 66.7 for pSS patients with SGE vs. 12.5 for pSS patients without] [38]. Thereafter, SGE has been validated as an independent risk factor for lymphoma development in multiple series of pSS-related NHL patients [11][18][24][28][29][32][39][40][41][42][43][44][45]. Since SGE is present in about one-third of pSS patients but only a subset of them develops lymphoma, Quartuccio et al. demonstrated that for patients with SGE, the presence of additional prognostic markers better defines the subgroup with increased risk for lymphoma development (namely, at least two of the following: cryoglobulinemia, low C4, leukopenia, anti-La/SSB positivity) [42]. The duration of parotid gland swelling also affects the risk for lymphoma development, with patients presenting parotid gland swelling for more than 12 months displaying a higher risk for lymphomagenesis compared to those with swelling durations ranging from 2 to 12 months [46].
Cutaneous vasculitic manifestations related to cryoglobulinemia, such as palpable purpura or skin ulcers, comprise another clinical entity independently associated with increased risk for lymphoma development [6][14][18][24][25][39][44]. Of note, it has been demonstrated that skin purpura is a clinical feature that can be used to distinguish patients with active lymphoma [43]. Other immune complex-mediated clinical manifestations, such as peripheral neuropathy [14][43] and glomerulonephritis [25][43][47], apart from their predictive value for future lymphoma development, tend to co-exist with active lymphoma in some pSS patients [43].
Lymphadenopathy, as a clinical manifestation of B-cell overactivity, has also been reported to be a possible predisposing factor for lymphoma development, mainly in earlier published studies [9][14][38][44][45], while only one study published in the last decade documents lymphadenopathy as a clinical predictor for lymphoma [29]. Splenomegaly [9][38] and Raynaud’s phenomenon [29] have been proposed as predisposing factors for pSS-associated lymphoma in fewer published studies.
2.3. Serological Predisposing Factors
The detection of serum IgMκ type II cryoglobulins has been demonstrated in almost 20% of pSS patients [48]. In 1996, Tzioufas et al. first proposed mixed monoclonal cryoglobulinemia as a serological marker predictive of pSS-related lymphoma development [49]. Ever since, cryoglobulinemia has been consistently reported in the published literature as a strong predisposing factor for lymphomagenesis in the setting of pSS [9][10][11][40][42][50][51][52]. In a more recent study by Chatzis et al. cryoglobulinemia has been demonstrated to be an independent prognostic factor, especially for pSS-associated MALT lymphoma [11]. Of note, in a large cohort of 1083 pSS patients, among which 10.6% had cryoglobulinemia, one-third of those who presented cryoglobulinemic vasculitis (CV) developed B-cell NHL within the first 5 years of the CV course [53].
Other cryoglobulin-related serological markers, such as C3/C4 hypocomplementemia, rheumatoid factor (RF) positivity, and the presence of monoclonal immunoglobulins, have also been proposed as predictive factors for lymphoma development. Skopouli et al. demonstrated low serum C4 levels (RR = 7.5, p = 0.0016) and cryoglobulinemia (RR = 7.9, p = 0.0012) to be strong predictors of lymphoma development [25]. C4 hypocomplementemia [9][10][18][24][25][29][32][42][43][50][52][54] is more frequently reported as a predisposing factor for lymphoma compared to C3 hypocomplementemia [6][10][18][32][50]. Brito-Zerón et al. reported low serum C4 levels as a prognostic serological marker for non-MALT B-NHL and low serum C3 levels for MALT lymphoma [10]. RF positivity, especially with concomitant C4 hypocomplementemia, is indicative of type II mixed cryoglobulinemia [49]. It has thus been associated with other predictors for lymphoma development and proposed as a possible predisposing factor [29][41][49][50]. Though a subset of studies supported the presence of monoclonal immunoglobulins as a serological finding conferring increased risk for pSS-associated lymphoma [29][43][45], Gottenberg et al. demonstrated an association only with pSS disease activity but not with lymphoma [55]. In an earlier study by Walters et al., serum and urine immunoglobulin light chains and their kappa/lambda ratio have been correlated with the burden of proliferating B-cells and have thus been proposed as a possible lymphoma biomarker [56]. More recent research, though, supports that higher levels of kappa- and lambda-free light chains associate only with higher pSS disease activity and not lymphoma [55].
Though Fragkioudaki et al. reported the presence of Ro/La antibodies as an independent predictor of pSS-related lymphoma [29], other studies of large pSS patients’ cohorts did not support a statistically significant association between anti-Ro/La positivity and lymphoma development [6][10][28][30]. A more recent study in a Greek cohort of 121 pSS-associated lymphoma patients reported an increased frequency of anti-La positivity at pSS diagnosis for patients subsequently developing MALT lymphoma compared to those that did not (51% vs. 34%, p = 0.0049), though anti-La positivity was not eventually proven to be an independent predisposing factor for MALT lymphoma development [11]. Of note, anti-Ro/La negative pSS patients demonstrate a lower frequency of manifestations associated with lymphoproliferation, as well as a lower risk of lymphoma development [57][58].
Higher baseline β2 microglobulin levels in pSS patients that subsequently developed lymphoma have been reported in a Finnish pSS patients’ cohort [59]. A more recent study, though, by Gottenberg et al., demonstrated that increased β2 microglobulin levels were associated with increased pSS disease activity, but no association with lymphoma was proven [55]. Notably, pSS patients with a history of lymphoma seem to maintain higher β2 microglobulin compared to those without lymphoma history [55].
Finally, Agmon-Levin et al. proposed a possible link between low vitamin-D levels and lymphoma predisposition in pSS patients [60].
2.4. Hematological Predisposing Factors
Fewer data are available in the published literature regarding hematologic aberrations as possible predisposing factors for pSS-related lymphoma. Theander et al. reported that CD4+ T lymphocytopenia and a CD4+/CD8+ T-cell ratio ≤ 0.8 correlated with an increased risk for DLBCL development in the setting of pSS [6]. In accordance, Baimpa et al. documented lymphocytopenia at pSS diagnosis as a predictive factor for DLBCL [9]. Leukopenia and neutropenia have also been identified as hematological parameters that could be used to discriminate pSS patients at increased risk for lymphoma development [10][14][18]. Finally, the presence of anemia has been correlated with DLBCL development in pSS patients [10].
2.5. Histological Predisposing Factors
The degree of inflammatory infiltration in the SG of pSS patients has been correlated with systemic involvement and disease severity [61]. Moreover, B-cells predominate over T-cells in more severe lesions, and as expected, their presence has been correlated with RF positivity, cryoglobulinemia, and low serum C4 levels [3], serologic features associated with increased risk for lymphoma development. High focus score (FS), defined as the number of lymphocytic foci per 4 mm2 of the SG tissue, has been documented in high-risk pSS patients who are more prone to develop systemic disease and have a poor outcome [62]. It also comprises a histological marker that can be used to discriminate the subset of pSS patients at higher risk for lymphomagenesis. Carubbi et al. showed that minor SG FS could be used as a prognostic histopathological feature for pSS-related lymphomagenesis [63]. Riselada et al. demonstrated that a minor SG FS ≥3 is an independent predictor of NHL development [64]. The independent prognostic value of minor SG FS is also supported by a more recent study additionally showing that patients who developed lymphoma with a minor SG FS ≥ 4 at pSS diagnosis were characterized by statistically significant shorter time intervals from pSS to lymphoma diagnosis compared to those with minor SG FS < 4 (4 vs. 9 years, respectively, p = 0.008) [65]. The same group also documented that FS is an independent prognostic risk factor, especially for pSS-related MALT lymphoma [11]. Researchers should note though that the above-mentioned studies assessed the prognostic value of minor SG FS [64][65], while another subset of studies supports that that parotid gland biopsy may be better for predicting early-stage lymphoma, given that pSS-associated lymphoma often primarily occurs in the parotid gland [66][67][68]. Considering that minor SG and parotid gland biopsy has been shown to have similar specificity and sensitivity for pSS diagnosis [68], as well as diagnostic accuracy based on the American College of Rheumatology–European League Against Rheumatism (ACR–EULAR) 2016 criteria [69], further studies are needed to confirm whether they also display the same prognostic value for lymphoma prediction in terms of FS.
One of the pSS hallmarks is antigen-driven B-cell activation and proliferation [70][71]. B-cell clones are detected with high prevalence in the SGs of pSS patients [72]. These B-cell clones use a limited repertoire of immunoglobulin (Ig) variable heavy (VH) gene repertoire homologous to RF [73][74]. It is believed that the locally produced IgG autoantibodies in the SGs of pSS patients may form immune complexes, which in turn chronically stimulate B-cells expressing RF B-cell receptors (BCRs) (as reviewed by Stergiou et al. [2]). The majority of patients with pSS-associated SG MALT lymphoma express somatically mutated BCRs that are selected for the monoreactive, high-affinity binding of IgG-Fc [75], while SG lymphomas seem to express BCRs with strong RF homology more frequently compared to MALT lymphomas arising in other anatomical sites (i.e., stomach, lung) [76]. The RF-expressing B-cell clones display antigen-dependent affinity maturation leading to lymphomagenesis [77]. Bende et al. demonstrated, though, that the BCR repertoire strongly biased towards stereotypic RFs in SG MALT lymphoma does not correspond to a similar repertoire in the inflamed SGs of pSS patients, concluding that, in the latter, the repertoire is based on a strong selection advantage of incidental stereotypic RF-expressing B-cells [78]. Of note, it has recently been demonstrated that lymphoma driver mutations are present in B-cells producing pathogenetic antibodies with RF activity [79]. The presence of RF-expressing B-cells may reflect a pre-lymphomatous condition in the setting of pSS [49][80][81]. However, given the observation that B-cell clones are found in approximately 50% of sialadenitis lesions without morphological or clinical evidence of lymphoma [73][82], further research is required to determine which of the patients with RF-expressing B-cell presence in their SGs are those at a higher risk for lymphoma development.
Though the presence of germinal center (GC)-like structures reflects high pSS disease activity [83], its prognostic value as a lymphoma predisposing factor remains controversial. A subset of studies supports the notion of increased lymphoma risk in pSS patients with GC structures in their SG biopsies [54][84][85]. Theander et al. reported that for pSS patients developing lymphoma, the median time from pSS to lymphoma diagnosis was 7 years for those presenting GC-like structures in their diagnostic SG biopsy [54]. On the contrary, other study groups demonstrated no association between ectopic GCs and risk for lymphoma development [66][83][86]. This discrepancy could be attributed to the underestimation of small GCs when an extensive immunohistochemical study is not applied in SG biopsies [87]. On the other hand, the overestimation of GC-like structures may result from the reliance on CD21 staining alone for the detection of follicular dendritic cell (FDC) networks [88][89][90][91]. At this point, researchers should highlight that the histopathological evaluation both of GCs and FS needs to be standardized when assessed in clinical trials in order to have more subjective calculations and to avoid possible discrepancies in the reported results [90][92]. Moreover, researchers should always consider the different sizes and characteristics of each study population which could confer to discrepancies in the reported results.
Investigating the role of ectopic GCs in class switch recombination, Bombardieri et al. described different activation-induced cytidine deaminase (AID) distribution patterns in SG biopsies of pSS patients with and without lymphoma. In the SG of pSS patients without MALT lymphoma, AID was expressed by follicular dendritic cells within ectopic GCs and large B-cells residing in a T-cell-rich zone outside the ectopic GC. On the other hand, in the SG of pSS patients with MALT lymphoma, AID expression was retained in residual GCs, whereas neoplastic marginal zone-like B-cells were consistently AID-negative [89]. This finding possibly reflects the multistep process toward lymphomagenesis.
The lymphoid organization in the SGs of pSS patients is mediated by lymphoid chemokines. Barone et al. demonstrated in the SGs of pSS patients with MALT lymphoma the lymphoid chemokines C-X-C motif chemokine ligand 13 (CXCL13) and C-C motif ligand 21 (CCL21) are selectively associated with areas of reactive lymphoid proliferation, while C-C motif ligand 21 (CXCL12) is observed predominantly in infiltrated ducts and malignant B-cells. These findings suggest that in SG MALT lymphoma, the lymphoid chemokines CXCL13 and CCL21 participate in the reactive lymphoid tissue organization, whereas CXCL12 is possibly involved in the regulation of malignant B-cell survival [93].
2.6. Disease Activity as a Predisposing Factor
The quantification of pSS disease by the EULAR SS disease activity index (ESSDAI) score incorporates a sum of domains, many of which have been shown to be independent prognostic factors for lymphoma development. A characteristic example is that of cryoglobulinemia, a validated strong predictor of pSS-related lymphoma. Cryoglobulin-positive patients present significantly higher ESSDAI scores compared to those without cryoglobulinemia [62][94]. This prompted researchers to investigate the effect of pSS activity on the risk for lymphomagenesis. The ESSDAI score (after excluding the lymphadenopathy domain) calculated at least 6 months before lymphoma diagnosis has been reported as an independent predictor of lymphoma development [41]. Brito-Zerón et al. demonstrated that the baseline ESSDAI score is associated with a higher risk of MALT lymphoma but not for non-MALT B-NHL [23]. In accordance, Chatzis et al. identified the ESSDAI score (after excluding the lymphadenopathy domain) calculated at pSS diagnosis as an independent predictor for MALT lymphoma development [11]. On the contrary, De Vita et al. observed no significant difference of the baseline ESSDAI score between pSS patients who developed lymphoma and those who did not [40]. The glandular domain of the baseline ESSDAI has been correlated with MALT lymphoma [23], while the glandular domain of the baseline clinical ESSDAI has been associated with any type of lymphoma [13]. In a cohort of pSS patients from Argentina, glandular and cutaneous ESSDAI domains have been associated with increased lymphoma risk [13]. Non-MALT B-NHL development has been shown to correlate with increased activity of the biological ESSDAI domain [10]. Of note, an increase in the median ESSDAI score (after excluding the effect of the domain lymphadenopathy and lymphoma) has been documented from baseline at pSS diagnosis (median 9, range 0–44) to the time of lymphoma diagnosis (median 18, range 2–50), with glandular and biological domains showing a statistically significant increase in their value [11].
2.7. Molecular and Genetic Predisposing Factors
More recent research focuses on the identification of molecular and genetic predisposing factors that could be used to discriminate pSS patients at a higher risk for lymphoma development. These newly proposed lymphoma biomarkers relate in terms of pathophysiology to B-cell differentiation, lymphoid organization, and immune responses.
2.8. Imaging-Defined Predisposing Factors
The effective detection of typical pSS SG structural abnormalities through SG ultrasonography (SGUS) has recently gained attention [95]. The retrospective studies of Theander et al. and Coiffier et al. reported that SGUS can identify patients at risk of developing lymphoma [96][97]. Lorenzon et al. prospectively assessed the OMERACT score in pSS patients with clinical findings suspicious for lymphoma development, who subsequently underwent US-guided core-needle biopsy. A higher OMERACT score and a more inhomogenous glandular pattern were documented in pSS patients diagnosed with lymphoma after the core-needle biopsy. For focal lesions, the authors identified eight suspicious lymphoma features (OMERACT grade 3, very hypoechoic, homogenous, oval shape, well-defined margins, presence of septa, color-Doppler vascularization, posterior acoustic enhancement), showing that the simultaneous presence of 6/8 and 7/8 features was significantly higher among pSS-lymphoma patients compared to pSS patients without lymphoma [98]. Overall, SGUS is considered to be the imaging modality of choice for the assessment of the SG parenchyma in patients with pSS, as well as for the identification of lesions suspicious for lymphoproliferative disease that need further histological assessment [99].
Magnetic resonance imaging (MRI) findings display good agreement with SGUS for pSS diagnostic work-up [100][101]. It should be noted, though, that the features of benign and malignant SG lesions in pSS seem to overlap in MRI [100][101], rendering this imaging technique ineffective for the identification of lymphoma. A single study reports that parotid gland solid cystic features could discriminate MALT from non-MALT lymphomas [102].
Bădărînză et al. investigated the role of bidimensional shear wave elastography in the identification of pSS parotid NHL, identifying features characteristic of parotid gland MALT lymphoma (hyperechoic bands in more than half of the glandular parenchyma, large hypoechoic area > 20 mm, traced gland area over 5 cm2, parotid US score greater than 13, and high stiffness, all p < 0.001). These features were shown to have high sensitivity (92.3%), specificity (100%), and positive (100%) and negative predictive values (98.3%) for NHL identification [103].
Since lymphadenopathy is a rather common clinical finding in pSS patients, contemporary imaging modalities could be used to distinguish patients with active lymphoma. To this end, Cohen et al. demonstrated that positron emission tomography/computed tomography (PET/CT) could be a useful diagnostic tool in the setting of pSS lymphadenopathy, since lymph node FDG uptake is marginally higher in pSS patients with lymphoma compared to those without lymphoma [maximum standardized uptake value (SUVmax) = 5.4 vs. 3.2, p = 0.05] [104]. In a more recent retrospective study by Keraen et al. a parotid gland SUVmax of ≥4.7 and/or the presence of focal lung lesions were associated with lymphoma diagnosis [105].
2.9. Predictive Models for Lymphoma Development
To improve the lymphoma risk stratification of pSS patients, models combining independent predisposing factors have been developed. Ioannidis et al. first proposed that the simultaneous presence of palpable purpura and low C4 levels at the initial evaluation could be used to distinguish pSS patients at high risk for lymphoma development from those at low risk [24]. Baimpa et al. constructed a prognostic model for pSS-associated MALT lymphoma based on the presence of five features, clinical, serological, and hematological as follows: cryoglobulinemia, neutropenia, low C4 levels, lymphadenopathy, and splenomegaly. The proportion of pSS patients who were diagnosed with MALT lymphoma by the number of predisposing features was 3.62%, 11.96%, 34.78%, 80%, and 100%, for patients with 0, 1, 2, 3, and 4 features, respectively [9]. Baldini et al. also developed a “bedside” clinical prediction model for the identification of high risk for lymphoma development in pSS patients, based on the presence of SGE, low C4 and/or C3 levels, and disease duration [32]. A predictive risk score for pSS-related NHL was developed by Fragkioudaki et al., including those features shown to be independent predictors for lymphoma development in their study: SGE, lymphadenopathy, Raynaud’s phenomenon, anti-Ro/SSA or/and anti-La/SSB positivity, RF positivity, the presence of monoclonal immunoglobulins, and C4 hypocomplementemia. The probability for NHL development was 3.8% for patients with fewer than two features, and 39.9% for those with three to six features, while the corresponding probability reached 100% in the presence of all seven features [29]. More recently, an attempt to combine classical clinical and serological predisposing factors for pSS-related lymphomagenesis with genetic variants has been undertaken [106].
2.10. “One Size Fits All?”: Distinct Predisposing Factors for Different Patient Subgroups and Lymphoma Subtypes, Predisposing Factors Detected at Different Time-Points before Lymphoma Development
Different predictive factors for lymphoma development have been shown to apply to pSS patients of different ages at disease onset. Regarding classical predisposing factors, cryoglobulinemia, C4 hypocomplementemia, lymphadenopathy, and SGE have been independently related to lymphomagenesis for pSS patients with disease onset at an age ≤ 35 years, while SGE, C4 hypocomplementemia, and the male gender were independently associated with lymphoma development for those with pSS onset at an age ≥ 65 years [31]. As for the more recently proposed lymphoma biomarkers, the TNFAIP3 rs2230926 variant and the functional LILRA3 gene variant could apply for the pSS patient subgroup with disease onset at an age ≤ 40 years [107][108], while BAFF-R His159Tyr mutation for the subgroup with disease onset between 31 and 40 years of age [109]. No significant differences in lymphoma predisposing factors have been so far described between female and male patients, despite the tendency of men to present more often SGE and lymphadenopathy [33][34].
Since the different pSS-related B-cell NHL subtypes are characterized by significantly different disease severity and prognosis, it is important to define subtype-specific predisposing factors, especially given the dismal outcomes of pSS patients with DLBCL. Cryoglobulinemia, neutropenia, low serum C4 levels, low serum C3 levels, lymphadenopathy, splenomegaly, FS at pSS diagnosis, and ESSDAI at pSS diagnosis have been specifically correlated with MALT lymphoma development, while lymphocytopenia, a CD4+/CD8+ T-cell ratio ≤0.8, anemia, and low serum C4 levels have been proposed as predisposing factors for DLBCL development [6][9][10][11][18].
Certain predisposing factors could be detected a long time before lymphoma development (Figure 1). The reported median time from the expression of a feature to lymphoma development could lead to the monitoring of pSS patients. Specifically, the median time to lymphoma diagnosis has been documented to be 108 months for SGE [38], 88 months for CD4+ lymphocytopenia [6], 84 months for lymphadenopathy/splenomegaly [38], 84 months for histologically detected GC-like structures [54], 68 months for an FS ≥ 3 [64], 48 months for an FS ≥ 4 [65], and 8 months for mixed monoclonal cryoglobulinemia [49]. Among the more recently proposed biomarkers, serum Flt-3L levels have been found to increase at a median time of 46 months before lymphoma diagnosis, [110], and low miR200p-5b expression in SGs can be detected at a median time of 36 months before lymphoma [111].
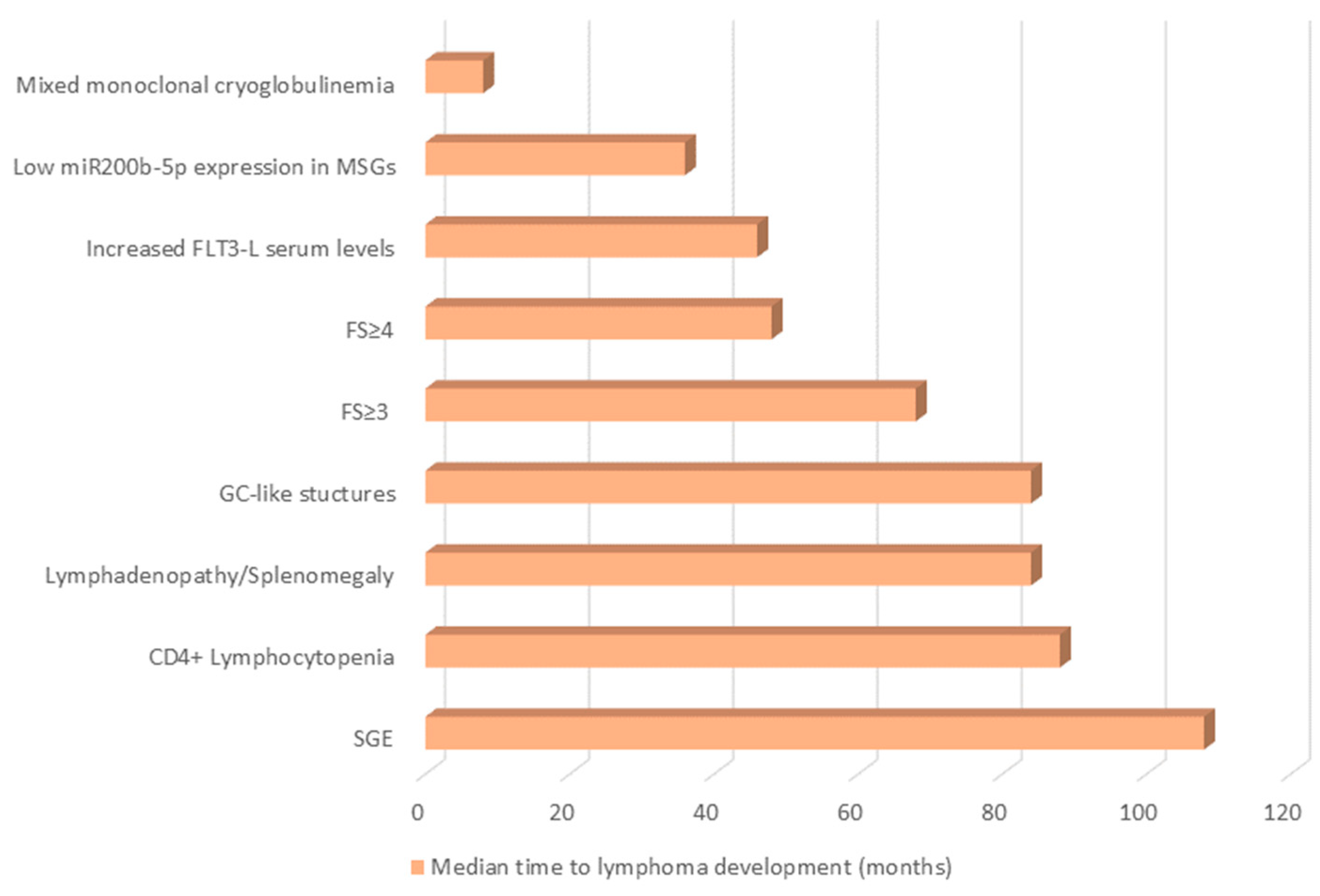
Figure 1. The median time (months) before lymphoma diagnosis that certain pSS-related lymphoma predisposing factors could be detected. FLT-3L, Fms-like tyrosine kinase 3 Ligand; FS, Focus Score; GC, Germinal Center; miR, micro-RNA; SGE, Salivary Gland Enlargement.
This entry is adapted from the peer-reviewed paper 10.3390/immuno2040037
References
- Goules, A.V.; Tzioufas, A.G. Lymphomagenesis in Sjögren’s syndrome: Predictive biomarkers towards precision medicine. Autoimmun. Rev. 2019, 18, 137–143.
- Stergiou, I.E.; Poulaki, A.; Voulgarelis, M. Pathogenetic Mechanisms Implicated in Sjögren’s Syndrome Lymphomagenesis: A Review of the Literature. J. Clin. Med. 2020, 9, 3794.
- Christodoulou, M.I.; Kapsogeorgou, E.K.; Moutsopoulos, H.M. Characteristics of the minor salivary gland infiltrates in Sjögren’s syndrome. J. Autoimmun. 2010, 34, 400–407.
- Goules, A.V.; Tzioufas, A.G. Primary Sjӧgren’s syndrome: Clinical phenotypes, outcome and the development of biomarkers. Autoimmun. Rev. 2016, 15, 695–703.
- Hernández-Molina, G.; Kostov, B.; Brito-Zerón, P.; Vissink, A.; Mandl, T.; Hinrichs, A.C.; Quartuccio, L.; Baldini, C.; Seror, R.; Szántó, A.; et al. Characterization and outcomes of 414 patients with primary SS who developed hematological malignancies. Rheumatology 2022.
- Theander, E.; Henriksson, G.; Ljungberg, O.; Mandl, T.; Manthorpe, R.; Jacobsson, L.T. Lymphoma and other malignancies in primary Sjögren’s syndrome: A cohort study on cancer incidence and lymphoma predictors. Ann. Rheum. Dis. 2006, 65, 796–803.
- Stergiou, I.E.; Papageorgiou, A.; Chatzis, L.G.; Tzioufas, A.G.; Voulgarelis, M.; Goules, A. T cell lymphoma in the setting of Sjögren’s syndrome: T cells gone bad? Report of five cases from a single centre cohort. Clin. Exp. Rheumatol. 2020, 38 (Suppl. 126), 125–129.
- Swerdlow, S.H.C.E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; IARC: Lyon, France, 2017.
- Baimpa, E.; Dahabreh, I.J.; Voulgarelis, M.; Moutsopoulos, H.M. Hematologic manifestations and predictors of lymphoma development in primary Sjögren syndrome: Clinical and pathophysiologic aspects. Medicine 2009, 88, 284–293.
- Brito-Zerón, P.; Kostov, B.; Fraile, G.; Caravia-Durán, D.; Maure, B.; Rascón, F.-J.; Zamora, M.; Casanovas, A.; Lopez-Dupla, M.; Ripoll, M.; et al. Characterization and risk estimate of cancer in patients with primary Sjögren syndrome. J. Hematol. Oncol. 2017, 10, 90.
- Chatzis, L.G.; Stergiou, I.E.; Goules, A.V.; Pezoulas, V.; Tsourouflis, G.; Fotiadis, D.; Tzioufas, A.G.; Voulgarelis, M. Clinical picture, outcome and predictive factors of lymphoma in primary Sjögren’s syndrome: Results from a harmonized dataset (1981–2021). Rheumatology 2022, 61, 3576–3585.
- Papageorgiou, A.; Ziogas, D.C.; Mavragani, C.P.; Zintzaras, E.; Tzioufas, A.G.; Moutsopoulos, H.M.; Voulgarelis, M. Predicting the outcome of Sjogren’s syndrome-associated non-hodgkin’s lymphoma patients. PLoS ONE 2015, 10, e0116189.
- Schenone, L.N.; Pellet, A.C.; Mamani, M.; Melo, F.; Adrover, M.; Barreira, J.; Dermarchi, J.; Escobar, C.S.; Santiago, L.; Salvatierra, G.; et al. Development of lymphoma in patients with primary Sjögren Syndrome OMICS Publishing Group. Int. J. Clin. Rheumatol. 2019, 14, 69–74.
- Voulgarelis, M.; Dafni, U.G.; Isenberg, D.A.; Moutsopoulos, H.M. Malignant lymphoma in primary Sjögren’s syndrome: A multicenter, retrospective, clinical study by the European Concerted Action on Sjögren’s Syndrome. Arthritis Rheum. 1999, 42, 1765–1772.
- Voulgarelis, M.; Ziakas, P.D.; Papageorgiou, A.; Baimpa, E.; Tzioufas, A.G.; Moutsopoulos, H.M. Prognosis and outcome of non-Hodgkin lymphoma in primary Sjögren syndrome. Medicine 2012, 91, 1–9.
- Royer, B.; Cazals-Hatem, D.; Sibilia, J.; Agbalika, F.; Cayuela, J.-M.; Soussi, T.; Maloisel, F.d.r.; Clauvel, J.-P.; Brouet, J.-C.; Mariette, X. Lymphomas in Patients with Sjögren’s Syndrome Are Marginal Zone B-Cell Neoplasms, Arise in Diverse Extranodal and Nodal Sites, and Are Not Associated with Viruses. Blood 1997, 90, 766–775.
- Vasaitis, L.; Nordmark, G.; Theander, E.; Backlin, C.; Smedby, K.E.; Askling, J.; Rönnblom, L.; Sundström, C.; Baecklund, E. Population-based study of patients with primary Sjögren’s syndrome and lymphoma: Lymphoma subtypes, clinical characteristics, and gender differences. Scand. J. Rheumatol. 2020, 49, 225–232.
- Solans-Laqué, R.; López-Hernandez, A.; Bosch-Gil, J.A.; Palacios, A.; Campillo, M.; Vilardell-Tarres, M. Risk, predictors, and clinical characteristics of lymphoma development in primary Sjögren’s syndrome. Semin. Arthritis Rheum. 2011, 41, 415–423.
- Tapinos, N.I.; Polihronis, M.; Moutsopoulos, H.M. Lymphoma development in Sjögren’s syndrome: Novel p53 mutations. Arthritis Rheum. 1999, 42, 1466–1472.
- Pisa, E.K.; Pisa, P.; Kang, H.I.; Fox, R.I. High frequency of t(14;18) translocation in salivary gland lymphomas from Sjögren’s syndrome patients. J. Exp. Med. 1991, 174, 1245–1250.
- Ihrler, S.; Baretton, G.B.; Menauer, F.; Blasenbreu-Vogt, S.; Löhrs, U. Sjögren’s Syndrome and MALT Lymphomas of Salivary Glands: A DNA-Cytometric and Interphase-Cytogenetic Study. Mod. Pathol. 2000, 13, 4–12.
- Alamanos, Y.; Tsifetaki, N.; Voulgari, P.V.; Venetsanopoulou, A.I.; Siozos, C.; Drosos, A.A. Epidemiology of primary Sjögren’s syndrome in north-west Greece, 1982–2003. Rheumatology 2006, 45, 187–191.
- Brito-Zerón, P.; Kostov, B.; Solans, R.; Fraile, G.; Suárez-Cuervo, C.; Casanovas, A.; Rascón, F.J.; Qanneta, R.; Pérez-Alvarez, R.; Ripoll, M.; et al. Systemic activity and mortality in primary Sjögren syndrome: Predicting survival using the EULAR-SS Disease Activity Index (ESSDAI) in 1045 patients. Ann. Rheum. Dis. 2016, 75, 348–355.
- Ioannidis, J.P.; Vassiliou, V.A.; Moutsopoulos, H.M. Long-term risk of mortality and lymphoproliferative disease and predictive classification of primary Sjögren’s syndrome. Arthritis Rheum. 2002, 46, 741–747.
- Skopouli, F.N.; Dafni, U.; Ioannidis, J.P.; Moutsopoulos, H.M. Clinical evolution, and morbidity and mortality of primary Sjögren’s syndrome. Semin. Arthritis Rheum. 2000, 29, 296–304.
- Theander, E.; Manthorpe, R.; Jacobsson, L.T. Mortality and causes of death in primary Sjögren’s syndrome: A prospective cohort study. Arthritis Rheum. 2004, 50, 1262–1269.
- Ramos-Casals, M.; Cervera, R.; Font, J.; Garcóa-Carrasco, M.; Espinosa, G.; Reino, S.; Pallarés, L.; Ingelmo, M. Young Onset of Primary Sjögren’s syndrome: Clinical and immunological characteristics. Lupus 1998, 7, 202–206.
- Brito-Zerón, P.; Ramos-Casals, M.; Bove, A.; Sentis, J.; Font, J. Predicting adverse outcomes in primary Sjogren’s syndrome: Identification of prognostic factors. Rheumatology 2007, 46, 1359–1362.
- Fragkioudaki, S.; Mavragani, C.P.; Moutsopoulos, H.M. Predicting the risk for lymphoma development in Sjogren syndrome: An easy tool for clinical use. Medicine 2016, 95, e3766.
- Johnsen, S.J.; Brun, J.G.; Gøransson, L.G.; Småstuen, M.C.; Johannesen, T.B.; Haldorsen, K.; Harboe, E.; Jonsson, R.; Meyer, P.A.; Omdal, R. Risk of non-Hodgkin’s lymphoma in primary Sjögren’s syndrome: A population-based study. Arthritis Care Res. 2013, 65, 816–821.
- Goules, A.V.; Argyropoulou, O.D.; Pezoulas, V.C.; Chatzis, L.; Critselis, E.; Gandolfo, S.; Ferro, F.; Binutti, M.; Donati, V.; Zandonella Callegher, S.; et al. Primary Sjögren’s Syndrome of Early and Late Onset: Distinct Clinical Phenotypes and Lymphoma Development. Front. Immunol. 2020, 11, 594096.
- Baldini, C.; Pepe, P.; Luciano, N.; Ferro, F.; Talarico, R.; Grossi, S.; Tavoni, A.; Bombardieri, S. A clinical prediction rule for lymphoma development in primary Sjögren’s syndrome. J. Rheumatol. 2012, 39, 804–808.
- Chatzis, L.; Pezoulas, V.C.; Ferro, F.; Gandolfo, S.; Donati, V.; Binutti, M.; Callegher, S.Z.; Venetsanopoulou, A.; Zampeli, E.; Mavrommati, M.; et al. Sjögren’s Syndrome: The Clinical Spectrum of Male Patients. J. Clin. Med. 2020, 9, 2620.
- Ramírez Sepúlveda, J.I.; Kvarnström, M.; Eriksson, P.; Mandl, T.; Norheim, K.B.; Johnsen, S.J.; Hammenfors, D.; Jonsson, M.V.; Skarstein, K.; Brun, J.G.; et al. Long-term follow-up in primary Sjögren’s syndrome reveals differences in clinical presentation between female and male patients. Biol. Sex Differ. 2017, 8, 25.
- Weng, M.Y.; Huang, Y.T.; Liu, M.F.; Lu, T.H. Incidence of cancer in a nationwide population cohort of 7852 patients with primary Sjogren’s syndrome in Taiwan. Ann. Rheum. Dis. 2012, 71, 524–527.
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Padala, S.A.; Barsouk, A.; Rawla, P. Epidemiology of Non-Hodgkin’s Lymphoma. Med. Sci. 2021, 9, 5.
- Gondran, G.; Fauchais, A.; Lambert, M.; Ly, K.; Launay, D.; Queyrel, V.; Benazahari, H.; Liozon, E.; Loustaud-Ratti, V.; Hachulla, E.; et al. Primary Sjogren’s syndrome in men. Scand. J. Rheumatol. 2008, 37, 300–305.
- Kassan, S.S.; Thomas, T.L.; Moutsopoulos, H.M.; Hoover, R.; Kimberly, R.P.; Budman, D.R.; Costa, J.; Decker, J.L.; Chused, T.M. Increased risk of lymphoma in sicca syndrome. Ann. Intern. Med. 1978, 89, 888–892.
- Abrol, E.; González-Pulido, C.; Praena-Fernández, J.M.; Isenberg, D.A. A retrospective study of long-term outcomes in 152 patients with primary Sjogren’s syndrome: 25-year experience. Clin. Med. 2014, 14, 157–164.
- De Vita, S.; Gandolfo, S.; Zandonella Callegher, S.; Zabotti, A.; Quartuccio, L. The evaluation of disease activity in Sjögren’s syndrome based on the degree of MALT involvement: Glandular swelling and cryoglobulinaemia compared to ESSDAI in a cohort study. Clin. Exp. Rheumatol. 2018, 36 (Suppl. 112), 150–156.
- Nocturne, G.; Virone, A.; Ng, W.F.; Le Guern, V.; Hachulla, E.; Cornec, D.; Daien, C.; Vittecoq, O.; Bienvenu, B.; Marcelli, C.; et al. Rheumatoid Factor and Disease Activity Are Independent Predictors of Lymphoma in Primary Sjögren’s Syndrome. Arthritis Rheumatol. 2016, 68, 977–985.
- Quartuccio, L.; Isola, M.; Baldini, C.; Priori, R.; Bartoloni Bocci, E.; Carubbi, F.; Maset, M.; Gregoraci, G.; Della Mea, V.; Salvin, S.; et al. Biomarkers of lymphoma in Sjögren’s syndrome and evaluation of the lymphoma risk in prelymphomatous conditions: Results of a multicenter study. J. Autoimmun. 2014, 51, 75–80.
- Risselada, A.P.; Kruize, A.A.; Bijlsma, J.W. Clinical features distinguishing lymphoma development in primary Sjögren’s Syndrome--a retrospective cohort study. Semin. Arthritis Rheum. 2013, 43, 171–177.
- Sutcliffe, N.; Inanc, M.; Speight, P.; Isenberg, D. Predictors of lymphoma development in primary Sjögren’s syndrome. Semin. Arthritis Rheum. 1998, 28, 80–87.
- Zhang, W.; Feng, S.; Yan, S.; Zhao, Y.; Li, M.; Sun, J.; Zhang, F.C.; Cui, Q.; Dong, Y. Incidence of malignancy in primary Sjogren’s syndrome in a Chinese cohort. Rheumatology 2010, 49, 571–577.
- De Vita, S.; Isola, M.; Baldini, C.; Goules, A.V.; Chatzis, L.G.; Quartuccio, L.; Zabotti, A.; Giovannini, I.; Donati, V.; Ferro, F.; et al. Predicting lymphoma in Sjögren’s syndrome and the pathogenetic role of parotid microenvironment through precise parotid swelling recording. Rheumatology 2022.
- Goules, A.; Masouridi, S.; Tzioufas, A.G.; Ioannidis, J.P.; Skopouli, F.N.; Moutsopoulos, H.M. Clinically significant and biopsy-documented renal involvement in primary Sjögren syndrome. Medicine 2000, 79, 241–249.
- Tzioufas, A.G.; Manoussakis, M.N.; Costello, R.; Silis, M.; Papadopoulos, N.M.; Moutsopoulos, H.M. Cryoglobulinemia in autoimmune rheumatic diseases. Evidence of circulating monoclonal cryoglobulins in patients with primary Sjögren’s syndrome. Arthritis Rheum. 1986, 29, 1098–1104.
- Tzioufas, A.G.; Boumba, D.S.; Skopouli, F.N.; Moutsopoulos, H.M. Mixed monoclonal cryoglobulinemia and monoclonal rheumatoid factor cross-reactive idiotypes as predictive factors for the development of lymphoma in primary Sjögren’s syndrome. Arthritis Rheum. 1996, 39, 767–772.
- Baldini, C.; Pepe, P.; Quartuccio, L.; Priori, R.; Bartoloni, E.; Alunno, A.; Gattamelata, A.; Maset, M.; Modesti, M.; Tavoni, A.; et al. Primary Sjogren’s syndrome as a multi-organ disease: Impact of the serological profile on the clinical presentation of the disease in a large cohort of Italian patients. Rheumatology 2014, 53, 839–844.
- Martel, C.; Gondran, G.; Launay, D.; Lalloué, F.; Palat, S.; Lambert, M.; Ly, K.; Loustaud-Ratti, V.; Bezanahary, H.; Hachulla, E.; et al. Active immunological profile is associated with systemic Sjögren’s syndrome. J. Clin. Immunol. 2011, 31, 840–847.
- Nocturne, G.; Seror, R.; Fogel, O.; Belkhir, R.; Boudaoud, S.; Saraux, A.; Larroche, C.; Le Guern, V.; Gottenberg, J.E.; Mariette, X. CXCL13 and CCL11 Serum Levels and Lymphoma and Disease Activity in Primary Sjögren’s Syndrome. Arthritis Rheumatol. 2015, 67, 3226–3233.
- Argyropoulou, O.D.; Pezoulas, V.; Chatzis, L.; Critselis, E.; Gandolfo, S.; Ferro, F.; Quartuccio, L.; Donati, V.; Treppo, E.; Bassoli, C.R.; et al. Cryoglobulinemic vasculitis in primary Sjögren’s Syndrome: Clinical presentation, association with lymphoma and comparison with Hepatitis C-related disease. Semin. Arthritis Rheum. 2020, 50, 846–853.
- Theander, E.; Vasaitis, L.; Baecklund, E.; Nordmark, G.; Warfvinge, G.; Liedholm, R.; Brokstad, K.; Jonsson, R.; Jonsson, M.V. Lymphoid organisation in labial salivary gland biopsies is a possible predictor for the development of malignant lymphoma in primary Sjögren’s syndrome. Ann. Rheum. Dis. 2011, 70, 1363–1368.
- Gottenberg, J.E.; Seror, R.; Miceli-Richard, C.; Benessiano, J.; Devauchelle-Pensec, V.; Dieude, P.; Dubost, J.J.; Fauchais, A.L.; Goeb, V.; Hachulla, E.; et al. Serum levels of beta2-microglobulin and free light chains of immunoglobulins are associated with systemic disease activity in primary Sjögren’s syndrome. Data at enrollment in the prospective ASSESS cohort. PLoS ONE 2013, 8, e59868.
- Walters, M.T.; Stevenson, F.K.; Herbert, A.; Cawley, M.I.; Smith, J.L. Urinary monoclonal free light chains in primary Sjögren’s syndrome: An aid to the diagnosis of malignant lymphoma. Ann. Rheum. Dis. 1986, 45, 210–219.
- Chatzis, L.G.; Pezoulas, V.; Voulgari, P.V.; Baldini, C.; Exarchos, T.P.; Fotiadis, D.I.; Mavragani, C.P.; Skopouli, F.N.; Moutsopoulos, H.M.; Tzioufas, A.G.; et al. Combined seronegativity in Sjögren’s syndrome. Clin. Exp. Rheumatol. 2021, 39 (Suppl. 133), 80–84.
- Quartuccio, L.; Baldini, C.; Bartoloni, E.; Priori, R.; Carubbi, F.; Corazza, L.; Alunno, A.; Colafrancesco, S.; Luciano, N.; Giacomelli, R.; et al. Anti-SSA/SSB-negative Sjögren’s syndrome shows a lower prevalence of lymphoproliferative manifestations, and a lower risk of lymphoma evolution. Autoimmun. Rev. 2015, 14, 1019–1022.
- Pertovaara, M.; Pukkala, E.; Laippala, P.; Miettinen, A.; Pasternack, A. A longitudinal cohort study of Finnish patients with primary Sjögren’s syndrome: Clinical, immunological, and epidemiological aspects. Ann. Rheum. Dis. 2001, 60, 467–472.
- Agmon-Levin, N.; Kivity, S.; Tzioufas, A.G.; López Hoyos, M.; Rozman, B.; Efes, I.; Shapira, Y.; Shamis, A.; Amital, H.; Youinou, P.; et al. Low levels of vitamin-D are associated with neuropathy and lymphoma among patients with Sjögren’s syndrome. J. Autoimmun. 2012, 39, 234–239.
- Gerli, R.; Muscat, C.; Giansanti, M.; Danieli, M.G.; Sciuto, M.; Gabrielli, A.; Fiandra, E.; Vitali, C. Quantitative assessment of salivary gland inflammatory infiltration in primary Sjögren’s syndrome: Its relationship to different demographic, clinical and serological features of the disorder. Br. J. Rheumatol. 1997, 36, 969–975.
- Brito-Zerón, P.; Retamozo, S.; Ramos-Casals, M. Phenotyping Sjögren’s syndrome: Towards a personalised management of the disease. Clin. Exp. Rheumatol. 2018, 36 (Suppl. 112), 198–209.
- Carubbi, F.; Alunno, A.; Cipriani, P.; Bartoloni, E.; Baldini, C.; Quartuccio, L.; Priori, R.; Valesini, G.; De Vita, S.; Bombardieri, S.; et al. A retrospective, multicenter study evaluating the prognostic value of minor salivary gland histology in a large cohort of patients with primary Sjögren’s syndrome. Lupus 2015, 24, 315–320.
- Risselada, A.P.; Kruize, A.A.; Goldschmeding, R.; Lafeber, F.P.; Bijlsma, J.W.; van Roon, J.A. The prognostic value of routinely performed minor salivary gland assessments in primary Sjögren’s syndrome. Ann. Rheum. Dis. 2014, 73, 1537–1540.
- Chatzis, L.; Goules, A.V.; Pezoulas, V.; Baldini, C.; Gandolfo, S.; Skopouli, F.N.; Exarchos, T.P.; Kapsogeorgou, E.K.; Donati, V.; Voulgari, P.V.; et al. A biomarker for lymphoma development in Sjogren’s syndrome: Salivary gland focus score. J. Autoimmun. 2021, 121, 102648.
- Haacke, E.A.; van der Vegt, B.; Vissink, A.; Spijkervet, F.K.L.; Bootsma, H.; Kroese, F.G.M. Germinal centres in diagnostic labial gland biopsies of patients with primary Sjögren’s syndrome are not predictive for parotid MALT lymphoma development. Ann. Rheum. Dis. 2017, 76, 1781–1784.
- Mossel, E.; Delli, K.; van Nimwegen, J.F.; Stel, A.J.; Kroese, F.G.M.; Spijkervet, F.K.L.; Vissink, A.; Arends, S.; Bootsma, H. Ultrasonography of major salivary glands compared with parotid and labial gland biopsy and classification criteria in patients with clinically suspected primary Sjögren’s syndrome. Ann. Rheum. Dis. 2017, 76, 1883–1889.
- Pijpe, J.; Kalk, W.W.I.; van der Wal, J.E.; Vissink, A.; Kluin, P.M.; Roodenburg, J.L.N.; Bootsma, H.; Kallenberg, C.G.M.; Spijkervet, F.K.L. Parotid gland biopsy compared with labial biopsy in the diagnosis of patients with primary Sjögren’s syndrome. Rheumatology 2007, 46, 335–341.
- van Nimwegen, J.F.; van Ginkel, M.S.; Arends, S.; Haacke, E.A.; van der Vegt, B.; Sillevis Smitt-Kamminga, N.; Spijkervet, F.K.L.; Kroese, F.G.M.; Stel, A.J.; Brouwer, E.; et al. Validation of the ACR-EULAR criteria for primary Sjögren’s syndrome in a Dutch prospective diagnostic cohort. Rheumatology 2018, 57, 818–825.
- Voulgarelis, M.; Moutsopoulos, H.M. Lymphoproliferation in autoimmunity and Sjögren’s syndrome. Curr. Rheumatol. Rep. 2003, 5, 317–323.
- Stott, D.I.; Hiepe, F.; Hummel, M.; Steinhauser, G.; Berek, C. Antigen-driven clonal proliferation of B cells within the target tissue of an autoimmune disease. The salivary glands of patients with Sjögren’s syndrome. J. Clin. Investig. 1998, 102, 938–946.
- Jordan, R.C.; Masaki, Y.; Takeshita, S.; Speight, P.M.; Sugai, S. High prevalence of B-cell monoclonality in labial gland biopsies of Japanese Sjögren’s syndrome patients. Int. J. Hematol. 1996, 64, 47–52.
- Miklos, J.A.; Swerdlow, S.H.; Bahler, D.W. Salivary gland mucosa-associated lymphoid tissue lymphoma immunoglobulin VH genes show frequent use of V1-69 with distinctive CDR3 features. Blood 2000, 95, 3878–3884.
- Visser, A.; Verstappen, G.M.; van der Vegt, B.; Vissink, A.; Bende, R.J.; Bootsma, H.; Bos, N.A.; Kroese, F.G.M. Repertoire Analysis of B-Cells Located in Striated Ducts of Salivary Glands of Patients with Sjögren’s Syndrome. Front. Immunol. 2020, 11, 234–239.
- Bende, R.J.; Janssen, J.; Beentjes, A.; Wormhoudt, T.A.M.; Wagner, K.; Haacke, E.A.; Kroese, F.G.M.; Guikema, J.E.J.; van Noesel, C.J.M. Salivary Gland Mucosa-Associated Lymphoid Tissue-Type Lymphoma From Sjögren’s Syndrome Patients in the Majority Express Rheumatoid Factors Affinity-Selected for IgG. Arthritis Rheumatol. 2020, 72, 1330–1340.
- Bende, R.J.; Aarts, W.M.; Riedl, R.G.; de Jong, D.; Pals, S.T.; van Noesel, C.J. Among B cell non-Hodgkin’s lymphomas, MALT lymphomas express a unique antibody repertoire with frequent rheumatoid factor reactivity. J. Exp. Med. 2005, 201, 1229–1241.
- Broeren, M.G.A.; Wang, J.J.; Balzaretti, G.; Groenen, P.; van Schaik, B.D.C.; Chataway, T.; Kaffa, C.; Bervoets, S.; Hebeda, K.M.; Bounova, G.; et al. Proteogenomic analysis of the autoreactive B cell repertoire in blood and tissues of patients with Sjögren’s syndrome. Ann. Rheum. Dis. 2022, 81, 644–652.
- Bende, R.J.; Slot, L.M.; Hoogeboom, R.; Wormhoudt, T.A.; Adeoye, A.O.; Guikema, J.E.; van Noesel, C.J. Stereotypic rheumatoid factors that are frequently expressed in mucosa-associated lymphoid tissue-type lymphomas are rare in the labial salivary glands of patients with Sjögren’s syndrome. Arthritis Rheumatol. 2015, 67, 1074–1083.
- Singh, M.; Jackson, K.J.L.; Wang, J.J.; Schofield, P.; Field, M.A.; Koppstein, D.; Peters, T.J.; Burnett, D.L.; Rizzetto, S.; Nevoltris, D.; et al. Lymphoma Driver Mutations in the Pathogenic Evolution of an Iconic Human Autoantibody. Cell 2020, 180, 878–894.e819.
- De Vita, S.; Boiocchi, M.; Sorrentino, D.; Carbone, A.; Avellini, C.; Dolcetti, R.; Marzotto, A.; Gloghini, A.; Bartoli, E.; Beltrami, C.A.; et al. Characterization of prelymphomatous stages of B cell lymphoproliferation in Sjögren’s syndrome. Arthritis Rheum. 1997, 40, 318–331.
- De Re, V.; De Vita, S.; Gasparotto, D.; Marzotto, A.; Carbone, A.; Ferraccioli, G.; Boiocchi, M. Salivary gland B cell lymphoproliferative disorders in Sjögren’s syndrome present a restricted use of antigen receptor gene segments similar to those used by hepatitis C virus-associated non-Hodgkins’s lymphomas. Eur. J. Immunol. 2002, 32, 903–910.
- Carbone, A.; Gloghini, A.; Ferlito, A. Pathological features of lymphoid proliferations of the salivary glands: Lymphoepithelial sialadenitis versus low-grade B-cell lymphoma of the malt type. Ann. Otol. Rhinol. Laryngol. 2000, 109, 1170–1175.
- Haacke, E.A.; van der Vegt, B.; Vissink, A.; Spijkervet, F.K.L.; Bootsma, H.; Kroese, F.G.M. Germinal Centers in Diagnostic Biopsies of Patients with Primary Sjögren’s Syndrome Are Not a Risk Factor for Non-Hodgkin’s Lymphoma but a Reflection of High Disease Activity: Comment on the Article by Sène et al. Arthritis Rheumatol. 2019, 71, 170–171.
- Risselada, A.P.; Looije, M.F.; Kruize, A.A.; Bijlsma, J.W.; van Roon, J.A. The role of ectopic germinal centers in the immunopathology of primary Sjögren’s syndrome: A systematic review. Semin. Arthritis Rheum. 2013, 42, 368–376.
- Sène, D.; Ismael, S.; Forien, M.; Charlotte, F.; Kaci, R.; Cacoub, P.; Diallo, A.; Dieudé, P.; Lioté, F. Ectopic Germinal Center-Like Structures in Minor Salivary Gland Biopsy Tissue Predict Lymphoma Occurrence in Patients with Primary Sjögren’s Syndrome. Arthritis Rheumatol. 2018, 70, 1481–1488.
- Johnsen, S.J.; Berget, E.; Jonsson, M.V.; Helgeland, L.; Omdal, R.; Jonsson, R. Evaluation of germinal center-like structures and B cell clonality in patients with primary Sjögren syndrome with and without lymphoma. J. Rheumatol. 2014, 41, 2214–2222.
- Nakshbandi, U.; Haacke, E.A.; Bootsma, H.; Vissink, A.; Spijkervet, F.K.L.; van der Vegt, B.; Kroese, F.G.M. Bcl6 for identification of germinal centres in salivary gland biopsies in primary Sjögren’s syndrome. Oral Dis. 2020, 26, 707–710.
- Barone, F.; Bombardieri, M.; Manzo, A.; Blades, M.C.; Morgan, P.R.; Challacombe, S.J.; Valesini, G.; Pitzalis, C. Association of CXCL13 and CCL21 expression with the progressive organization of lymphoid-like structures in Sjögren’s syndrome. Arthritis Rheum. 2005, 52, 1773–1784.
- Bombardieri, M.; Barone, F.; Humby, F.; Kelly, S.; McGurk, M.; Morgan, P.; Challacombe, S.; De Vita, S.; Valesini, G.; Spencer, J.; et al. Activation-induced cytidine deaminase expression in follicular dendritic cell networks and interfollicular large B cells supports functionality of ectopic lymphoid neogenesis in autoimmune sialoadenitis and MALT lymphoma in Sjögren’s syndrome. J. Immunol. 2007, 179, 4929–4938.
- Delli, K.; Haacke, E.A.; Ihrler, S.; van der Vegt, B.; Vissink, A.; Bootsma, H.; Spijkervet, F.K.; Kroese, F.G. Need for consensus guidelines to standardise the assessment of germinal centres and other histopathological parameters in salivary gland tissue of patients with primary Sjögren’s syndrome. Ann. Rheum. Dis. 2016, 75, e32.
- Jonsson, M.V.; Skarstein, K. Follicular dendritic cells confirm lymphoid organization in the minor salivary glands of primary Sjögren’s syndrome. J. Oral Pathol. Med. 2008, 37, 515–521.
- Fisher, B.A.; Jonsson, R.; Daniels, T.; Bombardieri, M.; Brown, R.M.; Morgan, P.; Bombardieri, S.; Ng, W.-F.; Tzioufas, A.G.; Vitali, C.; et al. Standardisation of labial salivary gland histopathology in clinical trials in primary Sjögren’s syndrome. Ann. Rheum. Dis. 2017, 76, 1161–1168.
- Barone, F.; Bombardieri, M.; Rosado, M.M.; Morgan, P.R.; Challacombe, S.J.; De Vita, S.; Carsetti, R.; Spencer, J.; Valesini, G.; Pitzalis, C. CXCL13, CCL21, and CXCL12 expression in salivary glands of patients with Sjogren’s syndrome and MALT lymphoma: Association with reactive and malignant areas of lymphoid organization. J. Immunol. 2008, 180, 5130–5140.
- Quartuccio, L.; Baldini, C.; Bartoloni, E.; Priori, R.; Carubbi, F.; Alunno, A.; Gandolfo, S.; Gattamelata, A.; Giacomelli, R.; Gerli, R.; et al. Correlation between ESSDAI and ClinESSDAI in a real-life cohort of patients with Sjögren’s syndrome. Clin. Exp. Rheumatol. 2017, 35, 546–547.
- Devauchelle-Pensec, V.; Zabotti, A.; Carvajal-Alegria, G.; Filipovic, N.; Jousse-Joulin, S.; De Vita, S. Salivary gland ultrasonography in primary Sjögren’s syndrome: Opportunities and challenges. Rheumatology 2019.
- Coiffier, G.; Martel, A.; Albert, J.D.; Lescoat, A.; Bleuzen, A.; Perdriger, A.; De Bandt, M.; Maillot, F. Ultrasonographic damages of major salivary glands are associated with cryoglobulinemic vasculitis and lymphoma in primary Sjogren’s syndrome: Are the ultrasonographic features of the salivary glands new prognostic markers in Sjogren’s syndrome? Ann. Rheum. Dis. 2021, 80, e111.
- Theander, E.; Mandl, T. Primary Sjögren’s syndrome: Diagnostic and prognostic value of salivary gland ultrasonography using a simplified scoring system. Arthritis Care Res. 2014, 66, 1102–1107.
- Lorenzon, M.; Tulipano Di Franco, F.; Zabotti, A.; Pegolo, E.; Giovannini, I.; Manfrè, V.; Mansutti, E.; De Vita, S.; Zuiani, C.; Girometti, R. Sonographic features of lymphoma of the major salivary glands diagnosed with ultrasound-guided core needle biopsy in Sjögren’s syndrome. Clin. Exp. Rheumatol. 2021, 39 (Suppl. 133), 175–183.
- Lorenzon, M.; Spina, E.; Tulipano Di Franco, F.; Giovannini, I.; De Vita, S.; Zabotti, A. Salivary Gland Ultrasound in Primary Sjögren’s Syndrome: Current and Future Perspectives. Open Access Rheumatol. 2022, 14, 147–160.
- Baldini, C.; Zabotti, A.; Filipovic, N.; Vukicevic, A.; Luciano, N.; Ferro, F.; Lorenzon, M.; De Vita, S. Imaging in primary Sjögren’s syndrome: The ‘obsolete and the new’. Clin. Exp. Rheumatol. 2018, 36 (Suppl. 112), 215–221.
- Grevers, G.; Ihrler, S.; Vogl, T.J.; Weiss, M. A comparison of clinical, pathological and radiological findings with magnetic resonance imaging studies of lymphomas in patients with Sjögren’s syndrome. Eur. Arch. Otorhinolaryngol. 1994, 251, 214–217.
- Zhu, L.; Wang, P.; Yang, J.; Yu, Q. Non-Hodgkin lymphoma involving the parotid gland: CT and MR imaging findings. Dentomaxillofac. Radiol. 2013, 42, 20130046.
- Bădărînză, M.; Serban, O.; Maghear, L.; Bocsa, C.; Micu, M.; Damian, L.; Felea, I.; Fodor, D. Shear wave elastography as a new method to identify parotid lymphoma in primary Sjögren Syndrome patients: An observational study. Rheumatol. Int. 2020, 40, 1275–1281.
- Cohen, C.; Mekinian, A.; Uzunhan, Y.; Fauchais, A.L.; Dhote, R.; Pop, G.; Eder, V.; Nunes, H.; Brillet, P.Y.; Valeyre, D.; et al. 18F-fluorodeoxyglucose positron emission tomography/computer tomography as an objective tool for assessing disease activity in Sjögren’s syndrome. Autoimmun. Rev. 2013, 12, 1109–1114.
- Keraen, J.; Blanc, E.; Besson, F.L.; Leguern, V.; Meyer, C.; Henry, J.; Belkhir, R.; Nocturne, G.; Mariette, X.; Seror, R. Usefulness of (18) F-Labeled Fluorodeoxyglucose-Positron Emission Tomography for the Diagnosis of Lymphoma in Primary Sjögren’s Syndrome. Arthritis Rheumatol. 2019, 71, 1147–1157.
- Kourou, K.D.; Pezoulas, V.C.; Georga, E.I.; Exarchos, T.; Papaloukas, C.; Voulgarelis, M.; Goules, A.; Nezos, A.; Tzioufas, A.G.; Moutsopoulos, E.M.; et al. Predicting Lymphoma Development by Exploiting Genetic Variants and Clinical Findings in a Machine Learning-Based Methodology with Ensemble Classifiers in a Cohort of Sjögren’s Syndrome Patients. IEEE Open J. Eng. Med. Biol. 2020, 1, 49–56.
- Nezos, A.; Gkioka, E.; Koutsilieris, M.; Voulgarelis, M.; Tzioufas, A.G.; Mavragani, C.P. TNFAIP3 F127C Coding Variation in Greek Primary Sjogren’s Syndrome Patients. J. Immunol. Res. 2018, 2018, 6923213.
- Argyriou, E.; Nezos, A.; Roussos, P.; Venetsanopoulou, A.; Voulgarelis, M.; Boki, K.; Tzioufas, A.G.; Moutsopoulos, H.M.; Mavragani, C.P. Leukocyte Immunoglobulin-Like Receptor A3 (LILRA3): A Novel Marker for Lymphoma Development among Patients with Young Onset Sjogren’s Syndrome. J. Clin. Med. 2021, 10, 644.
- Papageorgiou, A.; Mavragani, C.P.; Nezos, A.; Zintzaras, E.; Quartuccio, L.; De Vita, S.; Koutsilieris, M.; Tzioufas, A.G.; Moutsopoulos, H.M.; Voulgarelis, M. A BAFF receptor His159Tyr mutation in Sjögren’s syndrome-related lymphoproliferation. Arthritis Rheumatol. 2015, 67, 2732–2741.
- Tobón, G.J.; Saraux, A.; Gottenberg, J.E.; Quartuccio, L.; Fabris, M.; Seror, R.; Devauchelle-Pensec, V.; Morel, J.; Rist, S.; Mariette, X.; et al. Role of Fms-like tyrosine kinase 3 ligand as a potential biologic marker of lymphoma in primary Sjögren’s syndrome. Arthritis Rheum. 2013, 65, 3218–3227.
- Kapsogeorgou, E.K.; Papageorgiou, A.; Protogerou, A.D.; Voulgarelis, M.; Tzioufas, A.G. Low miR200b-5p levels in minor salivary glands: A novel molecular marker predicting lymphoma development in patients with Sjögren’s syndrome. Ann. Rheum. Dis. 2018, 77, 1200–1207.
This entry is offline, you can click here to edit this entry!
