Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Medicine, Research & Experimental
Numerous studies have shown that microglia are capable of producing a wide range of chemokines to promote inflammatory processes within the central nervous system (CNS). These cells share many phenotypical and functional characteristics with macrophages, suggesting that microglia participate in innate immune responses in the brain. Neuroinflammation induces neurometabolic alterations and increases in energy consumption. Microglia may constitute an important therapeutic target in neuroinflammation.
- Ghrelin
- Ghrelin receptor
- microglia
- inflammation
1. Introduction
Over the last few years, our knowledge of Ghrelin (Ghre) has increased significantly. In fact, the peptide Ghre is involved in several cellular activities affecting the gastrointestinal and immune systems. This orexigenic hormone not only regulates food intake and energy content but also modulates plasticity and cognition in the central nervous system (CNS). Ghre signaling deregulation is involved in the pathophysiology of obesity and may provide a link between metabolic syndromes and cognitive impairment [1]. Indeed, in obesity, impaired metabolic homeostasis is frequently associated with the severity of age-related cognitive decline, hippocampal function and neurodegenerative diseases [2]. These alterations are associated with increased microglia activation and synaptic profiles within microglia and lesser dendritic spines [2]. In fact, it has been reported that pharmacological inhibition of the phagocytic activity of microglia is sufficient to avoid cognitive deterioration [2].
During inflammation, proinflammatory cytokines and immune-derived cells stimulate hormone release and metabolism. Several reports show that Ghre and its receptor play a regulatory role during inflammation [3]. In the CNS, the main sources of proinflammatory molecules are glial cells, classifiable into two, large groups: microglia and macroglia. Microglial cells derive from the embryonic mesoderm. They protect the brain from injuries and diseases. Macroglia cells derive from the neuroectoderm and provide trophic support, maintain metabolism and homeostasis, and produce the myelin sheath around axons [4].
Both Ghre and microglia are involved in the pathophysiology of neurodegenerative diseases characterized by neuronal damage such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) [5,6].
2. Expression and Functions of Ghre and Ghre Receptor
Ghre is a small peptide of 28 amino acids, which is involved in several physiological functions [7]. Originally described as an endogenous growth hormone receptor ligand, Ghre was found to be largely produced by a population of neuroendocrine cells, rodent X/A-like cells and human P/D1 cells, found in the oxyntic mucosa of the stomach fundus [7]. Successively, Ghre gene expression was found in the hypothalamus, cerebral cortex, brainstem, heart, lung and testis [8], In addition, it is produced by neurons, glial cells, immune cells and endocrine cells located in other peripheral tissues, such as the intestines and pancreas [9]. In a sequence of catalytic steps, the precursor pre-proGhre is expressed, cleaved to pro-Ghre and transported to the Golgi body, where it is acylated by the action of the Ghre-O-acyltransferase enzyme, and undergoes a post-translational modification at serine residue 3 [10]. In the end, following translocation to the endoplasmic reticulum, pro-Ghre is further processed by prohormone convertase 1/3 to generate the anorexigenic hormone Ghre. Therefore, once secreted, it is released into the bloodstream in two distinct molecular structures: desacyl-Ghre and its acylated form [11]. This structural modification is an important step because through the circulatory system, Acyl-Ghre is able to cross the blood–brain barrier (BBB), carrying out its functions at the brain level on hypothalamic nuclei, portions of the cortex, amygdala, hippocampus and dorsal vagal complex [1,12,13] (Figure 1). In the acylated form, Ghre fulfills a wide range of physiological functions such as regulation of food intake, gastrointestinal motility and acid secretion, cardiac function, osteoblast proliferation, bone maturation and muscular/myoblast outgrowth, the formation of long-term memory, sleep cycle, the control of behaviors such as spontaneity, anxiety, and food/reward behavior, as well as the modulation of the circadian rhythm [11]. In addition, the metabolic hormone Acyl-Ghre leads the secretion of growth hormone by the pituitary gland, reduces insulin, increases glucagon secretion by pancreatic cells and promotes the hepatic release of glucose into the blood, retaining steady plasma glucose levels during fasting [14]. Furthermore, Acyl-Ghre induces the orexigenic peptide neuropeptide Y (NPY) expression and agouti-related protein (AgRP) in the hypothalamus to stimulate appetite [11].
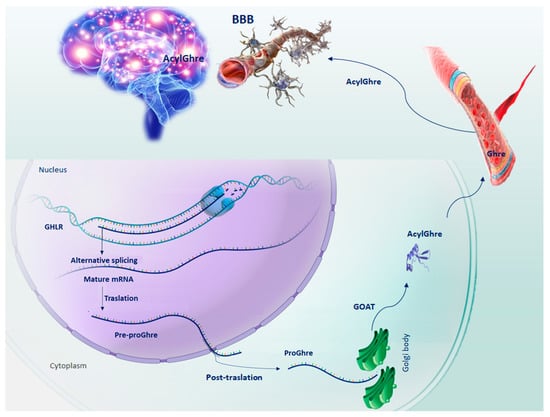
Figure 1. Schematic representation of the sequential steps of Ghrelin production. GHLR gene, located on the short arm of chromosome 3, through splicing alternative process, generates the mRNA from which the pre-proGhre precursor will originate. In turn, pre-proGhre is cleaved to pro-Ghre and transported to the Golgi body, where it is acylated by the action of the GOAT enzyme. The acylated form of Ghre through the circulatory system reaches and crosses the BBB, carrying out its functions in the brain. Abbreviation = AcylGhre, acylated Ghrelin; BBB, blood–brain barrier; Ghre, Ghrelin; GHLR, human ghrelin gene; GOAT, Ghre-O-acyltransferase; Pre-proGhre, pre-proGhrelin; ProGhre, pro-Ghrelin.
Acyl-Ghre exerts these functions, acting through its related G-protein-coupled receptor (GPCR), known as the growth hormone secretagogue receptor (GHSR). GHSR exists in two isoforms: GHSR-1A and its truncated and nonfunctional splicing variant GHSR-1B [15]. Only GHSR-1A is able to interact with Acyl-Ghre. GHSR-1A is broadly transcribed in various crucial regions of the brain, such as the hippocampus, hypothalamus, cortex, ventral tegmental area, spinal cord, dorsal and median raphe nuclei, sympathetic preganglionic nerves and endothelial cells of the cerebral vasculature. Further, it is expressed by several immune cells and in peripheral tissue [16]. In addition, Ghre and its receptor have been found to be expressed in B and T cells, monocytes and natural killer cells [17]. In CNS, Ghre and GHSR-1A act as neuropeptides in neural transmission and function. They have been identified in the olfactory bulb where they process olfactory signals [18,19]. GHSR-1A is induced by Ghre binding and triggers its signaling pathway [20]. In contrast, The GHSR-1B isoform is unable to bind Ghre but it modulates the GHSR-1A receptor through heterodimerization processes [21]. GHSR-1B causes a conformational restriction of the GHSR-1A receptor, inactivating it and exerting a negative effect [20,22]. GHSR-1A is able to interact with GHSR-1B and other G-coupled receptors, such as serotonin, dopamine (D1 and D2) and melanocortin [23].
3. Role of Ghre in Neuroinflammation and Neurometabolism
An unhealthy diet can generate obesity and altered metabolism, which induces a chronic proinflammatory metabolic phenotype (metaflammation) and associated brain damage [24]. The brain inflammation due to “wrong food habits” confirms the existence of the gut–brain axis. Metaflammation is produced by the dysfunction of the immune metabolism. The nutrition burden triggers signaling pathways and cascades without severe immune response symptoms, but is comparable to a chronic immune response for a long time [25]. The metaflammation can also damage the microstructure of brain regions and cause abnormal physiology. Therefore, pathological obesity leads not only to weight gain but also to disorders in the human immune system and neuroinflammation [26]. Bioenergetic changes and oxidative stress stimulate microglia-driven neuroinflammation. It is well known that the CNS is immune-privileged, largely protected from the circulating inflammatory pathways. Nevertheless, altered immune responses and proinflammatory mediators impair the BBB. Tight junctions and basal lamina due to loss of control in the production of matrix metalloproteinases (MMPs) and their inhibitors tissue inhibitors of metalloproteinases (TIMPs) causes structural and functional integrity of the BBB, which in turn promotes a massive migration of leukocytes through the BBB [27]. This process contributes to triggering the activation of microglia and the neuroinflammatory response. During brain impairment, BBB disruption can occur. Some studies have shown the ability of desacylated Ghre to enhance BBB integrity [28]. Ghre is involved not only in the regulation of energy intake but also in the adjustment of the immune response by inflammatory factors [29]. As previously reported, Ghre affects the physiologic processes of several systems acting on specialized innate cells, including glial cells, macrophages and dendritic cells [30]. Notably, Ghre significantly alleviates excessive inflammation and reduces damage to different target organs mainly by reducing the secretion of inflammatory cytokines, including interleukin-6 (IL-6), interleukin-1β (IL-1β), and tumor necrosis factor-α (TNF-α) [3,31]. Proinflammatory cytokine release and Ghre induction are related to transcription factor nuclear factor kappa-B (NF-κB) expression reduction and IL-8 secretion [32]. Moreover, it is well known how inflammasomes play a key role in the development and progression of diseases. Studies have shown that Ghre inhibits the NLR family pyrin domain containing 3 (NLRP3) inflammasome activation and IL-1β maturation [33]. In microglia, Ghre promotes inactivation, inhibiting the expression of TNFα, IL-1 and NOS [30]. The Ghre inhibitory effect also occurs in downstream proinflammatory cytokines such as the transcription factor high-mobility group protein B1 (HMGB1). In both humans and animals, during severe sepsis, it is possible to find high levels of systemic HMGB1. Chorny et al. demonstrated that Ghre administration reduced HMGB1 circulating levels, removing lethality [34].
This entry is adapted from the peer-reviewed paper 10.3390/ijms232113432
This entry is offline, you can click here to edit this entry!
