Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Infectious Diseases
C. jejuni is the leading cause of human foodborne illness associated with poultry, beef, and pork consumption. C. jejuni is highly prevalent in commercial poultry farms, where horizontal transmission from the environment is considered to be the primary source of C. jejuni.
- Campylobacter jejuni
- broilers
- feed additives
1. Introduction
C. jejuni was first recognized in 1886 by Escherich as he described the C. jejuni as a spiral bacteria isolated from the colon of dead children [1]. Escherich also identified the C. jejuni microscopically in stool specimens of children who suffered from diarrhea without being able to culture it on solid agar [1]. In 1909, a Vibrio-like bacterium was frequently isolated from aborted fetuses [2], later named Vibrio fetus [3]. Similar reports linked Vibrio-like organisms to sterility in cows [4], and dysentery in pigs, and later named Vibrio jejuni [5].
Similarly, several reports noted the presence of Vibrio fetus in the blood of pregnant women [6] and the blood of people associated with outbreaks related to consumption of milk contaminated with Vibrio fetus [7]. The absence of a proper isolation method for Vibrio fetus (C. jejuni) from feces resulted in fewer case reports despite the high prevalence of this pathogen. However, C. jejuni was successfully isolated from the stool of a patient suffering from acute enteritis [8]. The development of simpler isolation techniques for culturing C. jejuni led to the rapid isolation of this pathogen. In the mid-1980s, C. jejuni was recognized as one of the major causes of enterocolitis in humans [9].
C. jejuni is the leading cause of human foodborne illness associated with poultry, beef, and pork consumption [10]. C. jejuni is found in the gut of warm-blooded animals, with poultry species being the major reservoirs [11]. C. jejuni colonizes the ceca of chicken between 2 and 3 weeks of age and reaches around 1 × 109 CFU/g in the ceca at market age [12]. Furthermore, poultry carcass is cross-contaminated at the processing facility due to spillage of intestinal contents. Handling and consuming improperly cooked poultry products account for the majority of C. jejuni infections [13]. With the spread of antibiotic resistance across C. jejuni isolates, the burden of Campylobacteroisis has increased [14].
The poultry industry is facing several challenges with legislative restrictions on the subtherapeutic use of antibiotics, in addition to the shift in consumers’ preference for “zero” use of antibiotics in poultry production. Therefore, finding an antimicrobial alternative to control C. jejuni in poultry production is the need of the hour. Different antibiotic alternatives include prebiotics, probiotics, synbiotic, bacteriocins, bacteriophages, vaccines, and organic acids [15].
4. Pathogenesis of C. jejuni in Broilers
C. jejuni pathogenesis consists of four main steps: (1) ingestion, (2) acid tolerance and bile resistance, (3) reproduction in mucus, and (4) invasion of epithelial cells [12]. C. jejuni infection is transmitted between birds via the fecal–oral route [56]. A small dose of C. jejuni (around 35 CFU/mL) is sufficient for successful bird colonization [57]. As an enteric pathogen, C. jejuni expresses virulence factors regulated by a two-component system that mediates C. jejuni’s ability to survive the gut’s harsh conditions [58]. Campylobacter multidrug efflux pump (CmeABC) helps C. jejuni in eliminating toxic compounds such as antimicrobials, bile salts, and heavy metals. CmeABC comprises three proteins, a periplasmic protein, an inner membrane protein, and an outer membrane protein [59]. CmeABC gene encodes the multidrug efflux pump in C. jejuni and it is regulated through Cme repressor (CmeR) [60]. The presence of bile compounds stimulates the expression of CmeABC, increasing C. jejuni’s resistance to bile salts [60]. Mutations in regulator genes related to bile resistance block C. jejuni’s colonization ability [61].
C. jejuni depends on the two-component system consisting of CheY (cytoplasmic response regulator protein) and CheA (membrane-associated histidine auto kinase sensor) in responding to different chemoattractant/chemorepellents found in different environments [62]. In response to a stimulus, CheA is autophosphorylated, and a phosphate group is transferred to activate CheY. CheY interacts with the flagellar motor switch proteins leading to a clockwise rotation of the flagella [63]. The flagella play a central role in C. jejuni motility, adhesion, and invasion of the intestinal epithelial cells [64]. The flagellum consists of seven protofilaments of FlaA and FlaB subunits [64] and is attached to the basal structure through FlgE, which serves as a hook [64]. FlaA is the major Flagellin in C. jejuni and is regulated by σ28 promotor [20]. On the other hand, FlaB is the minor flagellin in C. jejuni and is regulated by the σ58 promoter [20]. Chemotaxis such as aspartate, glutamate, citrate, and L-fucose upregulates the σ58 gene [65]. FlaA plays a significant role in C. jejuni’s initial colonization of the chicken GIT [66]. The FlaA mutant has a ability to decrease the C. jejuni colonization in chicken [67]. Furthermore, the flagella include the type III secretion system (T3SS), which is responsible for delivering effector proteins needed for cellular invasion [20]. Thus, mutations in the flagellum lead to a decreased ability in colonization and invasion of intestinal epithelial cells.
The absence of immortalized chicken intestinal cell line hinders the capacity to characterize the mechanism of C. jejuni invasion of epithelial cells. In vitro, C. jejuni was capable of invading primary avian cells [68,69]. The presence of avian mucus protected the human cell line against the C. jejuni invasion [70]. It is well-known that C. jejuni survives and reproduces in avian mucus [71]. However, several factors interfere with C. jejuni’s capacity to invade the avian epithelial cells in vivo and might explain the near-commensal relationship of C. jejuni in avian species. Several differences are observed between humans and avian species in terms of body temperature (37 °C vs. 42 °C), mucus pH (the avian mucus is more acidic), and difference in mucus structure. C. jejuni upregulates genes related to metabolism and regulatory systems and downregulates genes related to periplasmic proteins at 37 °C in comparison with 42 °C [72]. This difference in gene expression may explain the C. jejuni adaptability and pathogenicity in humans’ intestinal tract. C. jejuni upregulates the CadF gene, which is responsible for cell adhesion at 37 °C and 42 °C, indicating the ability of C. jejuni to adhere to intestinal cells in humans and avian species [72]. On the other hand, C. jejuni isolates showed different gene expressions at 37 °C vs. 42 °C [73]. The difference in gene expression might explain some of the differences in C. jejuni’s pathogenicity. However, it might not be enough to justify the complete picture of C. jejuni pathogenicity in humans vs. the near-commensal relationship in avian species. It was hypothesized that the pH of the avian mucus confers protection for avian species against C. jejuni. However, through in vitro studies, the neutralization of the avian mucus did not diminish its anti-Campylobacter jejuni properties [68].
Purified chicken mucin inhibited the adherence and internalization of C. jejuni to a human intestinal cell line without affecting C. jejuni viability [70]. The oxidation of purified chicken mucin with sodium metaperiodate enabled C. jejuni to invade the intestinal cell line [70]. The results indicate the protecting role of o-glycosylated mucin structure in the intestinal cell line against C. jejuni.
The avian mucus is highly sulphated and sialylated compared with the human mucus [74]. The comparison between chicken and human mucin structures revealed thirty-three unique structures in chicken mucin [75]. The large intestine in chicken contains the highest sulphated structures, followed by the small intestine and cecum [75]. In chicken, C. jejuni colonizes mainly the ceca and, to a lesser extent, in the small and large intestines [76]. Evidence from in vitro studies shows that the purified chicken mucin from the large intestine had a higher inhibition ability against C. jejuni compared with the purified chicken mucin from the small intestine and cecum [75]. These results highlighted that presence of sulphated O-glycans is inversely correlated with the concentration of C. jejuni in the host. Furthermore, the increased sulfation and sialyation increase the anionic charge in the chicken mucin, creating a charge repulsion effect against C. jejuni [74]. These results indicate the role of chicken mucus in modulating C. jejuni virulence in avian species. C. jejuni pathogenesis is summarized in Figure 2.
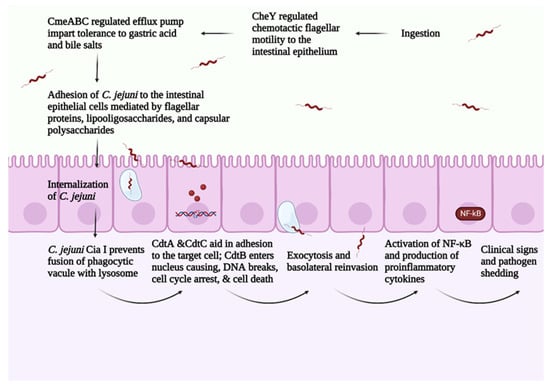
Figure 2. Overview of C. jejuni pathogenesis. Created with Biorender.com (accessed on 20 September 2022).
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms10112134
This entry is offline, you can click here to edit this entry!
