Double-stranded RNA-binding proteins (dsRBPs) are major players in the regulation of gene expression patterns. Among them, Nuclear Factor 90 (NF90) has a plethora of well-known functions in viral infection, transcription, and translation as well as RNA stability and degradation. In addition, NF90 has been identified as a regulator of microRNA (miRNA) maturation by competing with Microprocessor for the binding of pri-miRNAs in the nucleus. NF90 was recently shown to control the biogenesis of a subset of human miRNAs, which ultimately influences, not only the abundance, but also the expression of the host gene and the fate of the mRNA target repertoire.
- NF90
- miRNA
- RISC
- posttranscriptional regulation
- gene regulation
- dsRBP
1. Introduction
2. Functions of NF90
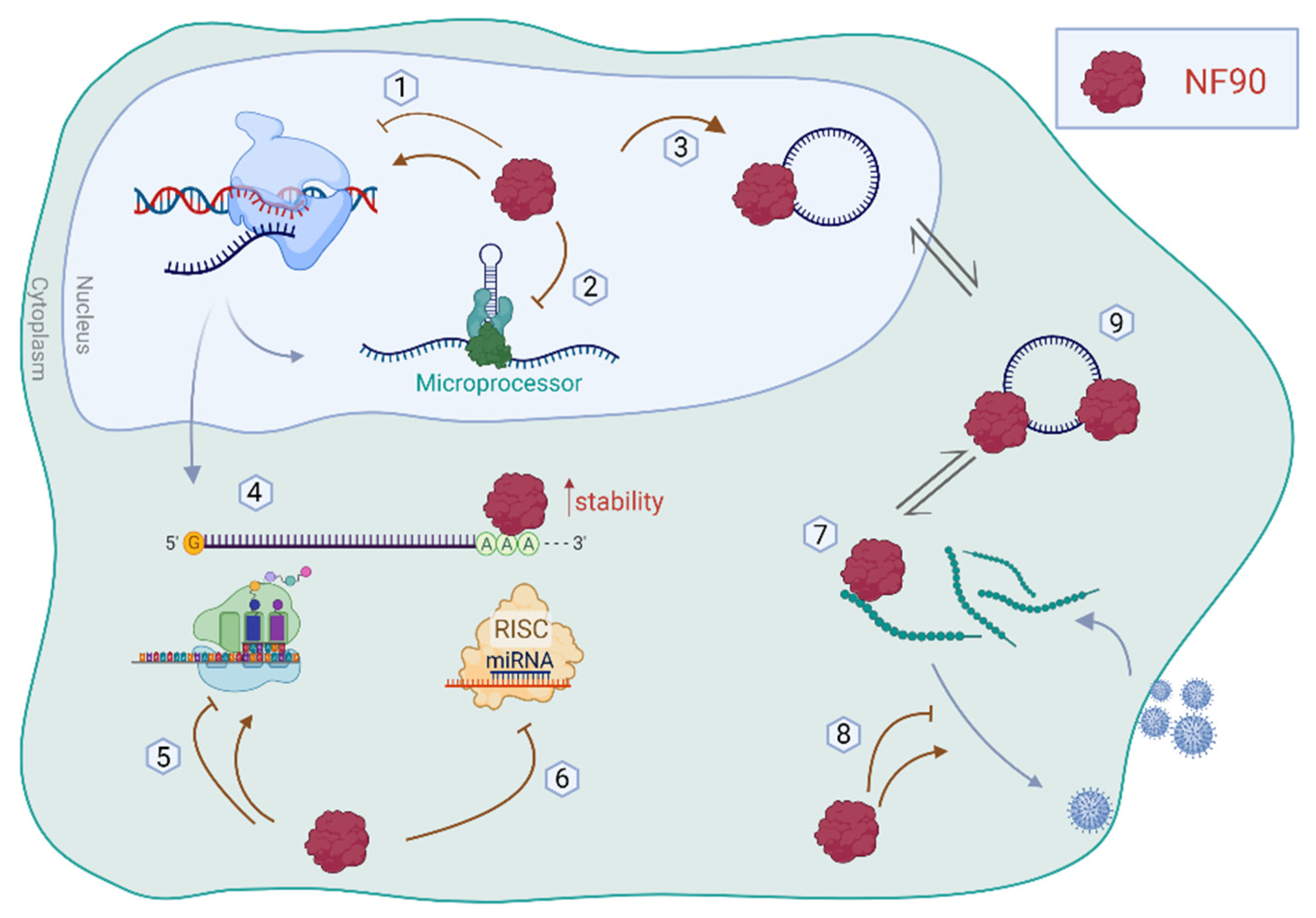
2.1. NF90 in Transcriptional Regulation
2.2. NF90 in the miRNA Biogenesis Pathway
2.3. NF90 in mRNA Translation, Stability, and Degradation
2.4. NF90 in Viral Replication
2.5. Interplay between NF90 and Circular RNAs
This entry is adapted from the peer-reviewed paper 10.3390/ijms232113584
References
- Audic, Y.; Hartley, R.S. Post-transcriptional regulation in cancer. Biol. Cell 2004, 96, 479–498.
- Corbett, A.H. Post-transcriptional regulation of gene expression and human disease. Curr. Opin. Cell Biol. 2018, 52, 96–104.
- Dolicka, D.; Sobolewski, C.; Correia de Sousa, M.; Gjorgjieva, M.; Foti, M. mRNA Post-Transcriptional Regulation by AU-Rich Element-Binding Proteins in Liver Inflammation and Cancer. Int. J. Mol. Sci. 2020, 21, 6648.
- Christou-Kent, M.; Dhellemmes, M.; Lambert, E.; Ray, P.F.; Arnoult, C. Diversity of RNA-Binding Proteins Modulating Post-Transcriptional Regulation of Protein Expression in the Maturing Mammalian Oocyte. Cells 2020, 9, 662.
- Uchida, Y.; Chiba, T.; Kurimoto, R.; Asahara, H. Post-transcriptional regulation of inflammation by RNA-binding proteins via cis-elements of mRNAs. J. Biochem. 2019, 166, 375–382.
- Saunders, L.R.; Perkins, D.J.; Balachandran, S.; Michaels, R.; Ford, R.; Mayeda, A.; Barber, G.N. Characterization of two evolutionarily conserved, alternatively spliced nuclear phosphoproteins, NFAR-1 and -2, that function in mRNA processing and interact with the double-stranded RNA-dependent protein kinase, PKR. J. Biol. Chem. 2001, 276, 32300–32312.
- Duchange, N.; Pidoux, J.; Camus, E.; Sauvaget, D. Alternative splicing in the human interleukin enhancer binding factor 3 (ILF3) gene. Gene 2000, 261, 345–353.
- Castella, S.; Bernard, R.; Corno, M.; Fradin, A.; Larcher, J.C. Ilf3 and NF90 functions in RNA biology. Wiley Interdiscip. Rev. RNA 2014, 6, 243–256.
- Masuda, K.; Kuwano, Y.; Nishida, K.; Rokutan, K.; Imoto, I. NF90 in posttranscriptional gene regulation and microRNA biogenesis. Int. J. Mol. Sci. 2013, 14, 17111–17121.
- Saunders, L.R.; Jurecic, V.; Barber, G.N. The 90- and 110-kDa human NFAR proteins are translated from two differentially spliced mRNAs encoded on chromosome 19p13. Genomics 2001, 71, 256–259.
- Xu, Y.H.; Grabowski, G.A. Molecular cloning and characterization of a translational inhibitory protein that binds to coding sequences of human acid beta-glucosidase and other mRNAs. Mol. Genet. Metab. 1999, 68, 441–454.
- Schmidt, T.; Knick, P.; Lilie, H.; Friedrich, S.; Golbik, R.P.; Behrens, S.E. The properties of the RNA-binding protein NF90 are considerably modulated by complex formation with NF45. Biochem. J. 2017, 474, 259–280.
- Guan, D.; Altan-Bonnet, N.; Parrott, A.M.; Arrigo, C.J.; Li, Q.; Khaleduzzaman, M.; Li, H.; Lee, C.G.; Pe’ery, T.; Mathews, M.B. Nuclear factor 45 (NF45) is a regulatory subunit of complexes with NF90/110 involved in mitotic control. Mol. Cell. Biol. 2008, 28, 4629–4641.
- Wolkowicz, U.M.; Cook, A.G. NF45 dimerizes with NF90, Zfr and SPNR via a conserved domain that has a nucleotidyltransferase fold. Nucleic Acids Res. 2012, 40, 9356–9368.
- Parrott, A.M.; Walsh, M.R.; Reichman, T.W.; Mathews, M.B. RNA binding and phosphorylation determine the intracellular distribution of nuclear factors 90 and 110. J. Mol. Biol. 2005, 348, 281–293.
- Li, X.; Liu, C.X.; Xue, W.; Zhang, Y.; Jiang, S.; Yin, Q.F.; Wei, J.; Yao, R.W.; Yang, L.; Chen, L.L. Coordinated circRNA Biogenesis and Function with NF90/NF110 in Viral Infection. Mol. Cell 2017, 67, 214–227.
- Vrakas, C.N.; Herman, A.B.; Ray, M.; Kelemen, S.E.; Scalia, R.; Autieri, M.V. RNA stability protein ILF3 mediates cytokine-induced angiogenesis. FASEB J. 2019, 33, 3304–3316.
- Zhang, W.; Xiong, Z.; Wei, T.; Li, Q.; Tan, Y.; Ling, L.; Feng, X. Nuclear factor 90 promotes angiogenesis by regulating HIF-1alpha/VEGF-A expression through the PI3K/Akt signaling pathway in human cervical cancer. Cell Death Dis. 2018, 9, 276.
- Vumbaca, F.; Phoenix, K.N.; Rodriguez-Pinto, D.; Han, D.K.; Claffey, K.P. Double-stranded RNA-binding protein regulates vascular endothelial growth factor mRNA stability, translation, and breast cancer angiogenesis. Mol. Cell. Biol. 2008, 28, 772–783.
- Gwizdek, C.; Ossareh-Nazari, B.; Brownawell, A.M.; Evers, S.; Macara, I.G.; Dargemont, C. Minihelix-containing RNAs mediate exportin-5-dependent nuclear export of the double-stranded RNA-binding protein ILF3. J. Biol. Chem. 2004, 279, 884–891.
- Wu, T.H.; Shi, L.; Adrian, J.; Shi, M.; Nair, R.V.; Snyder, M.P.; Kao, P.N. NF90/ILF3 is a transcription factor that promotes proliferation over differentiation by hierarchical regulation in K562 erythroleukemia cells. PLoS ONE 2018, 13, e0193126.
- Barbier, J.; Chen, X.; Sanchez, G.; Cai, M.; Helsmoortel, M.; Higuchi, T.; Giraud, P.; Contreras, X.; Yuan, G.; Feng, Z.; et al. An NF90/NF110-mediated feedback amplification loop regulates dicer expression and controls ovarian carcinoma progression. Cell Res. 2018, 28, 556–571.
- Ding, D.; Huang, H.; Li, Q.; Yu, W.; Wang, C.; Ma, H.; Wu, J.; Dang, Y.; Yu, L.; Jiang, W. NF90 stabilizes cyclin E1 mRNA through phosphorylation of NF90-Ser382 by CDK2. Cell Death Discov. 2020, 6, 3.
- Zhang, J.; Du, C.; Zhang, L.; Wang, Y.; Zhang, Y.; Li, J. LncRNA LINC00649 promotes the growth and metastasis of triple-negative breast cancer by maintaining the stability of HIF-1alpha through the NF90/NF45 complex. Cell Cycle 2022, 21, 1034–1047.
- Zhao, M.; Wang, Y.; Tan, F.; Liu, L.; Hou, X.; Fan, C.; Tang, L.; Mo, Y.; Wang, Y.; Yan, Q.; et al. Circular RNA circCCNB1 inhibits the migration and invasion of nasopharyngeal carcinoma through binding and stabilizing TJP1 mRNA. Sci. China Life Sci. 2022.
- Grasso, G.; Akkawi, C.; Franckhauser, C.; Nait-Saidi, R.; Bello, M.; Barbier, J.; Kiernan, R. NF90 interacts with components of RISC and modulates association of Ago2 with mRNA. BMC Biol. 2022, 20, 194.
- Grasso, G.; Higuchi, T.; Mac, V.; Barbier, J.; Helsmoortel, M.; Lorenzi, C.; Sanchez, G.; Bello, M.; Ritchie, W.; Sakamoto, S.; et al. NF90 modulates processing of a subset of human pri-miRNAs. Nucleic Acids Res. 2020, 48, 6874–6888.
- Patino, C.; Haenni, A.L.; Urcuqui-Inchima, S. NF90 isoforms, a new family of cellular proteins involved in viral replication? Biochimie 2015, 108, 20–24.
- Todaka, H.; Higuchi, T.; Yagyu, K.; Sugiyama, Y.; Yamaguchi, F.; Morisawa, K.; Ono, M.; Fukushima, A.; Tsuda, M.; Taniguchi, T.; et al. Overexpression of NF90-NF45 Represses Myogenic MicroRNA Biogenesis, Resulting in Development of Skeletal Muscle Atrophy and Centronuclear Muscle Fibers. Mol. Cell. Biol. 2015, 35, 2295–2308.
- Ye, J.; Jin, H.; Pankov, A.; Song, J.S.; Blelloch, R. NF45 and NF90/NF110 coordinately regulate ESC pluripotency and differentiation. RNA 2017, 23, 1270–1284.
- Corthesy, B.; Kao, P.N. Purification by DNA affinity chromatography of two polypeptides that contact the NF-AT DNA binding site in the interleukin 2 promoter. J. Biol. Chem. 1994, 269, 20682–20690.
- Shi, L.; Qiu, D.; Zhao, G.; Corthesy, B.; Lees-Miller, S.; Reeves, W.H.; Kao, P.N. Dynamic binding of Ku80, Ku70 and NF90 to the IL-2 promoter in vivo in activated T-cells. Nucleic Acids Res. 2007, 35, 2302–2310.
- Ting, N.S.; Kao, P.N.; Chan, D.W.; Lintott, L.G.; Lees-Miller, S.P. DNA-dependent protein kinase interacts with antigen receptor response element binding proteins NF90 and NF45. J. Biol. Chem. 1998, 273, 2136–2145.
- Kiesler, P.; Haynes, P.A.; Shi, L.; Kao, P.N.; Wysocki, V.H.; Vercelli, D. NF45 and NF90 regulate HS4-dependent interleukin-13 transcription in T cells. J. Biol. Chem. 2010, 285, 8256–8267.
- Sakamoto, S.; Morisawa, K.; Ota, K.; Nie, J.; Taniguchi, T. A binding protein to the DNase I hypersensitive site II in HLA-DR alpha gene was identified as NF90. Biochemistry 1999, 38, 3355–3361.
- Nakadai, T.; Fukuda, A.; Shimada, M.; Nishimura, K.; Hisatake, K. The RNA binding complexes NF45-NF90 and NF45-NF110 associate dynamically with the c-fos gene and function as transcriptional coactivators. J. Biol. Chem. 2015, 290, 26832–26845.
- Sakamoto, S.; Aoki, K.; Higuchi, T.; Todaka, H.; Morisawa, K.; Tamaki, N.; Hatano, E.; Fukushima, A.; Taniguchi, T.; Agata, Y. The NF90-NF45 complex functions as a negative regulator in the microRNA processing pathway. Mol. Cell. Biol. 2009, 29, 3754–3769.
- Higuchi, T.; Todaka, H.; Sugiyama, Y.; Ono, M.; Tamaki, N.; Hatano, E.; Takezaki, Y.; Hanazaki, K.; Miwa, T.; Lai, S.; et al. Suppression of MicroRNA-7 (miR-7) Biogenesis by Nuclear Factor 90-Nuclear Factor 45 Complex (NF90-NF45) Controls Cell Proliferation in Hepatocellular Carcinoma. J. Biol. Chem. 2016, 291, 21074–21084.
- Higuchi, T.; Morisawa, K.; Todaka, H.; Lai, S.; Chi, E.; Matsukawa, K.; Sugiyama, Y.; Sakamoto, S. A negative feedback loop between nuclear factor 90 (NF90) and an anti-oncogenic microRNA, miR-7. Biochem. Biophys. Res. Commun. 2018, 503, 1819–1824.
- Idda, M.L.; Lodde, V.; McClusky, W.G.; Martindale, J.L.; Yang, X.; Munk, R.; Steri, M.; Orru, V.; Mulas, A.; Cucca, F.; et al. Cooperative translational control of polymorphic BAFF by NF90 and miR-15a. Nucleic Acids Res. 2018, 46, 12040–12051.
- Chen, C.Y.; Shyu, A.B. AU-rich elements: Characterization and importance in mRNA degradation. Trends Biochem. Sci. 1995, 20, 465–470.
- Kuwano, Y.; Pullmann, R., Jr.; Marasa, B.S.; Abdelmohsen, K.; Lee, E.K.; Yang, X.; Martindale, J.L.; Zhan, M.; Gorospe, M. NF90 selectively represses the translation of target mRNAs bearing an AU-rich signature motif. Nucleic Acids Res. 2010, 38, 225–238.
- Song, D.; Huang, H.; Wang, J.; Zhao, Y.; Hu, X.; He, F.; Yu, L.; Wu, J. NF90 regulates PARP1 mRNA stability in hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2017, 488, 211–217.
- Jiang, W.; Huang, H.; Ding, L.; Zhu, P.; Saiyin, H.; Ji, G.; Zuo, J.; Han, D.; Pan, Y.; Ding, D.; et al. Regulation of cell cycle of hepatocellular carcinoma by NF90 through modulation of cyclin E1 mRNA stability. Oncogene 2015, 34, 4460–4470.
- Hoque, M.; Shamanna, R.A.; Guan, D.; Pe’ery, T.; Mathews, M.B. HIV-1 replication and latency are regulated by translational control of cyclin T1. J. Mol. Biol. 2011, 410, 917–932.
- Pfeifer, I.; Elsby, R.; Fernandez, M.; Faria, P.A.; Nussenzveig, D.R.; Lossos, I.S.; Fontoura, B.M.; Martin, W.D.; Barber, G.N. NFAR-1 and -2 modulate translation and are required for efficient host defense. Proc. Natl. Acad. Sci. USA 2008, 105, 4173–4178.
- Shamanna, R.A.; Hoque, M.; Pe’ery, T.; Mathews, M.B. Induction of p53, p21 and apoptosis by silencing the NF90/NF45 complex in human papilloma virus-transformed cervical carcinoma cells. Oncogene 2013, 32, 5176–5185.
- Gomila, R.C.; Martin, G.W.; Gehrke, L. NF90 binds the dengue virus RNA 3′ terminus and is a positive regulator of dengue virus replication. PLoS ONE 2011, 6, e16687.
- Li, Y.; Belshan, M. NF45 and NF90 Bind HIV-1 RNA and Modulate HIV Gene Expression. Viruses 2016, 8, 47.
- Li, Y.; Masaki, T.; Shimakami, T.; Lemon, S.M. hnRNP L and NF90 interact with hepatitis C virus 5′-terminal untranslated RNA and promote efficient replication. J. Virol. 2014, 88, 7199–7209.
- Shabman, R.S.; Leung, D.W.; Johnson, J.; Glennon, N.; Gulcicek, E.E.; Stone, K.L.; Leung, L.; Hensley, L.; Amarasinghe, G.K.; Basler, C.F. DRBP76 associates with Ebola virus VP35 and suppresses viral polymerase function. J. Infect. Dis. 2011, 204 (Suppl. 3), S911–S918.
- Harashima, A.; Guettouche, T.; Barber, G.N. Phosphorylation of the NFAR proteins by the dsRNA-dependent protein kinase PKR constitutes a novel mechanism of translational regulation and cellular defense. Genes Dev. 2010, 24, 2640–2653.
- Langland, J.O.; Kao, P.N.; Jacobs, B.L. Nuclear factor-90 of activated T-cells: A double-stranded RNA-binding protein and substrate for the double-stranded RNA-dependent protein kinase, PKR. Biochemistry 1999, 38, 6361–6638.
- Wang, M.; Yu, F.; Wu, W.; Zhang, Y.; Chang, W.; Ponnusamy, M.; Wang, K.; Li, P. Circular RNAs: A novel type of non-coding RNA and their potential implications in antiviral immunity. Int. J. Biol. Sci. 2017, 13, 1497–1506.
- Ma, Y.; Zheng, B.; Zhang, X.H.; Nie, Z.Y.; Yu, J.; Zhang, H.; Wang, D.D.; Shi, B.; Bai, Y.; Yang, Z.; et al. circACTA2 mediates Ang II-induced VSMC senescence by modulation of the interaction of ILF3 with CDK4 mRNA. Aging 2021, 13, 11610–11628.
