1. Anti-Inflammatory Drugs Used as an Antiaging Approach
Chronic inflammation is one of the major contributors to age-associated diseases and aging and disrupts the normal functioning of tissues [
1,
8,
9]. Increased activity of proinflammatory pathways accompanies inflammation with age [
10,
11]. Serum concentrations of proinflammatory cytokines (IL-1, IL-2, IL-6, IL-8, IL-12, IL-15, IL-17, IL-18, IL-22, IL-23, tumor necrosis factor-α, and interferon-γ) are significantly increased in normal process aging compared with younger individuals in a normal stage [
12,
13,
14]. A chronic proinflammatory status is a pervasive feature of aging, and increased systemic inflammation is closely associated with aging and age-related diseases [
15,
16]. The term “inflammaging” is used to describe aging induced by chronic and persistent inflammation. Anti-inflammatory agents block certain substances in the body that cause inflammation, and various studies have shown that anti-inflammatory agents are linked to antiaging [
17]. The most important drivers of age-dependent inflammation are derived at the cellular and molecular levels. In a cell, the proinflammatory senescence-associated secretory phenotype (SASP) is associated with cellular senescence that is triggered by agents such as radiation and viruses and by continuous exposure to cellular debris [
18] and cellular senescence [
19]. In a molecule, ROS (reactive oxygen species) and other agents can trigger inflammatory DNA damage responses that affect DNA and telomeres [
20] and activate the inflammasomes and NF-kB pathway [
21]. Inhibition of the inflammatory processes by genetic and pharmacological intervention is considered an effective and verified antiaging strategy [
2]. Nonsteroidal anti-inflammatory drugs (NSAIDs) not only prevent certain age-associated features but also increase the lifespan in various model organisms, such as yeasts [
22], nematodes [
23], mice [
24,
25], and flies [
22]; however, their effectiveness for neurodegenerative disorders (Alzheimer’s disease and Huntington’s disease [
26]) is not clear, and there is a search for anti-inflammatory bioactive compounds [
27,
28,
29,
30,
31]. Anti-inflammatory drugs might be considered to have great potential for extending the lifespan. In some studies, spermidine (polyamines) and their action on the expression of pro- and anti-inflammatory cytokines can directly reduce inflammation and indirectly alter inflammation and cell growth by the action of autophagy [
32]. Spermidine has been reported to slow down aging due to its antiaging effects [
33]. Aspirin, a potent anti-inflammatory and antioxidant compound, may affect oxidant production and cytokine responses and block glycoxidation reactions that protect against oxidative stress, as well as extend the lifespan of
Caenorhabditis elegans and mice [
34,
35,
36]. Ibuprofen (NSAID) has been shown to reduce the risk of age-related pathologies and increase the lifespan of
Saccharomyces cerevisiae,
Caenorhabditis elegans, and
Drosophila melanogaster [
37,
38]. A novel NSAID, M2000, could modify oxidative stress pathways by lowering the expression levels of the SOD
2, GST, iNOS, and MPO genes and reduce the risk of inflammatory diseases through its immunosuppressive effects, with no adverse side effects on the enzymatic and nonenzymatic determinants [
39]. This can be recommended as an antiaging drug. MAAs (mycosporine-like amino acids), such as M2G (mycosporine-2-glycine), exhibit antioxidant, anti-inflammatory, anti-protein-glycation, and collagenase inhibition activities and show the ability to protect DNA against UV damage [
40,
41]. Many nutraceuticals (apigenin, quercetin, kaempferol, naringenin, catechins, epigallocatechin, genistein, cyanidin, resveratrol, etc.) and functional foods possess antioxidant activity that might play an important role in delaying aging and be effective in various human neurodegenerative diseases [
30,
42,
43,
44,
45].
2. Antioxidant Activity
Phytochemicals such as phenolic acids and flavonoids have antioxidant activity, which acts by scavenging free radicals and increasing the levels of antioxidant enzymes in plasma [
46]. The function of a primary antioxidant enzyme is to protect organisms from the damaging effects of superoxide radicals, which are quickened by their dismutation into hydrogen peroxide and oxygen [
47]. Several studies have confirmed that quercetin is a strong antioxidant that accumulates in nematodes and displays reactive oxygen species (ROS) scavenging activity and has been demonstrated to have a positive effect on longevity and stress resistance in various animal models [
48,
49,
50,
51]. Many studies have demonstrated that NSAIDs have antioxidant activity that is mediated by free radical scavenging and antioxidant enzyme activation [
52]. The antioxidant activity of NSAIDs has been witnessed in membranes, cells, and at the organismal level [
52,
53,
54].
3. Telomere Reactivation
Telomeres are conserved microsatellite repeats TTAGGG that protect the ends of chromosomes from DNA breakage and prevent DNA end-joining, recombination, and DNA repair [
55]. DNA polymerases are incapable of fully replicating the linear chromosomes owing to end replication in somatic cells, and telomeres become gradually shortened after each cell division [
56]. This shortening of telomeres is usually fulfilled by the telomerase enzyme, but most somatic cells and adult stem cells do not express enough telomerase to compensate for the telomere length that leads to entering ‘replicative senescence’, which might be followed by cell death [
57,
58,
59]. Telomere shortening occurs during normal aging and is an important biomarker of aging and longevity that is influenced by several factors, such as genetics, epigenetics, and environments [
60,
61,
62,
63]. It is also associated with many age-related diseases, such as osteoarthritis, atherosclerosis, coronary heart disease, and atrial fibrillation [
64,
65,
66]. Several studies have reported that aging can be inhibited by the overexpression of telomerase; however, it can enhance tumorigenesis [
67,
68,
69]. A telomerase activator, telomerase expression activator, and telomerase gene therapy have been developed as telomerase-based antiaging strategies in recent years. TA-65 is an extract of a Chinese plant (
Astragalus membranaceus), a telomerase activator that can restore telomere length without cancer occurrence and improve age-related indicators, including glucose tolerance, bone health, and skin quality [
70]. Additionally, some studies found that TERT transcription activator and sex hormones are directly involved in activating telomerase, which rescued telomere shortening and enhanced the lifespan [
71,
72]. Evidence has suggested that the reactivation of telomerase expression by using a gene therapy approach is the best example of the lifespan extension of mice and delay aging without cancer occurrence [
73]. A recent study has found that Metadichol, a telomerase activator, is used to overcome organ failure by enriching cells with telomerase and is a safer alternative [
74]. Another study explained that natural compounds such as 08AGTLF
(Centella asiatica), Nutrient 4 (
Astragalus), TA-65 (
Astragalus membranaceus), OA (oleanolic acid), and MA (maslinic acid); and Nutrients 1, 2, and 3 have telomerase activation, and among all, 08AGTLF has the greatest potential to activate telomerase [
75]. The impact of telomeric length on humans has been evidenced by the fact that the expression of telomerase in normal cells may extend a healthy lifespan; however, inhibition of telomerase in cancer cells may be a viable target for anticancer therapeutics [
76].
4. Antiaging Approaches Using Epigenetic Drugs
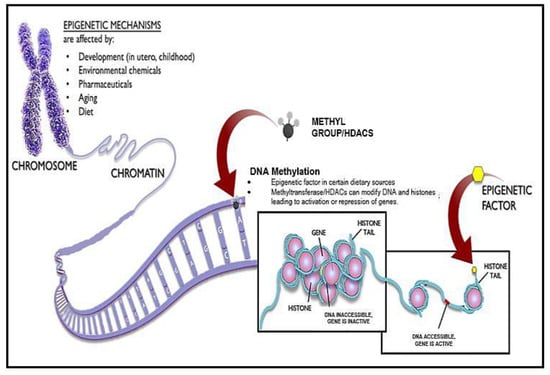
The effects of chromatin on aging are probably complex and bidirectional. Chromatin remodeling appears to counter aging and age-associated diseases and extend organismal lifespan [
77]. Chromatin is intensely altered during aging owing to the decreased level of histone proteins and adequate changes in histone modification that were found in recent studies in budding yeast and human fibroblast cells [
78]. Elevating histone expression, reducing H4 K16 acetylation, reducing H3 N-terminal acetylation, inactivating the HDAC Rpd3, and inactivating the H3K4 methylase might be capable of extending lifespan or reverting the aged phenotype to a more youthful state of chromatin [
79]. These epigenetic factors are influenced by diet, lifestyle and exogenous stress, which raises the possibility of enhancing age-related cellular dysfunction [
80,
81]. Several studies revealed age-associated changes in DNA methylation patterns leading to a global reduction in DNA methylation [
82] (
Figure 2); site-specific hypermethylation, specifically at CpG islands and polycomb target sites [
83]; site-specific hypomethylation specifically at gene-poor regions, tissue-specific promoters, and polycomb protein regions; and hypermethylation in different tissues but hypomethylation, to be more tissue specific [
84,
85]. These changes accumulate gradually; such changes are indicative of the aging process and strongly associated with epigenetic changes such as replicative senescence [
86]. Diet, lifestyle, environmental interventions, and inhibitors of epigenetic enzymes have proven to be effective in promoting longevity, as seen in various experiments [
87]. Natural substances such as spermidine and resveratrol have been found to lead to deacetylation of chromatin, indicating that it has the potential to extend the lifespan in humans [
88]. Many studies report that HDAC (histone deacetylase) inhibitors show evidence as an antiaging strategy [
89]. HDAC inhibitors have the potential to reverse the aging process that allows healthy aging [
90].
Figure 2. Epigenetic Mechanisms via DNA Methylation and histone modification. Epigenetics can be altered via developmental mechanisms, environmental chemicals, pharmaceuticals, aging, and diet. Some dietary sources can lead to a direct production of DNA methylation, allowing for the overexpression or repression of genes, thereby increasing the aging process.
5. Activation of Chaperons and the Proteolytic System against Aging
Loss of proteostasis is a common feature of aging that leads to protein aggregation, unfolding, oxidative damage, posttranslational modification, and an altered rate of protein turnover and, ultimately, to cellular dysfunction [
91,
92,
93,
94,
95]. Two proteolytic systems—the ubiquitin–proteasome system (UPS) and autophagy–lysosome system (ALS)—and chaperones play a major role in maintaining proteostasis [
96]. The alteration or deterioration of these pathways impairs normal cell functioning and cell physiology, causing aging [
2]. Many studies have found proteostasis changes with age owing to reduced activity of heat-shocked protein chaperones [
97,
98]. To compensate for this decline, increasing the chaperone protein level has been shown to beneficially impact longevity in worms and flies [
99,
100,
101,
102]. A study reported that the aggregation of Hsp104, a chaperone, has been associated with aging and has increased the lifespan in
Saccharomyces cerevisiae [
103,
104,
105]. The UPS and ALS systems are the main proteolytic systems that influence the cellular fate and aging process [
106,
107]. The availability of the chaperone is extremely compromised in aged cells in which the proteostasis collapses by decreased G1-cyclin function that causes an irreversible arrest in G1, configuring a molecular pathway claiming proteostasis deterioration leads to cell senescence [
108]. Promoting proteasomal activity via overexpression of the proteasomal β5 subunit either in
Caenorhabditis elegans [
109] or in human fibroblast [
110] and the overexpression of Rpn11 in
Drosophila melanogaster [
111] increases the lifespan and stress resistance. The compound 18α-glycyrrhetinic acid and loss of IGF (insulin-like growth factor) signaling due to mutated daf-2 induce proteasomal activation and extend the lifespan of
Caenorhabditis elegans [
112]. Spermidine, metformin, rapamycin, and resveratrol are pharmaceutical approaches well-known to activate the autophagy system [
113]. In a recent study, it was found that minocycline, JZL184, monorden, and paxilline directly targeted the 18S rRNA/ribosome, FAAH-4, Hsp90, and the SLO-1 BK channel, significantly increasing the lifespan of
Caenorhabditis elegans [
114]. The proteostatic system governs the synthesis and conformation of target proteins, and the ubiquitin—proteasome system and autophagy act as the main scavengers of misfolded or excessive proteins. The main cause of Alzheimer’s disease is the accumulation of misfolded proteins as Aβ plaques and tau aggregates owing to dysregulation of proteostasis, which contributes to the accumulation of proteotoxins in Alzheimer’s disease [
115]. It has been reported that a decrease in the efficiency of the autophagy and ubiquitin—proteasome systems might lead to aging and neurodegenerative diseases such as AD, PD, and ALS [
116]. The prion diseases in mammals are related to altered versions of PrP
c (cellular), which is a key component of the infectious agent responsible for transmission, and the disease-associated version of PrP
c can be partially resistant to the protease–digestion system, designated PrP
sc (scrapie) [
117]. Various cellular components, predominantly chaperones such as Hsp104, Hsp40s, HSP42, and HSP70s, can lead to the curing of yeast prions by their deficiency or overproduction. These studies have revealed the requirements for prion propagation and conditions that affect prion stability, which have led to the discovery of anti-prion systems. Btn2p, a component of the yeast anti-prion system, has the aggregate-sequestering abilities; it can cure an artificial and natural yeast prion, and works on a variety of non-prion aggregates as well [
118]. This system might be advantageous for neurodegenerative diseases that result from aggregates of proteo-toxins.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10102515