2. Functions of NF90
NF90 is a polyvalent factor that, since its discovery, has been linked to a variety of functions such as transcriptional and translational regulation, viral replication, and miRNA biogenesis (
Figure 2). Moreover, deregulation of NF90 was observed for several diseases such as cancer and muscular atrophy, and it was implicated in the immune response, particularly against viruses [
22,
29,
40]. More recently, NF90 was also shown to regulate embryonic stem cell pluripotency and differentiation [
41].
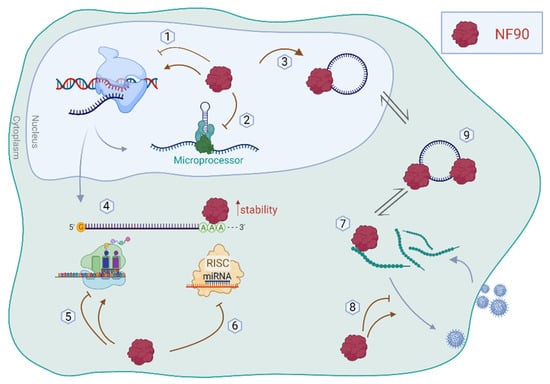
Figure 2. Schematic representation of the functions of NF90 in the cytoplasm and nucleus. NF90 is involved in (1) transcription regulation, (2) miRNA biogenesis, and (3) circRNA biogenesis, in the nucleus. In the cytoplasm, NF90 can regulate (4) mRNA stability, (5) translation of mRNA, and (6) RISC-mediated silencing. In addition, NF90 is implicated in viral infection by (7,8) binding viral genome/proteins. (9) NF90 can be sponged by circRNAs, modulating its availability in the cytoplasm.
2.1. NF90 in Transcriptional Regulation
Although NF90 does not contain a known DNA-binding motif, evidence strongly suggests that it could have a similar function to canonical DNA-binding proteins. NF90/NF45 was originally described as a DNA-binding complex, acting as a transcription factor for the cytokine, interleukin 2 (IL2), during T-cell activation. In particular, it was shown that NF90/NF45 binds the antigen recognition response element 2 (ARRE-2) present in the
IL2 promoter and enhances its transcription [
45,
46]. It was subsequently demonstrated that the interaction between the complex and target DNA is indirect, being mediated by several protein partners such as eukaryotic Translation Initiation Factor 2 (eIF2), Ku proteins, and DNA-protein kinase (PK) [
46,
47].
In keeping with its role in viral replication and T-cell activation, NF90 was also shown to regulate the transcription of another cytokine, Interleukin 13 (IL13), by binding to a DNase I hypersensitive site (DHS) [
48]. On the other hand, transcription inhibition by NF90/NF45 was observed for the major histocompatibility complex class II HLA-DR, mediated by DHS binding in B-cells [
49].
More recently, NF90/NF45 was reported to be involved in the upregulation of
c-FOS transcription upon serum induction. NF90/NF45 was shown to bind to the
c-FOS enhancer/promoter region while cooperating with general co-activator factors [
50]. Similarly, ChIP-seq data in K562 erythroleukemia cells strongly suggests that NF90/NF110, by associating with promoter regions, significantly activates the expression of transcription factors that are drivers of growth and proliferation [
21]. Therefore, NF90 seems to indirectly mediate transcription regulation, with a marked implication in the immune response and cancer progression.
2.2. NF90 in the miRNA Biogenesis Pathway
The role of NF90 in the regulation of miRNA biogenesis was recently described. The first evidence for the involvement of NF90/NF45 in the maturation of miRNAs was shown by Sakamoto and colleagues [
51]. In this study, they showed that NF90/NF45 behaves as a negative regulator of Microprocessor activity for the maturation of pri-let-7a, competing with Drosha for the binding to pri-miRNA. Therefore, the maturation of the pri-miRNA to pre-miRNA is inhibited by the binding of the NF90/NF45 complex by impairing access of Microprocessor to the pri-miRNAs.
Since this finding, different miRNAs have been shown to be modulated by NF90/NF45. The complex was found to downregulate myogenic miRNAs such as miR-133a, leading to significant loss and maturation of skeletal muscle and atrophy in NF90/NF45 double-transgenic mice [
40]. More recently, NF90/NF45 was shown to inhibit the maturation of miR-7 in HCC. MiR-7 is a tumor suppressor miRNA and increased expression of NF90 leads to the inhibition of miR-7 maturation, followed by an elevated proliferation rate in HCC [
52]. NF90 was therefore reported to be an oncogenic factor for HCC. Interestingly, the existence of a negative feedback loop between miR-7 and NF90 was later shown, in which mature miR-7 was able to target the 3′ UTR of NF90, leading to its translational repression [
53].
It was recently demonstrated that NF90/NF45 is able to inhibit the maturation of miR-3173, a miRNA embedded in the first intron of Dicer pre-mRNA, by preventing binding of Microprocessor to pri-miR-3173. Furthermore, in the absence of NF90, the level of pre-miR-3173 increases while Dicer pre-mRNA exhibits splicing defects that lead to its downregulation. Increased progression and metastasis were observed in ovarian cancer cells. Therefore, it was established that NF90 can act as a tumor suppressor in ovarian cancer models. Interestingly, the mature form of miR-3173 is able to target NF90 mRNA by binding to its 3′ UTR, leading to translational repression, mediating a feedback amplification loop that controls Dicer expression and ovarian carcinoma progression [
22].
More recently, the extent of the effect of NF90 on miRNA maturation was uncovered using genome-wide approaches [
27]. Data indicate that NF90 is able to directly bind and modulate the processing of a specific subset of human miRNA precursors that are weakly bound by Microprocessor, suggesting that NF90 and Microprocessor might be in competition for the binding of pri-miRNAs. Moreover, in agreement with other studies [
20], it was found that NF90-bound and modulated pri-miRNAs are highly stable RNA structures, displaying significantly longer duplexes with fewer and smaller bulges compared to all human pri-miRNAs [
27].
2.3. NF90 in mRNA Translation, Stability, and Degradation
In addition to controlling mRNA fate by binding and modulating the processing of miRNAs, NF90 can directly regulate mRNAs’ translation, stability, and decay [
19,
58]. AU-rich regions are frequently found in 3′ UTRs of mRNAs and their recognition by RBPs often determines their fate [
3,
59]. Ribonucleoprotein immunoprecipitation (RIP) analysis showed that NF90 is able to bind an AU-rich signature motif, known as NF90m, found in a large subset of mRNAs [
39]. However, the consequence of NF90 binding can vary depending on the target mRNA or on the condition studied. For example, NF90 binding to 3′ UTRs is able to regulate the stability of mRNAs and their translation, either positively or negatively [
16,
23,
39,
58,
60].
While NF90 has been shown to modulate the translation of bound mRNAs, its effect can be either positive or negative, depending on the RNA target. NF90 can inhibit translation by affecting the initiation step, or by retaining target mRNAs in the nucleus [
8]. For instance, insertion of the AU-rich NF90m into a reporter gene did not affect mRNA stability but rather inhibited its translation by preventing its association with actively translating ribosomes [
39].
NF90 has also been shown to activate the translation of a limited subset of mRNAs, such as Vascular Endothelial Growth Factor (VEGF) and cyclin T1 mRNA [
17,
61]. Under hypoxic conditions, NF90 is able to interact with the 3′ UTR stem-loop hypoxia stability region in
VEGF mRNA, promoting its loading onto polysomes and increasing its stability [
19]. After Human Immunodeficiency Virus 1 (HIV1) infection, NF90 promotes viral replication and latency by binding to cyclin T1 mRNA 3′ UTR and facilitating the recruitment of translation initiation factors [
62].
2.4. NF90 in Viral Replication
Besides acting as a cellular mRNA binding factor that controls RNA metabolism and translation, NF90 also binds viral RNA or DNA [
66]. The consequence of its binding can vary, supporting or inhibiting viral replication and viral genome expression, depending on the type of virus [
29]. Numerous viruses have been shown to exploit NF90 to support their replication such as hepatitis C virus (HCV), HIV, human papilloma virus (HPV), and Dengue virus (DV) [
43,
67,
68,
69]. For instance, NF90 was shown to bind the 5′-terminal sequence of the HCV RNA genome upon infection and promote HCV replication by possibly associating with the replication complex [
69]. Upon HIV infection, NF90 shows a pleiotropic effect by stimulating the viral gene expression as well as stabilizing HIV RNA [
68]. On the other hand, NF90 is able to act as a host antiviral factor for other types of viruses such as the influenza A virus (IAV) and Ebola virus (EBOV) [
70]. For example, NF90 was found to suppress EBOV replication by associating with Viral Protein 35 (VP35) and impairing the function of EBOV replication complex [
16,
70]. Moreover, it was recently shown that viral infection promotes the translocation of NF90 from the nucleus to the cytoplasm. NF90 in the cytoplasm is able to bind viral mRNAs, leading to the inhibition of viral infection [
16]. Similarly, following viral infection, the interferon-inducible kinase, PKR, phosphorylates NF90, leading to its dissociation from NF45 and export from the nucleus [
33,
71]. Phosphorylated NF90 accumulates on ribosomes where it associates with viral RNA, inhibiting their translation [
33].
Despite the numerous and diverse examples of NF90 activity during the response to viral infection, the exact mechanism underlying its role is yet to be fully elucidated. However, it is possible that the complicated contribution of NF90 to antiviral immunity might occur through different mechanisms depending on the type of virus.
2.5. Interplay between NF90 and Circular RNAs
The most recent described role of NF90 involves circular RNA (circRNA) biology. In fact, it was found that NF90 is a key factor for the biogenesis of circRNAs in the nucleus while their mature form might act as a molecular reservoir of NF90 in the cytoplasm for prompt immune response following viral infection [
16]. In particular, in the nucleus, NF90 promotes back-splicing of circRNAs by binding to flanking introns of circularized exons and stabilizing the transient RNA duplexes that juxtapose the splice sites [
72]. Interestingly, upon viral infection, the nuclear pool of NF90 translocates into the cytoplasm, reducing the formation of circRNAs in the nucleus. On the other hand, the re-distribution of NF90 in the cytoplasm increases its binding to circular ribonucleoprotein (circRNP) complexes that compete with viral RNAs for the binding of NF90 [
16].
It was recently shown that circular Actin Alpha 2 (circACTA2) RNA can compete with CDK4 mRNA for the binding of NF90 in vascular smooth muscle cells (VSMC). In particular, increased circACTA2 expression, induced by angiotensin II (Ang II) stimulation in VSMC, reduced the association of NF90 with CDK4 mRNA, leading to Ang II mediated senescence in VSMC [
73]. Although this newly discovered function of NF90 is not yet supported by extensive literature, the regulatory potential of circRNAs and NF90 highlights the significance of this mechanism and encourages further investigation.