Magnetic resonance imaging linear accelerators (MRLINAC) technology allows clinicians to leverage imaging information gathered during radiation therapy to adapt therapy for a patient while actively undergoing treatment. There is a significant need to understand how imaging changes may correlate to outcomes during treatment for glioblastoma (GBM) as these tumors have a poor prognosis and treatment tailored to the tumor characteristics may improve outcomes. Some of the advantages of MR guided therapy include facilitating a more detailed study of tumor and normal tissue response during chemo-radiation therapy, providing a mechanism to adapt therapy based on imaging changes, identifying new imaging biomarkers for tumor response as well as normal tissue response. These avenues could provide a more tangible way to evaluate pseudoprogression and radiation necrosis with radiogenomics as a mechanism to correlate imaging findings to genomic biomarkers.
- GBMs
- radiotherapy
- MRI
1. Molecular Basis of Glioblastoma (GBM)
2. Role of Radiotherapy in GBM
3. Types of Radiotherapy Devices for GBM
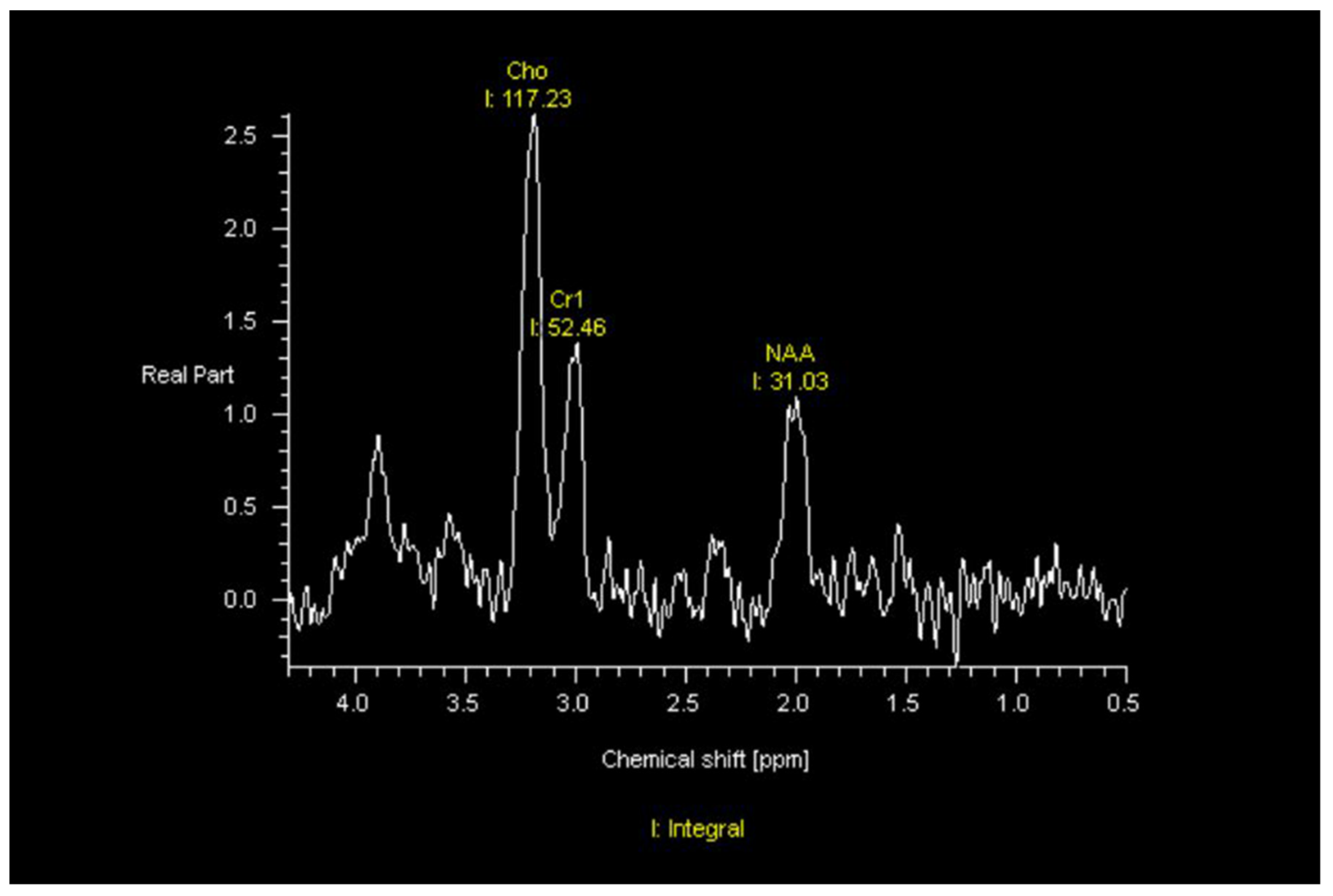
4. Imaging Characteristics of GBM on MRI
5. Radiomic and Radiogenomic Differentiation of Molecular Markers, Sex Differences, and Morphologic Subtypes of GBMs
6. MRI Guided Machines
This entry is adapted from the peer-reviewed paper 10.3390/jcm11195961
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251.
- Labussiere, M.; Boisselier, B.; Mokhtari, K.; Di Stefano, A.L.; Rahimian, A.; Rossetto, M.; Ciccarino, P.; Saulnier, O.; Paterra, R.; Marie, Y.; et al. Combined analysis of TERT, EGFR, and IDH status defines distinct prognostic glioblastoma classes. Neurology 2014, 83, 1200–1206.
- Network, C.G.A.R. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008, 455, 1061–1068.
- Verhaak, R.G.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110.
- Zhang, P.; Xia, Q.; Liu, L.; Li, S.; Dong, L. Current Opinion on Molecular Characterization for GBM Classification in Guiding Clinical Diagnosis, Prognosis, and Therapy. Front. Mol. Biosci. 2020, 7, 562798.
- Wang, Q.; Hu, B.; Hu, X.; Kim, H.; Squatrito, M.; Scarpace, L.; deCarvalho, A.C.; Lyu, S.; Li, P.; Li, Y.; et al. Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer Cell 2017, 32, 42–56.e46.
- Brennan, C.W.; Verhaak, R.G.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The somatic genomic landscape of glioblastoma. Cell 2013, 155, 462–477.
- Nie, S.; Zhu, Y.; Yang, J.; Xin, T.; Xue, S.; Sun, J.; Mu, D.; Chen, Z.; Sun, P.; Yu, J.; et al. Clinicopathologic analysis of microscopic tumor extension in glioma for external beam radiotherapy planning. BMC Med. 2021, 19, 269.
- Walker, M.D.; Strike, T.A.; Sheline, G.E. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int. J. Radiat. Onco.l Biol. Phys. 1979, 5, 1725–1731.
- Walker, M.D.; Alexander, E.; Hunt, W.E.; MacCarty, C.S.; Mahaley, M.S.; Mealey, J.; Norrell, H.A.; Owens, G.; Ransohoff, J.; Wilson, C.B.; et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J. Neurosurg. 1978, 49, 333–343.
- Walker, M.D.; Alexander, E.; Hunt, W.E.; Leventhal, C.M.; Mahaley, M.S.; Mealey, J.; Norrell, H.A.; Owens, G.; Ransohoff, J.; Wilson, C.B.; et al. Evaluation of mithramycin in the treatment of anaplastic gliomas. J. Neurosurg. 1976, 44, 655–667.
- Walker, M.D.; Strike, T.A. Evaluation of methyl-CCNU, BCNU, and radiotherapy in the treatment of malignant glioma (abstr.). Proc. Am. Assoc. Cancer Res. 1976, 17, 652.
- Nelson, D.F.; Diener-West, M.; Horton, J.; Chang, C.H.; Schoenfeld, D.; Nelson, J.S. Combined modality approach to treatment of malignant gliomas—Re-evaluation of RTOG 7401/ECOG 1374 with long-term follow-up: A joint study of the Radiation Therapy Oncology Group and the Eastern Cooperative Oncology Group. NCI Monogr. 1988, 6, 279–284.
- Murray, K.J.; Nelson, D.F.; Scott, C.; Fischbach, A.J.; Porter, A.; Farnan, N.; Curran, W.J., Jr. Quality-adjusted survival analysis of malignant glioma. Patients treated with twice-daily radiation (RT) and carmustine: A report of Radiation Therapy Oncology Group (RTOG) 83-02. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 453–459.
- Ali, A.N.; Zhang, P.; Yung, W.K.A.; Chen, Y.; Movsas, B.; Urtasun, R.C.; Jones, C.U.; Choi, K.N.; Michalski, J.M.; Fischbach, A.J.; et al. NRG oncology RTOG 9006: A phase III randomized trial of hyperfractionated radiotherapy (RT) and BCNU versus standard RT and BCNU for malignant glioma patients. J. Neurooncol. 2018, 137, 39–47.
- Tsien, C.I.; Brown, D.; Normolle, D.; Schipper, M.; Piert, M.; Junck, L.; Heth, J.; Gomez-Hassan, D.; Ten Haken, R.K.; Chenevert, T.; et al. Concurrent temozolomide and dose-escalated intensity-modulated radiation therapy in newly diagnosed glioblastoma. Clin. Cancer Res. 2012, 18, 273–279.
- Gondi, V.; Pugh, S.; Tsien, C.; Chenevert, T.; Gilbert, M.; Omuro, A.; McDonough, J.; Aldape, K.; Srinivasan, A.; Rogers, C.L.; et al. Radiotherapy (RT) Dose-intensification (DI) Using Intensity-modulated RT (IMRT) versus Standard-dose (SD) RT with Temozolomide (TMZ) in Newly Diagnosed Glioblastoma (GBM): Preliminary Results of NRG Oncology BN001. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, S22–S23.
- Aydin, H.; Sillenberg, I.; von Lieven, H. Patterns of failure following CT-based 3-D irradiation for malignant glioma. Strahlenther Onkol. 2001, 177, 424–431.
- Wallner, K.E.; Galicich, J.H.; Krol, G.; Arbit, E.; Malkin, M.G. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int. J. Radiat. Oncol. Biol. Phys. 1989, 16, 1405–1409.
- Kruser, T.J.; Bosch, W.R.; Badiyan, S.N.; Bovi, J.A.; Ghia, A.J.; Kim, M.M.; Solanki, A.A.; Sachdev, S.; Tsien, C.; Wang, T.J.C.; et al. NRG brain tumor specialists consensus guidelines for glioblastoma contouring. J. Neurooncol. 2019, 143, 157–166.
- Giordano, F.A.; Brehmer, S.; Murle, B.; Welzel, G.; Sperk, E.; Keller, A.; Abo-Madyan, Y.; Scherzinger, E.; Clausen, S.; Schneider, F.; et al. Intraoperative Radiotherapy in Newly Diagnosed Glioblastoma (INTRAGO): An Open-Label, Dose-Escalation Phase I/II Trial. Neurosurgery 2019, 84, 41–49.
- Sarria, G.R.; Sperk, E.; Han, X.; Sarria, G.J.; Wenz, F.; Brehmer, S.; Fu, B.; Min, S.; Zhang, H.; Qin, S.; et al. Intraoperative radiotherapy for glioblastoma: An international pooled analysis. Radiother. Oncol. 2020, 142, 162–167.
- Gessler, D.J.; Neil, E.C.; Shah, R.; Levine, J.; Shanks, J.; Wilke, C.; Reynolds, M.; Zhang, S.; Özütemiz, C.; Gencturk, M.; et al. GammaTile® brachytherapy in the treatment of recurrent glioblastomas. Neurooncol. Adv. 2022, 4, vdab185.
- Guckenberger, M.; Klement, R.J.; Allgauer, M.; Appold, S.; Dieckmann, K.; Ernst, I.; Ganswindt, U.; Holy, R.; Nestle, U.; Nevinny-Stickel, M.; et al. Applicability of the linear-quadratic formalism for modeling local tumor control probability in high dose per fraction stereotactic body radiotherapy for early stage non-small cell lung cancer. Radiother. Oncol. 2013, 109, 13–20.
- Song, C.W.; Kim, M.S.; Cho, L.C.; Dusenbery, K.; Sperduto, P.W. Radiobiological basis of SBRT and SRS. Int. J. Clin. Oncol. 2014, 19, 570–578.
- Soliman, H.; Ruschin, M.; Angelov, L.; Brown, P.D.; Chiang, V.L.S.; Kirkpatrick, J.P.; Lo, S.S.; Mahajan, A.; Oh, K.S.; Sheehan, J.P.; et al. Consensus Contouring Guidelines for Postoperative Completely Resected Cavity Stereotactic Radiosurgery for Brain Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 436–442.
- Souhami, L.; Seiferheld, W.; Brachman, D.; Podgorsak, E.B.; Werner-Wasik, M.; Lustig, R.; Schultz, C.J.; Sause, W.; Okunieff, P.; Buckner, J.; et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: Report of Radiation Therapy Oncology Group 93-05 protocol. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 853–860.
- Redmond, K.J.; Mehta, M. Stereotactic Radiosurgery for Glioblastoma. Cureus 2015, 7, e413.
- Harrabi, S.B.; Bougatf, N.; Mohr, A.; Haberer, T.; Herfarth, K.; Combs, S.E.; Debus, J.; Adeberg, S. Dosimetric advantages of proton therapy over conventional radiotherapy with photons in young patients and adults with low-grade glioma. Strahlenther. Onkol. 2016, 192, 759–769.
- Brown, P.D.; Chung, C.; Liu, D.D.; McAvoy, S.; Grosshans, D.; Al Feghali, K.; Mahajan, A.; Li, J.; McGovern, S.L.; McAleer, M.F.; et al. A prospective phase II randomized trial of proton radiotherapy vs intensity-modulated radiotherapy for patients with newly diagnosed glioblastoma. Neuro Oncol. 2021, 23, 1337–1347.
- Snyder, J.M.; Huang, R.Y.; Bai, H.; Rao, V.R.; Cornes, S.; Barnholtz-Sloan, J.S.; Gutman, D.; Fasano, R.; Van Meir, E.G.; Brat, D.; et al. Analysis of morphological characteristics of IDH-mutant/wildtype brain tumors using whole-lesion phenotype analysis. Neurooncol. Adv. 2021, 3, vdab088.
- Bangalore Yogananda, C.G.; Shah, B.R.; Vejdani-Jahromi, M.; Nalawade, S.S.; Murugesan, G.K.; Yu, F.F.; Pinho, M.C.; Wagner, B.C.; Mickey, B.; Patel, T.R.; et al. A novel fully automated MRI-based deep-learning method for classification of IDH mutation status in brain gliomas. Neuro Oncol. 2020, 22, 402–411.
- Yogananda, C.G.B.; Shah, B.R.; Nalawade, S.S.; Murugesan, G.K.; Yu, F.F.; Pinho, M.C.; Wagner, B.C.; Mickey, B.; Patel, T.R.; Fei, B.; et al. MRI-Based Deep-Learning Method for Determining Glioma. AJNR Am. J. Neuroradiol. 2021, 42, 845–852.
- Akbari, H.; Bakas, S.; Pisapia, J.M.; Nasrallah, M.P.; Rozycki, M.; Martinez-Lage, M.; Morrissette, J.J.D.; Dahmane, N.; O’Rourke, D.M.; Davatzikos, C. In vivo evaluation of EGFRvIII mutation in primary glioblastoma patients via complex multiparametric MRI signature. Neuro Oncol. 2018, 20, 1068–1079.
- Yun, J.; St Aubin, J.; Rathee, S.; Fallone, B.G. Brushed permanent magnet DC MLC motor operation in an external magnetic field. Med. Phys. 2010, 37, 2131–2134.
- St Aubin, J.; Santos, D.M.; Steciw, S.; Fallone, B.G. Effect of longitudinal magnetic fields on a simulated in-line 6 MV linac. Med. Phys. 2010, 37, 4916–4923.
- Burke, B.; Wachowicz, K.; Fallone, B.G.; Rathee, S. Effect of radiation induced current on the quality of MR images in an integrated linac-MR system. Med. Phys. 2012, 39, 6139–6147.
- Liney, G.P.; Whelan, B.; Oborn, B.; Barton, M.; Keall, P. MRI-Linear Accelerator Radiotherapy Systems. Clin. Oncol. (R Coll Radiol.) 2018, 30, 686–691.
- Kluter, S. Technical design and concept of a 0.35 T MR-Linac. Clin. Transl. Radiat. Oncol. 2019, 18, 98–101.
- Winkel, D.; Bol, G.H.; Kroon, P.S.; van Asselen, B.; Hackett, S.S.; Werensteijn-Honingh, A.M.; Intven, M.P.W.; Eppinga, W.S.C.; Tijssen, R.H.N.; Kerkmeijer, L.G.W.; et al. Adaptive radiotherapy: The Elekta Unity MR-linac concept. Clin. Transl. Radiat. Oncol. 2019, 18, 54–59.
