Esculetin is a coumarin compound, which belongs to the class of benzopyrone enriched in various plants such as Sonchus grandifolius, Aesculus turbinata, etc. Free radicals lead to the development of oxidative stress causing inflammation, arthritis, cancer, diabetes, fatty liver disease, etc. These further reduce the efficacy of anticancer drugs, activate inflammatory signaling pathways, degrade joints and cartilage, and disrupt the glycemic index and normal function of liver enzymes. For instance, the current treatment modalities used in arthritis such as non-steroidal anti-inflammatory drugs, disease-modifying anti-rheumatoid drugs, and lipoxygenase inhibitors present limited efficacy and adverse effects. Thus, there is a constant need to find newer and safer alternatives. Esculetin has an immense antioxidative potential thereby alleviating arthritis, diabetes, malignancies, and hepatic disorders.
- esculetin
- cancer
- oxidative stress
- inflammation
- arthritis
- diabetes
1. Introduction
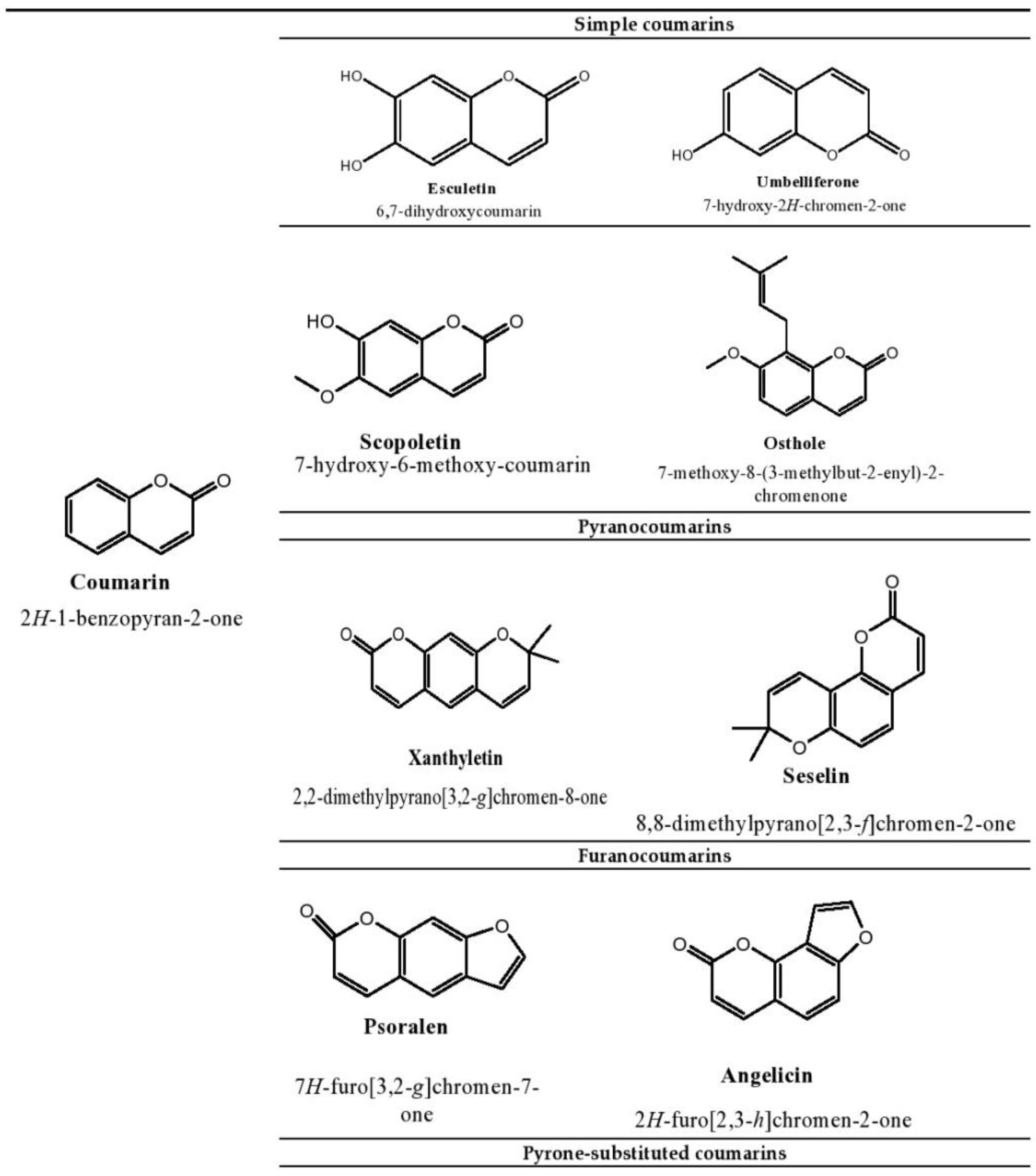
2. Therapeutic Applications of Esculetin
2.1. The Role of Esculetin in Cancer Treatment
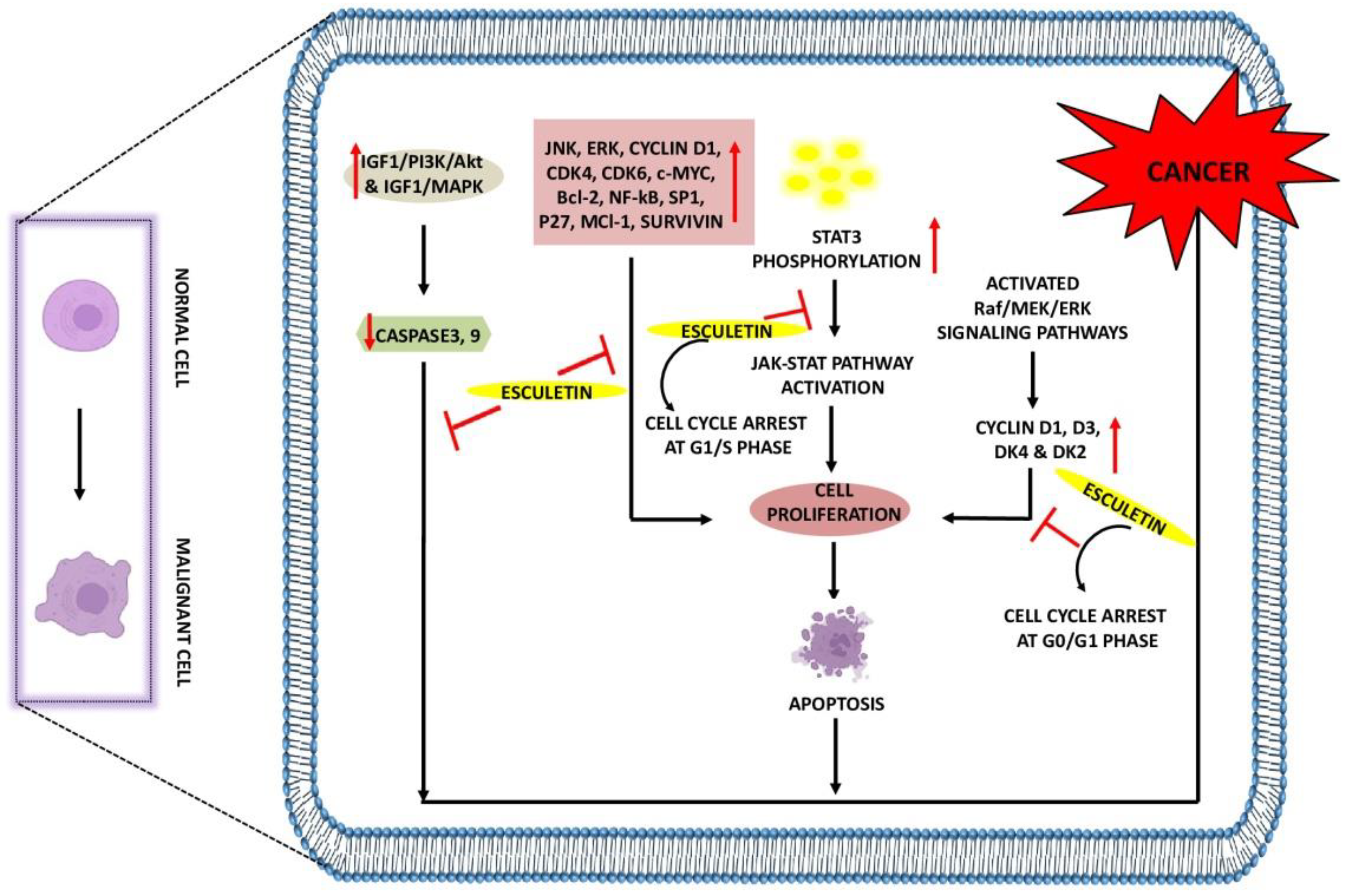
| Pharmacological Mechanism | Inhibition/Activation/ Downregulation/Upregulation |
Model Used | Dosage | Application | Reference |
|---|---|---|---|---|---|
| Cell-cycle arrest at G1-phase Activate ARE pathway and impede binding interactions between Nrf2 and KEAP-1 Attenuate NF-κB pathway |
Human PANC-1 cells | 100 µM | In vitro | [43] | |
| Inhibit cell proliferation Induce autophagy by forming autophagic-vesicles Downregulate cyclin D1, D3, DK4 and DK2 Induce cell-cycle arrest at G0/G1-phase Block MEK/ERK phosphorylation by inhibiting Raf/MEK/ERK signaling |
Human leukemia cells (HL-60 cells) | 20 µM | In vitro | [45] | |
| Downregulate JNK/ERK signaling | Human leukemia cells (U937 cells) | 30 µM | In vitro | [46] | |
| Downregulate Bcl-2 and NF-κB expressions Induce apoptosis |
Benzo[a]pyrene-induced lung carcinogenesis in Swiss-albino mice | 50 mg/kg | In vivo | [48] | |
| Anti-cancer | Activate MAPK signaling Activate caspase-3 and 9 and cause apoptosis Release cytochrome c into cytosol Increase mitochondrial membrane depolarization Increase Bax expression |
Human colon cancer cells (HT-29 cells) | 55 µg/mL | In vitro | [49] |
| Suppress SP1, p27, cyclin D1, Mcl-1, survivin expressions Induce apoptosis |
Oral squamous cancer (HN22 and HSC4 cells) | 20 µg/mL | In vitro | [50] | |
| Downregulate STAT3 phosphorylation Inhibition of JAK/STAT pathways Induce cell-cycle arrest at G1/S-phase |
Laryngeal cancer (Hep2 cells) | 2, 10 µM | In vitro, In vivo | [51] | |
| Cell-cycle arrest at S-phase Elevate caspase-3, 9 expressions Reduce mitochondrial membrane potential Increase Bax expression Downregulate Bcl-2 expression |
Hepatocellular carcinoma (C57BL/6 mice were implanted with Hepa1–6 cells and SMMC-7721 cells) | 2.24 mM | In vitro, In vivo | [52] | |
| Suppress IGF-1/PI3K/Akt and IGF-1/MAPK signaling Reduce mitochondrial membrane potential Release cytochrome c from mitochondria Increase Bax, Bcl-2, caspase-3, 9 Expressions |
Human gastric cancer (MGC-803 and GES-1 cells) | 850 µM | In vitro | [53] | |
| Inhibit proliferation, migration and invasion of renal cancerous cells Induce cell-cycle arrest at G0/G1 and G2-phase Downregulate cyclin D1, CDK4, CDK6 and c-Myc expressions Increase E-cadherin level by decreasing N-cadherin and vimentin expressions |
Renal carcinoma (786-O and SN12-PM6 cells) | 200 µg/mL | In vitro | [54] |
2.2. The Role of Esculetin in Oxidative Stress Treatment
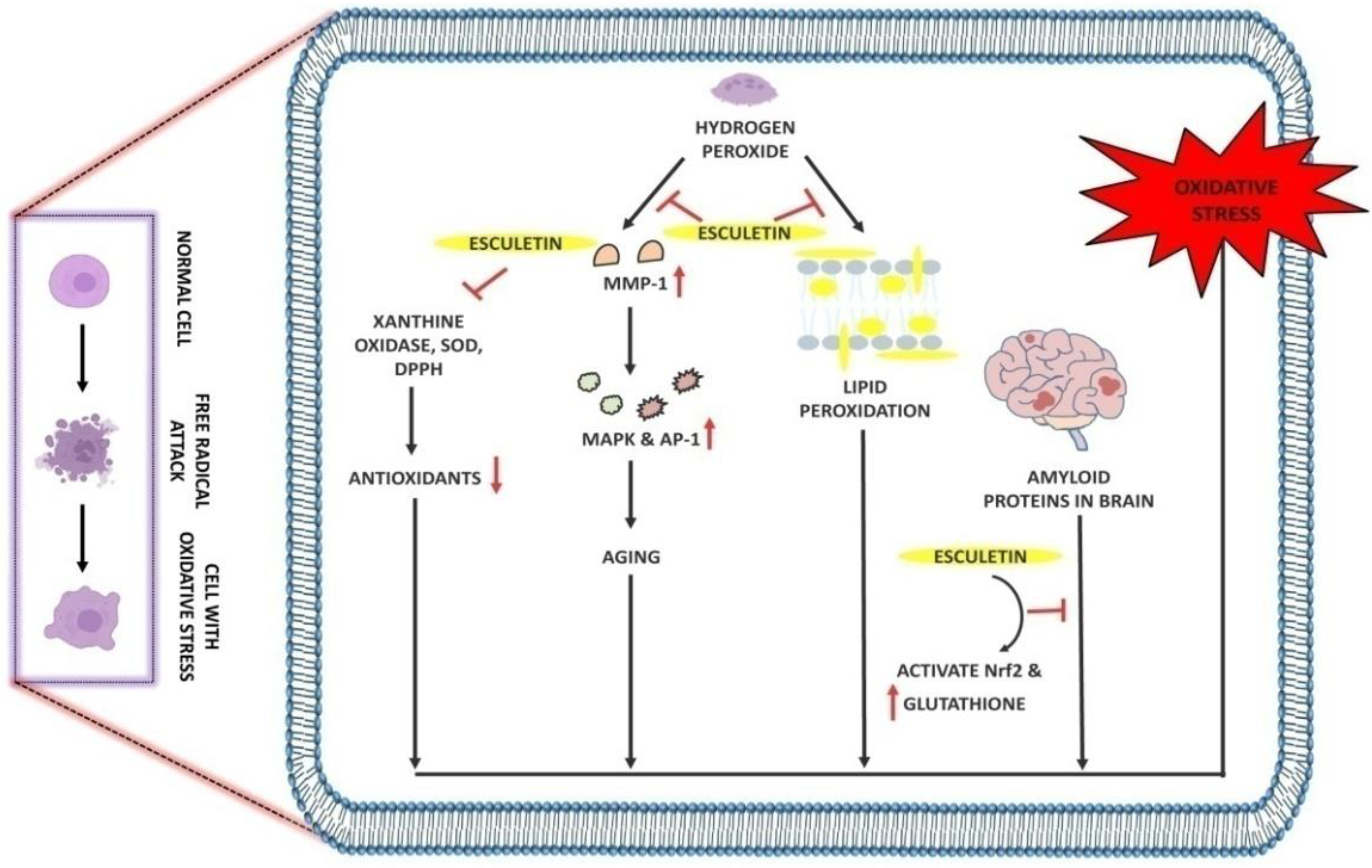
| Pharmacological Mechanism | Inhibition/Activation/ Downregulation/Upregulation |
Model Used | Dosage | Application | Reference |
|---|---|---|---|---|---|
| Antioxidant | Increase phosphorylation of Nrf2 and NQO1 Activate ERK signaling pathways Show protective effect against H2O2-induced oxidative stress |
H2O2-induced oxidative stress in C2C12 myoblasts cells | 5 µM | In vitro | [55] |
| Scavenge DPPH, hydroxyl and intracellular ROS Inhibit lipid peroxidation, protein carbonyl and DNA-damage induced by H2O2 |
Chinese hamster lung fibroblast cells (V79-4 cells) | 10 µg/mL | In vitro | [56] | |
| Scavenge free radicals Inhibition of lipid peroxidation, AST, ALT and ALP in liver |
CCl4-induced acute hepatotoxicity in male Sprague Dawley rats | 35 mg/kg | In vivo | [57] | |
| Activate Nrf2 Increase phosphorylation of ERK signaling and Akt signaling Increase glutathione levels |
Amyloid protein-induced oxidative stress and neuronal death in SH-SY5Y cells | 20 µM | In vitro | [58] | |
| Inhibit DPPH, Xanthine oxidase, superoxide radicals Downregulate MMP-1 expression |
Oxidative stress in human dermal fibroblasts cells (HDF-cells) | 0.6 µg/mL and 2.1 µg/mL |
In vitro | [59] | |
| Inhibit phospho-MEK1, phospho-ERK1/2, phospho-SEK1 and phospho-JNK1/2 along with intracellular Ca2+ levels Inhibit MMP-1 expression |
H2O2-induced oxidative stress in Human HaCaT keratinocytes cells | 5 µg/mL | In vitro | [60] | |
| Scavenge hydroxyl radicals and protect DNA from oxidative damage | Lipid-hydroperoxide-induced oxidative damage in human diploid fibroblast cells (TIG-7 cells) | 50 µL | In vitro | [61] |
2.3. The Role of Esculetin in Inflammation Treatment
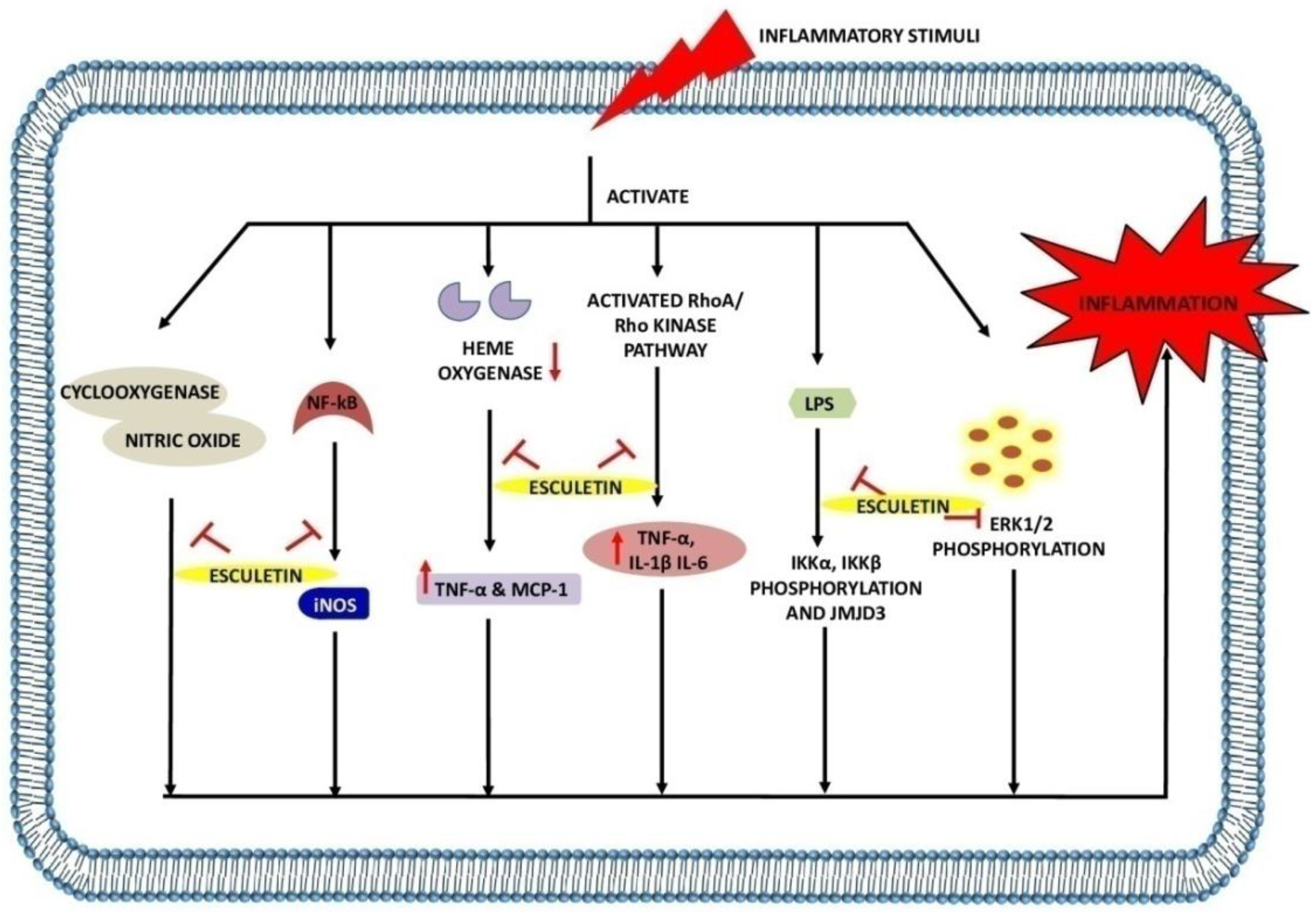
| Pharmacological Mechanism | Inhibition/Activation/ Downregulation/Upregulation |
Model Used | Dosage | Application | Reference |
|---|---|---|---|---|---|
| Downregulate inflammatory cytokines and chemokines (TNF-α, IL-1β, IL-6, CCL2 and iNOS) Inhibit NF-κB, STAT1 and STAT3 expression in macrophage Attenuate IKKα/β, IKBα phosphorylation and p65 levels in LPS-stimulated macrophage Inhibit translocation of p65 from cytoplasm to nucleus in LPS-stimulated macrophage Downregulate phosphorylation of ERK1/2, JNK and p38 levels in macrophage Suppress STAT1 and STAT3 activation in LPS-induced macrophage and sepsis mice |
E. coli-induced mice sepsis mice and LPS-stimulated macrophage of lung injury (RAW 264.7 cells) | 20, 40 and 60 mg/kg | In vitro and in vivo | [65] | |
| Decrease iNOS and COX-2 level Inhibition of NO and PGE2 production Inhibit TNF-α, IL-1β expression Inhibit LPS-mediated nuclear translocation of NF-κBp65 by suppressing IKβ-α degradation Inhibit ROS generation |
LPS-induced inflammation in RAW 264.7 cells | 12 µg/mL | In vitro | [66] | |
| Reverse LTA-induced IkB degradation Reverse NF-κBp65 phosphorylation Increase Nrf2 activity and scavenge DPPH radicals Inhibit NF-κBp65 translocation to nucleus |
RAW 264.7 cells | 20 µM | In vitro | [67] | |
| Reduce IL-1β, IL-6, TNF-α in serum and hippocampus Downregulate iNOS and COX-2 in hippocampus Inhibit LPS-induced pIKK-α, pIKK-β, pIKB-α and p-NF-kB65 activation Upregulate p-TrKB protein expression in hippocampus due to activation of BDNF/TrKB signaling pathway, thus exhibit neuroprotective activity |
LPS-induced neuro-inflammation in mice and hippocampus protein extract | 20, 40 mg/kg | In vivo | [70] | |
| Increase endocytic activity and augmented NO and iNOS levels in LPS-treated macrophage |
LPS-induced inflammation in RAW 264.7 cells and BALB/c mice | 80 and 120 µM | In vitro and in vivo | [71] | |
| Reduce MMP-1 in cartilage Reduce NO and PGE2 in synovial fluid |
Knee OA model of rabbit | 100 and 200 mg/kg | In vivo | [72] | |
| Decrease NO, TNF-α and MCP-1 expression Inhibit PPARϒ and CCAAT/enhancer binding protein-α in adipocyte Inhibit iNOS level in macrophage Increase silencing of heme oxygenase |
Adipose tissue inflammation model (RAW264.7 cells and 3T3-L1 adipocyte cells) | 100 µM | In vitro | [73] | |
| Inhibit pro-inflammatory cytokines (IL-2, IL-1β, TNF-α, INF-ϒ) in colon Inhibit ROS generation Inhibit MPO and ALP Decrease GSH depletion |
TNBS-induced colitis in male Wistar rats and RAW 264.7 cells | 5 mg/kg, 100 µM | In vitro and in vivo | [75] | |
| Increase GSH and serotonin (5-HT) level in brain tissue Decrease TBARS, TNF-α, IL-1β levels in brain tissue |
Reserpine-induced fibromyalgia in female Swiss albino mice | 100 mg/kg | In vivo | [80] | |
| Suppress histamine-induced expressions and secretion of IL-6, IL-8, MUC5AC by inhibiting NF-kB signaling pathway Suppress histamine-induced p-p65 expression and p-IKBα degradation |
Allergic rhinitis model (Human nasal epithelial cells) | 10, 20 and 40 µmol/L | In vivo | [81] | |
| Anti-inflammatory | Reduction in ear swelling Decrease DFE/DNCB-induced scratching Decrease epidermal and dermal thickness Decrease accumulation of mast cells Decrease TNF-α, INF-ϒ, IL-4, IL-13, IL-31, IL-17A-induced phosphorylation of STAT1 and NF-κB (p65) translocation by degrading IKBα |
DNCB/DFE—induced atopic skin inflammation model (Female BALB/c mice and Human HaCaT keratinocytes cells) | 2, 10, 50 mg/kg and 10 µM |
In vitro and in vivo | [82] |
| Decrease attenuation of LPS-induced phosphorylation of ERK1/2 and NF-κB expression Protect cells from LPS-induced apoptosis and necrosis Decrease LPS-induced TRAIL, IL-1β, TNFR expression Inhibit LPS-induced MnSOD and GPx Downregulate IL-6, IL-12, VEGF expressions |
LPS-induced inflammation in Human retinal pigment epithelial cells (ARPE-19 cells) | 5 µM | In vitro | [76] | |
| Decrease MPO, IL-6, TNF-α, IL-1β expression Inhibit neutrophils infiltration Inhibit LPS-induced RhoA/Rho kinase pathway Block NF-κB activation |
LPS-induced acute lung injury (lung epithelial A549 cells and BALB/c mice) | 20, 40 mg/kg and 0.1, 1 and 10 µM | In vitro and in vivo | [77] | |
| Decrease MPO, COX-2, iNOS levels Activate HIF-1in HCT116 cells and increase HIF-1α protein expression Increase secretion of VEGF in HCT116 cells Inhibit HIF-prolyl hydroxylase-2 Enzyme |
TNBC-induced colitis (Human colon carcinoma HCT116 cells and Sprague Dawley colitic rats) |
100 and 200 µM | In vitro and in vivo | [78] | |
| Ameliorate skin lesion of psoriatic mice Inhibit CD3+ and CD8+ T-cell infiltration in psoriatic mice skin Decrease Ki67 and K10 mRNA expression Lower effector CD8+ T-cells in lymph nodes and spleen Inhibit NF-κB signaling by suppressing phosphor-IKKα and phosphor-p65 expression Increase CD4+ FOXp3+ Treg frequency in lymph node and spleen Downregulate IL-6, TNF-α, IFN-ϒ, IL-17A, IL-22, IL-23 |
Imiquimod-induced psoriasis in BALB/c mice | 50 and 100 mg/kg | In vivo | [79] |
This entry is adapted from the peer-reviewed paper 10.3390/ijms232012643
References
- Vuolo, M.M.; Lima, V.S.; Junior, M.R.M. Chapter 2—Phenolic Compounds: Structure, Classification, and Antioxidant Power. In Bioactive Compounds: Health Benefits and Potential Applications; Woodhead Publishing: Sawston, UK, 2019; pp. 33–50.
- Garg, S.S.; Gupta, J.; Sharma, S.; Sahu, D. An insight into the therapeutic applications of coumarin compounds and their mechanism of action. Eur. J. Pharm. Sci. 2020, 152, 105424.
- Aykin-Burns, N.; Ahmad, I.M.; Zhu, Y.; Oberley, L.W.; Spitz, D.R. Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation. Biochem. J. 2009, 418, 29–37.
- Singh, A.; Misra, V.; Thimmulappa, R.K.; Lee, H.; Ames, S.; Hoque, M.O.; Herman, J.G.; Baylin, S.B.; Sidransky, D.; Gabrielson, E.; et al. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006, 3, e420.
- Kotamraju, S.; Chitambar, C.R.; Kalivendi, S.V.; Joseph, J.; Kalyanaraman, B. Transferrin receptor-dependent iron uptake is responsible for doxorubicin-mediated apoptosis in endothelial cells: Role of oxidant-induced iron signaling in apoptosis. J. Biol. Chem. 2002, 277, 17179–17187.
- Djavaheri-Mergny, M.; Wietzerbin, J.; Besançon, F. 2-Methoxyestradiol induces apoptosis in Ewing sarcoma cells through mitochondrial hydrogen peroxide production. Oncogene 2003, 22, 2558–2567.
- Kachadourian, R.; Liochev, S.I.; Cabelli, D.E.; Patel, M.N.; Fridovich, I.; Day, B.J. 2-methoxyestradiol does not inhibit superoxide dismutase. Arch. Biochem. Biophys. 2001, 392, 349–353.
- Mooberry, S.L. Mechanism of action of 2-methoxyestradiol: New developments. Drug Resist. Updates 2003, 6, 355–361.
- Heo, J.R.; Kim, S.M.; Hwang, K.A.; Kang, J.H.; Choi, K.C. Resveratrol induced reactive oxygen species and endoplasmic reticulum stress-mediated apoptosis, and cell cycle arrest in the A375SM malignant melanoma cell line. Int. J. Mol. Med. 2018, 42, 1427–1435.
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902.
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167.
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84.
- Kanazawa, H.; Kurihara, N.; Hirata, K.; Takeda, T. The role of free radicals in airway obstruction in asthmatic patients. Chest 1991, 100, 1319–1322.
- Shanmugasundaram, K.R.; Kumar, S.S.; Rajajee, S. Excessive free radical generation in the blood of children suffering from asthma. Clin. Chim. Acta 2001, 305, 107–114.
- Samimi, L.N.; Farhadi, E.; Tahmasebi, M.N.; Jamshidi, A.; Vaziri, A.S.; Mahmoudi, M. NF-κB signaling in rheumatoid arthritis with focus on fibroblast-like synoviocytes. Auto. Immun. Highlights 2020, 11, 11.
- Liang, Y.; Zhou, Y.; Shen, P. NF-kappa B and its regulation on the immune system. Cell. Mol. Immunol. 2004, 1, 343–350.
- Jeong, S.R.; Park, H.Y.; Lee, K.W. Methylglyoxal-derived advanced glycation end products induce matrix metalloproteinases through activation of ERK/JNK/NF-κB pathway in kidney proximal epithelial cells. Food Sci. Biotechnol. 2019, 29, 675–682.
- Xue, M.; McKelvey, K.; Shen, K.; Minhas, N.; March, L.; Park, S.Y.; Jackson, C.J. Endogenous MMP-9 and not MMP-2 promotes rheumatoid synovial fibroblast survival, inflammation and cartilage degradation. Rheumatology 2014, 53, 2270–2279.
- Jacques, C.; Gosset, M.; Berenbaum, F.; Gabay, C. The role of IL-1 and IL-1Ra in joint inflammation and cartilage degradation. Vitamin. Horm. 2006, 74, 371–403.
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed. Pharmacother. 2020, 129, 110452.
- Rolo, A.P.; Palmeira, C.M. Diabetes and mitochondrial function: Role of hyperglycemia and oxidative stress. Toxicol. Appl. Pharmacol. 2006, 212, 167–178.
- Haidara, M.A.; Yassin, H.Z.; Rateb, M.; Ammar, H.; Zorkani, M.A. Role of oxidative stress in development of cardiovascular complications in diabetes mellitus. Curr. Vasc. Pharmacol. 2006, 4, 215–227.
- Bajaj, S.; Khan, A. Antioxidants and diabetes. Ind. J. Endocrinol. Metab. 2012, 16, S267–S271.
- Aghadavod, E.; Khodadadi, S.; Baradaran, A.; Nasri, P.; Bahmani, M.; Rafieian-Kopaei, M. Role of Oxidative Stress and Inflammatory Factors in Diabetic Kidney Disease. Iran. J. Kidney Dis. 2016, 10, 337–343.
- Sarwar, R.; Pierce, N.; Koppe, S. Obesity and nonalcoholic fatty liver disease: Current perspectives. Diabetes Metab. Syndr. Obes. 2018, 11, 533–542.
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689.
- Bhatt, H.B.; Smith, R.J. Fatty liver disease in diabetes mellitus. Hepatobiliary Surg. Nutr. 2015, 4, 101–108.
- Hazlehurst, J.M.; Woods, C.; Marjot, T.; Cobbold, J.F.; Tomlinson, J.W. Non-alcoholic fatty liver disease and diabetes. Metabolism 2016, 65, 1096–1108.
- Tomah, S.; Alkhouri, N.; Hamdy, O. Nonalcoholic fatty liver disease and type 2 diabetes: Where do Diabetologists stand? Clin. Diabetes Endocrinol. 2020, 6, 9.
- Kim, E.J.; Kim, B.H.; Seo, H.S.; Lee, Y.J.; Kim, H.H.; Son, H.H.; Choi, M.H. Cholesterol induced non-alcoholic fatty liver disease and atherosclerosis aggravated by systemic inflammation. PLoS ONE 2014, 9, e97841.
- Enjoji, M.; Yasutake, K.; Kohjima, M.; Nakamuta, M. Nutrition and nonalcoholic Fatty liver disease: The significance of cholesterol. Int. J. Hepatol. 2012, 2012, 925807.
- Malhotra, P.; Gill, R.K.; Saksena, S.; Alrefai, W.A. Disturbances in Cholesterol Homeostasis and Non-alcoholic Fatty Liver Diseases. Front. Med. 2020, 7, 467.
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxid. Med. Cell Longev. 2018, 2018, 9547613.
- Serviddio, G.; Bellanti, F.; Vendemiale, G. Free radical biology for medicine: Learning from nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2013, 65, 952–968.
- Morita, M.; Ishida, N.; Uchiyama, K.; Yamaguchi, K.; Itoh, Y.; Shichiri, M.; Yoshida, Y.; Hagihara, Y.; Naito, Y.; Yoshikawa, T.; et al. Fatty liver induced by free radicals and lipid peroxidation. Free Radic. Res. 2012, 46, 758–765.
- Basu, S. Carbon tetrachloride-induced lipid peroxidation: Eicosanoid formation and their regulation by antioxidant nutrients. Toxicology 2003, 189, 113–127.
- Von Minden, H.M.; Brandenburg, K.; Seydel, U.; Koch, M.H.; Garamus, V.; Willmeit, R.; Vill, V. Thermotropic and lyotropic properties of long chain alkyl glycopyranosides. Part II. Disaccharide headgroups. Chem. Phys. Lipids 2000, 106, 157–179.
- Traykova, M.; Kostova, I. Coumarin Derivatives and Oxidative Stress. Int. J. Pharmacol. 2005, 1, 29–32.
- Kostova, I.; Bhatia, S.; Grigorov, P.; Balkansky, S.; Parmar, V.S.; Prasad, A.K.; Saso, L. Coumarins as antioxidants. Curr. Med. Chem. 2011, 18, 3929–3951.
- Vianna, D.R.; Bubols, G.; Meirelles, G.; Silva, B.V.; da Rocha, A.; Lanznaster, M.; Monserrat, J.M.; Garcia, S.C.; von Poser, G.; Eifler-Lima, V.L. Evaluation of the antioxidant capacity of synthesized coumarins. Int. J. Mol. Sci. 2012, 13, 7260–7270.
- Zhu, J.J.; Jiang, J.G. Pharmacological and Nutritional Effects of Natural Coumarins and Their Structure-Activity Relationships. Mol. Nutr. Food Res. 2018, 62, e1701073.
- Najmanová, I.; Doseděl, M.; Hrdina, R.; Anzenbacher, P.; Filipský, T.; Říha, M.; Mladěnka, P. Cardiovascular effects of coumarins besides their antioxidant activity. Curr. Top. Med. Chem. 2015, 15, 830–849.
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress-A concise review. Saudi Pharm. J. 2016, 24, 547–553.
- Arora, R.; Sawney, S.; Saini, V.; Steffi, C.; Tiwari, M.; Saluja, D. Esculetin induces antiproliferative and apoptotic response in pancreatic cancer cells by directly binding to KEAP1. Mol. Cancer. 2016, 15, 64.
- Wang, X.; Yang, C.; Zhang, Q.; Wang, C.; Zhou, X.; Zhang, X.; Liu, S. In vitro anticancer effects of esculetin against human leukemia cell lines involves apoptotic cell death, autophagy, G0/G1 cell cycle arrest and modulation of Raf/MEK/ERK signalling pathway. JBUON 2019, 24, 1686–1691.
- Park, C.; Jin, C.Y.; Kim, G.Y.; Choi, I.W.; Kwon, T.K.; Choi, B.T.; Lee, S.J.; Lee, W.H.; Choi, Y.H. Induction of apoptosis by esculetin in human leukemia U937 cells through activation of JNK and ERK. Toxicol. Appl. Pharmacol. 2008, 227, 219–228.
- Mortenson, M.M.; Galante, J.G.; Gilad, O.; Schlieman, M.G.; Virudachalam, S.; Kung, H.J.; Bold, R.J. BCL-2 functions as an activator of the AKT signaling pathway in pancreatic cancer. J. Cell. Biochem. 2007, 102, 1171–1179.
- Anand, J.R.; Rijhwani, H.; Malapati, K.; Kumar, P.; Saikia, K.; Lakhar, M. Anticancer activity of esculetin via-modulation of Bcl-2 and NF-κB expression in benzo pyrene induced lung carcinogenesis in mice. Biomed. Prev. Nutr. 2013, 3, 107–112.
- Kim, A.D.; Han, X.; Piao, M.J.; Hewage, S.R.; Hyun, C.L.; Cho, S.J.; Hyun, J.W. Esculetin induces death of human colon cancer cells via the reactive oxygen species-mediated mitochondrial apoptosis pathway. Environ. Toxicol. Pharmacol. 2015, 39, 982–989.
- Cho, J.H.; Shin, J.C.; Cho, J.J.; Choi, Y.H.; Shim, J.H.; Chae, J.I. Esculetin (6,7-dihydroxycoumarin): A potential cancer chemopreventive agent through suppression of Sp1 in oral squamous cancer cells. Int. J. Oncol. 2015, 46, 265–271.
- Zhang, G.; Xu, Y.; Zhou, H.F. Esculetin Inhibits Proliferation, Invasion, and Migration of Laryngeal Cancer In Vitro and In Vivo by Inhibiting Janus Kinas (JAK)-Signal Transducer and Activator of Transcription-3 (STAT3) Activation. Med. Sci. Monit. 2019, 25, 7853–7863.
- Wang, J.; Lu, M.L.; Dai, H.L.; Zhang, S.P.; Wang, H.X.; Wei, N. Esculetin, a coumarin derivative, exerts in vitro and in vivo antiproliferative activity against hepatocellular carcinoma by initiating a mitochondrial-dependent apoptosis pathway. Braz. J. Med. Biol. Res. 2015, 48, 245–253.
- Wang, G.; Lu, M.; Yao, Y.; Wang, J.; Li, J. Esculetin exerts antitumor effect on human gastric cancer cells through IGF-1/PI3K/Akt signaling pathway. Eur. J. Pharmacol. 2017, 814, 207–215.
- Duan, J.; Shi, J.; Ma, X.; Xuan, Y.; Li, P.; Wang, H.; Fan, Y.; Gong, H.; Wang, L.; Pang, Y.; et al. Esculetin inhibits proliferation, migration, and invasion of clear cell renal cell carcinoma cells. Biomed. Pharmacother. 2020, 125, 110031.
- Han, M.H.; Park, C.; Lee, D.S.; Hong, S.H.; Choi, I.W.; Kim, G.Y.; Choi, S.H.; Shim, J.H.; Chae, J.I.; Yoo, Y.H.; et al. Cytoprotective effects of esculetin against oxidative stress are associated with the upregulation of Nrf2-mediated NQO1 expression via the activation of the ERK pathway. Int. J. Mol. Med. 2017, 39, 380–386.
- Kim, S.H.; Kang, K.A.; Zhang, R.; Piao, M.J.; Ko, D.O.; Wang, Z.H.; Chae, S.W.; Kang, S.S.; Lee, K.H.; Kang, H.K.; et al. Protective effect of esculetin against oxidative stress-induced cell damage via scavenging reactive oxygen species. Acta. Pharmacol. Sin. 2008, 29, 1319–1326.
- Bilgin, H.M.; Atmaca, M.; Deniz Obay, B.; Ozekinci, S.; Taşdemir, E.; Ketani, A. Protective effects of coumarin and coumarin derivatives against carbon tetrachloride induced acute hepatotoxicity in rats. Exp. Toxicol. Pathol. 2011, 63, 325–330.
- Pruccoli, L.; Morroni, F.; Sita, G.; Hrelia, P.; Tarozzi, A. Esculetin as a Bifunctional Antioxidant Prevents and Counteracts the Oxidative Stress and Neuronal Death Induced by Amyloid Protein in SH-SY5Y Cells. Antioxidants 2020, 9, 551.
- Lee, B.C.; Lee, S.Y.; Lee, H.J.; Sim, G.S.; Kim, J.H.; Kim, J.H.; Cho, Y.H.; Lee, D.H.; Pyo, H.B.; Choe, T.B.; et al. Anti-oxidative and photoprotective effects of coumarins isolated from Fraxinus chinensis. Arch. Pharm. Res. 2007, 30, 1293–1301.
- Zhe, A.X.; Piao, M.J.; Kang, K.A.; Fernando, P.D.S.M.; Kang, H.K.; Koh, Y.S.; Hyun, J.W. Esculetin Prevents the Induction of Matrix Metalloproteinase-1 by Hydrogen Peroxide in Skin Keratinocytes. J. Cancer. Prev. 2019, 24, 123–128.
- Kaneko, T.; Tahara, S.; Takabayashi, F. Suppression of lipid hydroperoxide-induced oxidative damage to cellular DNA by esculetin. Biol. Pharm. Bull. 2003, 26, 840–844.
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627–635.
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000.
- Zinatizadeh, M.R.; Schock, B.; Chalbatani, G.M.; Zarandi, P.K.; Jalali, S.A.; Miri, S.R. The Nuclear Factor Kappa B (NFκB) signaling in cancer development and immune diseases. Genes Dis. 2020, 8, 287–297.
- Cheng, Y.J.; Tian, X.L.; Zeng, Y.Z.; Lan, N.; Guo, L.F.; Liu, K.F.; Fang, H.L.; Fan, H.Y.; Peng, Z.L. Esculetin protects against early sepsis via attenuating inflammation by inhibiting NF-κB and STAT1/STAT3 signaling. Chin. J. Nat. Med. 2021, 19, 432–441.
- Hong, S.H.; Jeong, H.K.; Han, M.H.; Park, C.; Choi, Y.H. Esculetin suppresses lipopolysaccharide-induced inflammatory mediators and cytokines by inhibiting nuclear factor-κB translocation in RAW 264.7 macrophages. Mol. Med. Rep. 2014, 10, 3241–3246.
- Jayakumar, T.; Huang, C.J.; Yen, T.L.; Hsia, C.W.; Sheu, J.R.; Bhavan, P.S.; Huang, W.C.; Hsieh, C.Y.; Hsia, C.H. Activation of Nrf2 by Esculetin Mitigates Inflammatory Responses through Suppression of NF-kB Signaling Cascade in RAW 264.7 Cells. Molecules 2022, 27, 5143.
- Soufli, I.; Toumi, R.; Rafa, H.; Touil-Boukoffa, C. Overview of cytokines and nitric oxide involvement in immuno-pathogenesis of inflammatory bowel diseases. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 353–360.
- Andrew, P.J.; Mayer, B. Enzymatic function of nitric oxide synthases. Cardiovasc. Res. 1999, 43, 521–531.
- Zhu, L.; Nang, C.; Luo, F.; Pan, H.; Zhang, K.; Liu, J.; Zhou, R.; Gao, J.; Chang, X.; He, H.; et al. Esculetin attenuates lipopolysaccharide (LPS)-induced neuroinflammatory processes and depressive-like behavior in mice. Physiol. Behav. 2016, 163, 184–192.
- Leung, K.N.; Leung, P.Y.; Kong, L.P.; Leung, P.K. Immunomodulatory effects of esculetin (6,7-dihydroxycoumarin) on murine lymphocytes and peritoneal macrophages. Cell. Mol. Immunol. 2005, 2, 181–188.
- Liu, S.Q.; He, L.; Peng, H. Effect of esculetin on osteoarthritis in rabbit. Med. J. Wuhan Univ. 2004, 9, 567–570.
- Kim, Y.; Park, Y.; Namkoong, S.; Lee, J. Esculetin inhibits the inflammatory response by inducing heme oxygenase-1 in cocultured macrophages and adipocytes. Food Funct. 2014, 5, 2371–2377.
- Koelman, L.; Pivovarova-Ramich, O.; Pfeiffer, A.F.H.; Grune, T.; Aleksandrova, K. Cytokines for evaluation of chronic inflammatory status in ageing research: Reliability and phenotypic characterisation. Immun. Ageing 2019, 16, 11.
- Witaicenis, A.; Luchini, A.C.; Hiruma-Lima, C.A.; Felisbino, S.L.; Justulin, L.A., Jr.; Garrido-Mesa, N.; Utrilla, P.; Gálvez, J.; Di Stasi, L.C. Mechanism and effect of esculetin in an experimental animal model of inflammatory bowel disease. Eur. J. Inflamm. 2013, 11, 433–446.
- Ozal, S.A.; Turkekul, K.; Gurlu, V.; Guclu, H.; Erdogan, S. Esculetin Protects Human Retinal Pigment Epithelial Cells from Lipopolysaccharide-induced Inflammation and Cell Death. Curr. Eye Res. 2018, 43, 1169–1176.
- Chen, T.; Guo, Q.; Wang, H.; Zhang, H.; Wang, C.; Zhang, P.; Meng, S.; Li, Y.; Ji, H.; Yan, T. Effects of esculetin on lipopolysaccharide (LPS)-induced acute lung injury via regulation of RhoA/Rho Kinase/NF-κB pathways in vivo and in vitro. Free Radic. Res. 2015, 49, 1459–1468.
- Yum, S.; Jeong, S.; Lee, S.; Kim, W.; Nam, J.; Jung, Y. HIF-prolyl hydroxylase is a potential molecular target for esculetin-mediated anti-colitic effects. Fitoterapia 2015, 103, 55–62.
- Chen, Y.; Zhang, Q.; Liu, H.; Lu, C.; Liang, C.L.; Qiu, F.; Han, L.; Dai, Z. Esculetin Ameliorates Psoriasis-Like Skin Disease in Mice by Inducing CD4+Foxp3+ Regulatory T Cells. Front. Immunol. 2018, 9, 2092.
- Singh, L.; Kaur, A.; Garg, S.; Singh, A.P.; Bhatti, R. Protective Effect of Esculetin, Natural Coumarin in Mice Model of Fibromyalgia: Targeting Pro-Inflammatory Cytokines and MAO-A. Neurochem. Res. 2020, 45, 2364–2374.
- Sun, B.; Wang, B.; Xu, M. Esculetin inhibits histamine-induced expression of inflammatory cytokines and mucin in nasal epithelial cells. Clin. Exp. Pharmacol. Physiol. 2019, 46, 821–827.
- Jeong, N.H.; Yang, E.J.; Jin, M.; Lee, J.Y.; Choi, Y.A.; Park, P.H.; Lee, S.R.; Kim, S.U.; Shin, T.Y.; Kwon, T.K.; et al. Esculetin from Fraxinus rhynchophylla attenuates atopic skin inflammation by inhibiting the expression of inflammatory cytokines. Int. Immunopharmacol. 2018, 59, 209–216.
