Organosilicon compounds are organometallic compounds containing carbon–silicon bonds. Organosilicon chemistry is the corresponding science of their preparation and properties. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air. Silicon carbide is an inorganic compound.
- organometallic compounds
- organosilicon compounds
- organosilicon
1. Occurrence and Applications
Organosilicon compounds are widely encountered in commercial products. Most common are sealants, caulks (sealant), adhesives, and coatings made from silicones. Other important uses include synthesis of polyhedral oligomeric silsesquioxanes, agricultural and plant control adjuvants commonly used in conjunction with herbicides and fungicides.[1]
1.1. Biology and Medicine
Carbon–silicon bonds are naturally absent in biology, however enzymes have been used to artificially create carbon-silicon bonds in living microbes.[2][3][4] Silicates, on the other hand, have known existence in diatoms.[5] Silafluofen is an organosilicon compound that functions as a pyrethroid insecticide. Several organosilicon compounds have been investigated as pharmaceuticals.[6][7]
2. Properties of Si–C, Si–O, and Si–F Bonds
In most organosilicon compounds, Si is tetravalent with tetrahedral molecular geometry. Carbon–silicon bonds compared to carbon–carbon bonds are longer (186 pm vs. 154 pm) and weaker with bond dissociation energy 451 kJ/mol vs. 607 kJ/mol.[1][8] The C–Si bond is somewhat polarised towards carbon due to carbon's greater electronegativity (C 2.55 vs Si 1.90). The Si–C bond can be broken more readily than typical C–C bonds. One manifestation of bond polarization in organosilanes is found in the Sakurai reaction.[9] Certain alkyl silanes can be oxidized to an alcohol in the Fleming–Tamao oxidation.
Another manifestation is the β-silicon effect describes the stabilizing effect of a β-silicon atom on a carbocation with many implications for reactivity.
Si–O bonds are much stronger (809 kJ/mol compared to 538 kJ/mol) than a typical C–O single bond. The favorable formation of Si–O bonds drives many organic reactions such as the Brook rearrangement and Peterson olefination.
3. Preparation
The first organosilicon compound, tetraethylsilane, was prepared by Charles Friedel and James Crafts in 1863 by reaction of tetrachlorosilane with diethylzinc.
The bulk of organosilicon compounds derive from organosilicon chlorides (CH3)4-xSiClx. These chlorides are produced by the "Direct process", which entails the reaction of methyl chloride with a silicon-copper alloy. The main and most sought-after product is dimethyldichlorosilane:
- 2 CH3Cl + Si → (CH3)2SiCl2
A variety of other products are obtained, including trimethylsilyl chloride and methyltrichlorosilane. About 1 million tons of organosilicon compounds are prepared annually by this route. The method can also be used for phenyl chlorosilanes.[10]
3.1. Hydrosilylation
After the Direct Process, a second major method for the formation of Si-C bonds is hydrosilylation (also called hydrosilation).[11] In this process, compounds with Si-H bonds (hydrosilanes) add to unsaturated substrates. Commercially, the main substrates are alkenes. Other unsaturated functional groups—alkynes, imines, ketones, and aldehydes. An example is the hydrosilation of phenylacetylene:[12]
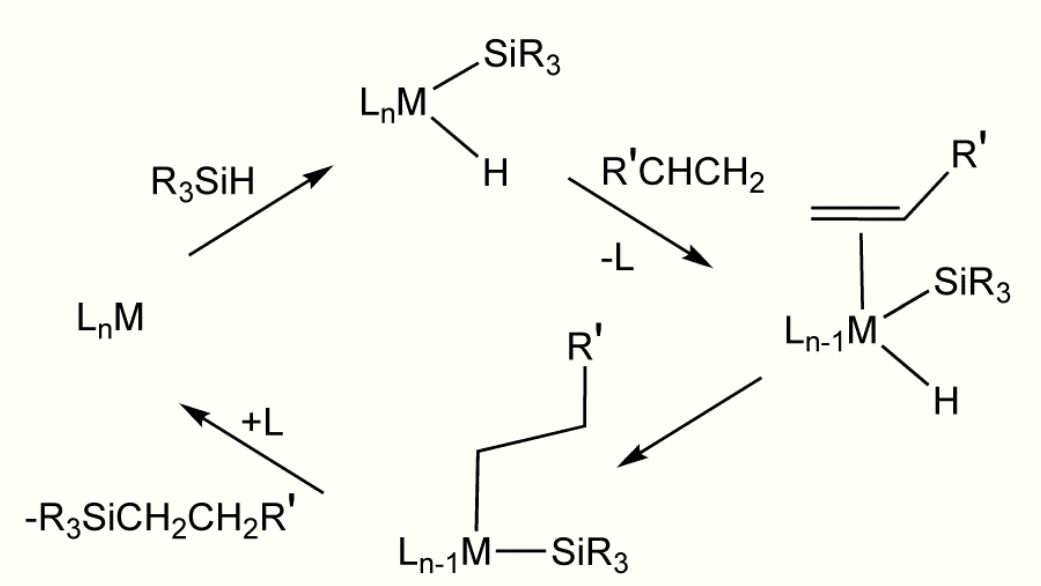
Hydrosilylation requires metal catalysts, especially those based on platinum group metals.
In the related silylmetalation, a metal replaces the hydrogen atom.
4. Functional Groups
Silicon is a component of many functional groups. Most of these are analogous to organic compounds. The overarching exception is the rarity of multiple bonds to silicon, as reflected in the double bond rule.
4.1. Silanols, Siloxides, and Siloxanes
Silanols are analogues of alcohols. They are generally prepared by hydrolysis of silyl chlorides:[13]
- R3SiCl + H2O → R3SiOH + HCl
Less frequently silanols are prepared by oxidation of silyl hydrides, a reaction that uses a metal catalyst:
- 2 R3SiH + O2 → 2 R3SiOH
Many silanols have been isolated including (CH3)3SiOH and (C6H5)3SiOH. They are about 500x more acidic than the corresponding alcohols. Siloxides are the deprotonated derivatives of silanols:[13]
- R3SiOH + NaOH → R3SiONa + H2O
Silanols tend to dehydrate to give siloxanes:
- 2 R3SiOH → R3Si-O-SiR3 + H2O
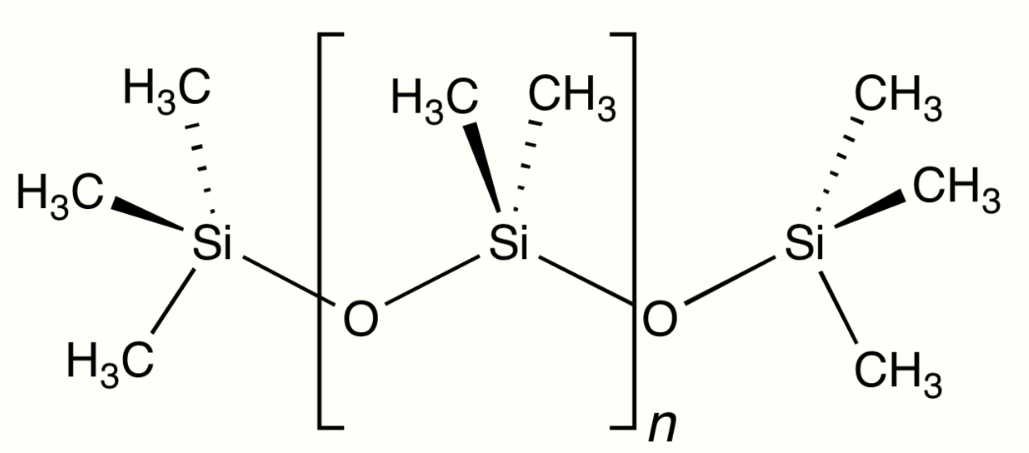
Polymers with repeating siloxane linkages are called silicones. Compounds with an Si=O double bond called silanones are extremely unstable.
4.2. Silyl Ethers
Silyl ethers have the connectivity Si-O-C. They are typically prepared by the reaction of alcohols with silyl chlorides:
- (CH3)3SiCl + ROH → (CH3)3Si-O-R + HCl
Silyl ethers are extensively used as protective groups for alcohols.
Exploiting the strength of the Si-F bond, fluoride sources such as tetra-n-butylammonium fluoride (TBAF) are used in deprotection of silyl ethers:
- (CH3)3Si-O-R + F− + H2O → (CH3)3Si-F + H-O-R + OH−
4.3. Silyl Chlorides
Organosilyl chlorides are important commodity chemicals. They are mainly used to produce silicone polymers as described above. Especially important silyl chlorides are dimethyldichlorosilane (Me2SiCl2), methyltrichlorosilane (MeSiCl3), and trimethylsilyl chloride (Me3SiCl). More specialized derivatives that find commercial applications include dichloromethylphenylsilane, trichloro(chloromethyl)silane, trichloro(dichlorophenyl)silane, trichloroethylsilane, and phenyltrichlorosilane.
Although proportionately a minor outlet, organosilicon compounds are widely used in organic synthesis. Notably trimethylsilyl chloride Me3SiCl is the main silylating agent. One classic method called the Flood reaction for the synthesis of this compound class is by heating hexaalkyldisiloxanes R3SiOSiR3 with concentrated sulfuric acid and a sodium halide.[14]
4.4. Silyl Hydrides
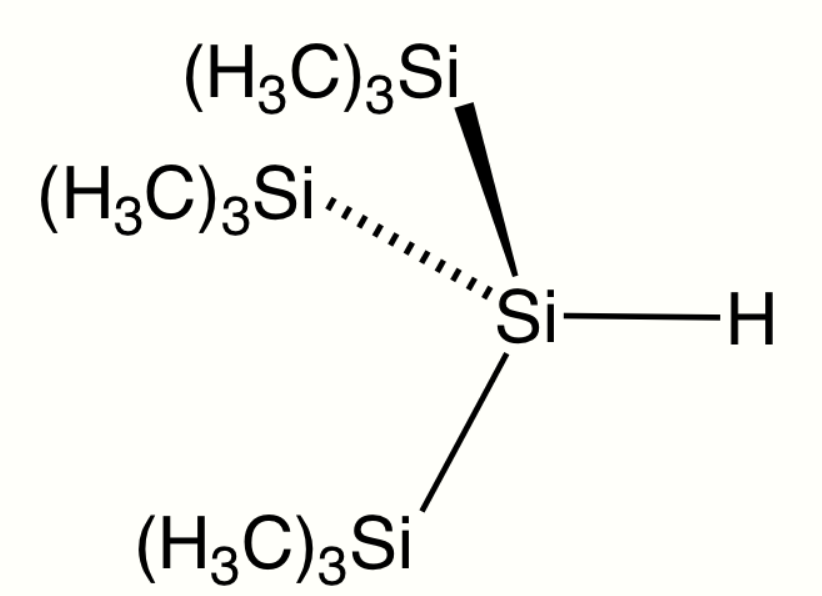
The silicon to hydrogen bond is longer than the C–H bond (148 compared to 105 pm) and weaker (299 compared to 338 kJ/mol). Hydrogen is more electronegative than silicon hence the naming convention of silyl hydrides. Commonly the presence of the hydride is not mentioned in the name of the compound. Triethylsilane has the formula Et3SiH. Phenylsilane is PhSiH3. The parent compound SiH4 is called silane.
4.5. Silenes
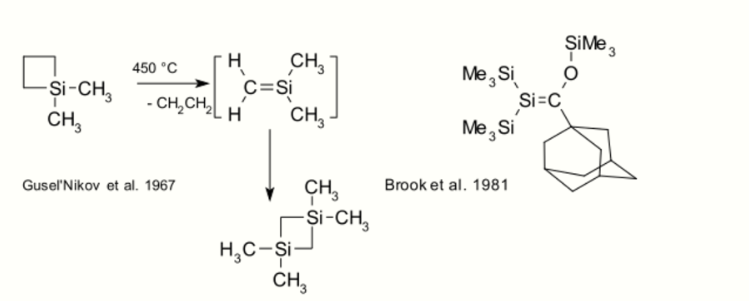
Organosilicon compounds, unlike their carbon counterparts, do not have a rich double bond chemistry.[16] Compounds with silene Si=C bonds (also known as alkylidenesilanes) are laboratory curiosities such as the silicon benzene analogue silabenzene. In 1967, Gusel'nikov and Flowers provided the first evidence for silenes from pyrolysis of dimethylsilacyclobutane.[17] The first stable (kinetically shielded) silene was reported in 1981 by Brook.[18][19]
Disilenes have Si=Si double bonds and disilynes are silicon analogues of an alkyne. The first Silyne (with a silicon to carbon triple bond) was reported in 2010 [20]. https://handwiki.org/wiki/index.php?curid=2081530
4.6. Siloles
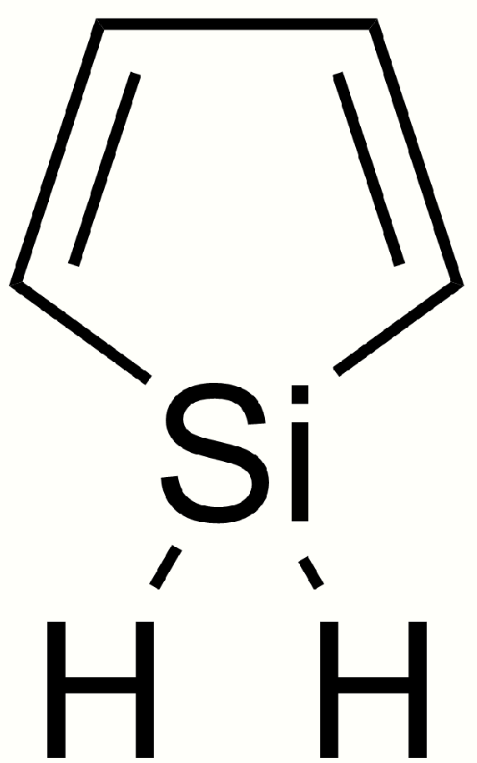
Siloles, also called silacyclopentadienes, are members of a larger class of compounds called metalloles. They are the silicon analogs of cyclopentadienes and are of current academic interest due to their electroluminescence and other electronic properties.[21][22] Siloles are efficient in electron transport. They owe their low lying LUMO to a favorable interaction between the antibonding sigma silicon orbital with an antibonding pi orbital of the butadiene fragment.
4.7. Pentacoordinated Silicon
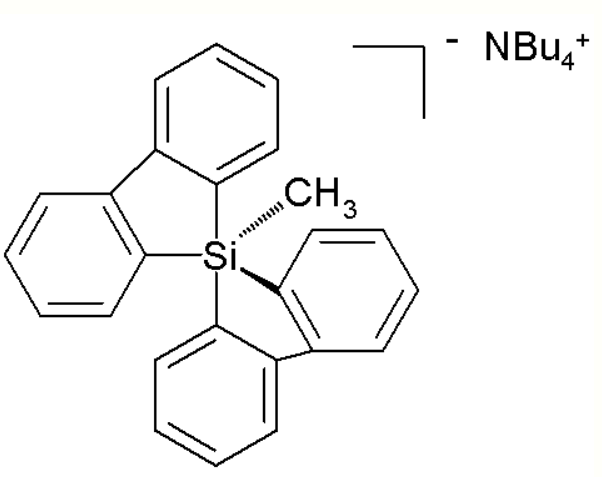
Unlike carbon, silicon compounds can be coordinated to five atoms as well in a group of compounds ranging from so-called silatranes, such as phenylsilatrane, to a uniquely stable pentaorganosilicate:[23]
Pentaorganosilicate. https://handwiki.org/wiki/index.php?curid=1683343
The stability of hypervalent silicon is the basis of the Hiyama coupling, a coupling reaction used in certain specialized organic synthetic applications. The reaction begins with the activation of Si-C bond by fluoride:
R-SiR'3 + R"-X + F− → R-R" + R'3SiF + X−
5. Various Reactions
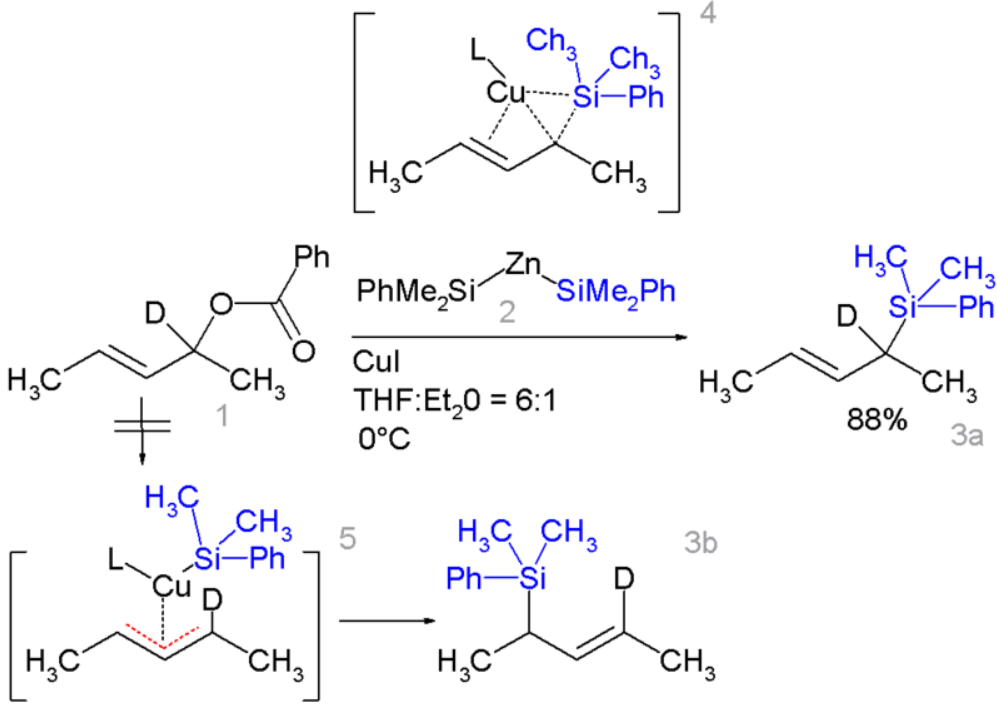
Certain allyl silanes can be prepared from allylic esters such as 1 and monosilylcopper compounds such as 2, in:[24][25]
In this reaction type, silicon polarity is reversed in a chemical bond with zinc and a formal allylic substitution on the benzoyloxy group takes place. https://handwiki.org/wiki/index.php?curid=1610996
6. Environmental Effects
Organosilicon compounds affect bee (and other insect) immune expression, making them more susceptible to viral infection.[7][26]
The content is sourced from: https://handwiki.org/wiki/Chemistry:Organosilicon
References
- Janeta, Mateusz; Szafert, Sławomir (2017-10-01). "Synthesis, characterization and thermal properties of T8 type amido-POSS with p-halophenyl end-group" (in en). Journal of Organometallic Chemistry 847: 173–183. doi:10.1016/j.jorganchem.2017.05.044. ISSN 0022-328X. https://dx.doi.org/10.1016%2Fj.jorganchem.2017.05.044
- Choi, Charles. "Possibility Of Silicon Based Life Grows". https://www.astrobio.net/news-exclusive/possibility-silicon-based-life-grows/.
- Mark B. Frampton, Paul M. Zelisko (2009), "Organosilicon Biotechnology" Silicon, 2009, 1, 147-163, doi:10.1007/s12633-009-9021-3 https://doi.org/10.1007%2Fs12633-009-9021-3
- Organosilicon Chemistry S. Pawlenko Walter de Gruyter New York 1986
- Stephen D. Kinrade, Ashley-M. E. Gillson and Christopher T. G. Knight (2002), Silicon-29 NMR evidence of a transient hexavalent silicon complex in the diatom Navicula pelliculosa. J. Chem. Soc., Dalton Trans., 307–309, doi:10.1039/b105379p https://doi.org/10.1039%2Fb105379p
- Bains, W.; Tacke, R. "Silicon chemistry as a novel source of chemical diversity in drug design" Curr. Opin. Drug Discov. Devel. 2003 Jul;6(4):526-43.
- https://phys.org/news/2017-01-common-crop-chemical-bees-susceptible.html#ms
- Handbook of Chemistry and Physics, 81st Edition CRC Press ISBN:0-8493-0481-4
- Silicon in Organic Synthesis Colvin, E. Butterworth: London 1981
- Röshe, L.; John, P.; Reitmeier, R. "Organic Silicon Compounds" Ullmann's Encyclopedia of Industrial Chemistry, 2003, Wiley-VCH, Weinheim. doi:10.1002/14356007.a24_021. https://doi.org/10.1002%2F14356007.a24_021
- B. Marciniec (ed.), Hydrosilylation, Advances in Silicon Science, doi:10.1007/978-1-4020-8172-9_1, Springer, 2009. https://doi.org/10.1007%2F978-1-4020-8172-9_1
- Effect of the synthetic method of Pt/MgO in the hydrosilylation of phenylacetylene Eulalia Ramírez-Oliva, Alejandro Hernández, J. Merced Martínez-Rosales, Alfredo Aguilar-Elguezabal, Gabriel Herrera-Pérez, and Jorge Cervantesa Arkivoc 2006 (v) 126-136 Link https://www.arkat-usa.org/ARKIVOC/JOURNAL_CONTENT/manuscripts/2006/EL-1973AP%20as%20published%20mainmanuscript.pdf
- Paul D. Lickiss "The Synthesis and Structure of Organosilanols" Advances in Inorganic Chemistry Volume 42, 1995, Pages 147–262. doi:10.1016/S0898-8838(08)60053-7 https://doi.org/10.1016%2FS0898-8838%2808%2960053-7
- Preparation of Triethylsilicon Halides E. A. Flood J. Am. Chem. Soc.; 1933; 55(4) pp 1735–1736; doi:10.1021/ja01331a504 https://doi.org/10.1021%2Fja01331a504
- Chryssostomos Chatgilialoglu, Carla Ferreri, Yannick Landais, Vitaliy I. Timokhin (2018). "Thirty Years of (TMS)3SiH: A Milestone in Radical-Based Synthetic Chemistry". Chemical Reviews 118: 6516–6572. doi:10.1021/acs.chemrev.8b00109. PMID 29938502. https://dx.doi.org/10.1021%2Facs.chemrev.8b00109
- Henrik Ottosson and Patrick G. Steel Silylenes, Silenes, and Disilenes: Novel Silicon-Based Reagents for Organic Synthesis? Chem. Eur. J. 2006, 12, 1576–1585 doi:10.1002/chem.200500429 https://doi.org/10.1002%2Fchem.200500429
- The thermal decomposition of 1,1-dimethyl-1-silacyclobutane and some reactions of an unstable intermediate containing a silicon–carbon double bond L. E. Gusel'Nikov and M. C. Flowers Chem. Commun. (London), 1967, 864–865, doi:10.1039/C19670000864 https://doi.org/10.1039%2FC19670000864
- A solid silaethene: isolation and characterization Adrian G. Brook, Fereydon Abdesaken, Brigitte Gutekunst, Gerhard Gutekunst, R. Krishna Kallury J. Chem. Soc., Chem. Commun., 1981, 191–192, doi:10.1039/C39810000191 https://doi.org/10.1039%2FC39810000191
- Brook silenes: inspiration for a generation Kim M. Baines Chem. Commun., 2013, 6366-6369. doi:10.1039/C3CC42595A https://doi.org/10.1039%2FC3CC42595A
- Gau, D., Kato, T., Saffon-Merceron, N., De Cózar, A., Cossío, F. and Baceiredo, A. (2010), Synthesis and Structure of a Base-Stabilized C-Phosphino-Si-Amino Silyne. Angewandte Chemie International Edition, 49: 6585–6588. doi:10.1002/anie.201003616 https://doi.org/10.1002%2Fanie.201003616
- Direct synthesis of 2,5-dihalosiloles Organic Syntheses 2008, 85, 53-63 http://www.orgsynth.org/orgsyn/pdfs/V85P0053.pdf
- Synthesis of new dipyridylphenylaminosiloles for highly emissive organic electroluminescent devices Laurent Aubouy, Philippe Gerbier, Nolwenn Huby, Guillaume Wantz, Laurence Vignau, Lionel Hirsch and Jean-Marc Jano New J. Chem., 2004, 28, 1086–1090, doi:10.1039/b405238b https://doi.org/10.1039%2Fb405238b
- Tetraalkylammonium pentaorganosilicates: the first highly stable silicates with five hydrocarbon ligands Sirik Deerenberg, Marius Schakel, Adrianus H. J. F. de Keijzer, Mirko Kranenburg, Martin Lutz, Anthony L. Spek, Koop Lammertsma, Chem. Commun., 2002, (4), 348-349 doi:10.1039/b109816k https://doi.org/10.1039%2Fb109816k
- Mechanistic insight into copper-catalysed allylic substitutions with bis(triorganosilyl) zincs. Enantiospecific preparation of -chiral silanes Eric S. Schmidtmann and Martin Oestreich Chem. Commun., 2006, 3643–3645, doi:10.1039/b606589a https://doi.org/10.1039%2Fb606589a
- By isotopic desymmetrisation on the substrate (replacing hydrogen by deuterium) it can be demonstrated that the reaction proceeds not through the symmetrical π-allyl intermediate 5 which would give an equal mixture of 3a and 3b but through the Π-δ intermediate 4 resulting in 3a only, through an oxidative addition or reductive elimination step
- Fine, Julia D.; Cox-Foster, Diana L.; Mullin, Christopher A. (2017-01-16). "An Inert Pesticide Adjuvant Synergizes Viral Pathogenicity and Mortality in Honey Bee Larvae" (in en). Scientific Reports 7: 40499. doi:10.1038/srep40499. ISSN 2045-2322. PMID 28091574. Bibcode: 2017NatSR...740499F. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=5238421