1. Introduction
A great number of studies on SACs have rapidly emerged in recent decades. In general, the detailed surface structure of a single atom can be obtained by a scanning tunneling microscope (STM) and aberration-corrected scanning transmission electron microscopy (STEM). X-ray absorption near edge spectra (XANES) and extended X-ray absorption fine structure spectra (EXAFS) can provide the valence states of the metal atoms and the properties and oxidation states of the neighbor species, which are the most effective ways to determine metal coordination numbers and judge whether SACs have been successfully prepared. Nuclear magnetic resonance spectroscopy (NMR) is used to provide coordinate information of metal species and ligands during in situ reactions. Density functional theory (DFT) calculations can reveal the geometrical configuration and strong interaction of the SACs according to the bond lengths, bond energies, electron transfer, binding energies, orbital hybridization, and density of the states. SACs exhibit excellent catalytic activity and high stability in heterogeneous catalytic reactions owing to their specific geometries, unique electronic structures, and strong interactions.
2. CO Oxidation
CO oxidation is the most widely studied field of SACs. As early as 1999, researchers studied CO oxidation over the Pt/MgO catalyst and put forward the correlation between monodisperse Pt cluster size and catalytic activity [
74]. The concept of SACs was first proposed by Zhang and colleagues when they concluded that partially vacant orbitals of high-valence Pt atoms contributed to reducing the adsorption energy of CO and the activation energy barrier of CO oxidation, thereby increasing the catalytic activity [
11]. At present, the most commonly studied catalysts in SACs take Pd and Au as the active sites; FeO
x [
11], CeO
2 [
34,
57,
97], TiO
2 [
98], Co
3O
4 [
59], and Al
2O
3 [
88] are used as the supports for CO oxidation. Generally speaking, the catalytic performance of CO oxidation over SACs is better than that of its corresponding NPs, such as Pt
1/FeO
x [
11], Pt/
m-Al
2O
3 [
88], and Au
1/Co
3O
4 [
89]. The activity of CO oxidation over the Pt/
m-Al
2O
3 SAC was still maintained after 60 circles of testing in a month, while the temperature went up dramatically after 10 testing circles of the NPs catalyst [
88]. The excellent activity and high stability of CO oxidation over the Au
1/Co
3O
4 SAC depend upon the interaction between Au
1 atoms and neighboring Co or O atoms (or oxygen vacancies), not only the individual Au
1 atoms [
89]. Nevertheless, SACs do not always exhibit a better performance than NPs catalysts—for example, the catalytic activity of Pt SAC/SiO
2 is lower than that of Pt NPs/SiO
2 [
99]. The Pt SACs prepared by electrostatic adsorption and high-temperature atomic capture were not active for CO oxidation at low temperatures, but the CO oxidation activities of both catalysts were improved after the catalysts were treated in a CO atmosphere at 275 °C, which was mainly attributed to the reduction in lattice oxygen in the CeO
2 support [
100]. Another study reported that the active sites were the Pt−O−Pt sites, rather than the Pt−O−Ce interface. The Pt−O−Pt sites were more likely to activate oxygen molecules, and its CO oxidation activity was 100–1000 times higher than that of Pt
1/CeO
2 [
101]. At the same time, the oxidation activity of CO was related to the migration of Pt sites to the position of clusters containing a small number of Pt atoms [
102]. The reduced Au sites (Au
0), which are the pivotal active sites in CO oxidation, demonstrate a higher CO adsorption capacity compared with the completely oxidized Au species [
103]. In conclusion, the advantages of SACs in CO oxidation depend upon the interaction between metal atoms and support, but they cannot be simply generalized.
The activities of CO oxidation can also be further improved by doping other components to modified SACs. First, 1 wt% Ce was introduced to modify the TiO
2 support particles, which could stabilize Pt single atoms through the strong electronic interaction. The as-formed CeO
x−TiO
2 mixed oxide interface could stabilize Pt single atoms through the strong electronic interaction. Compared with the Pt/TiO
2 catalyst, the mass activity of the compact Pt SAC formed on CeO
x/TiO
2 mixed oxide increased by about 15 times [
98]. Fe atoms, displaying a similar oxygen affinity to Pt, were inserted in nitrogen-doped graphene to prevent CO poisoning [
90]. On the atomic scale, Luo et al. visualized the evolution of the AuCu alloy NPs supported on CeO
2 during the CO oxidation process and found that CO exposure led to the formation of the metal-CO bonds, while O
2 exposure resulted in the formation of the Cu
2O–AuCu interface, thereby promoting CO–O interaction [
97].
3. Methane Combustion
Methane is abundant in natural reserve, and it is also the main component of natural gas and biogas. It is used as low-value fuel at all times, and its emission leads to increased methane concentration in the atmosphere, not only causing a waste of resources but also aggravating the greenhouse effect and bringing great environmental pressure. Therefore, it is desirable to study the complete oxidation of methane and convert methane to high-value chemicals for air pollution control and the utilization of low-carbon alkanes, respectively. In view of the highly symmetric tetrahedral stability and strong C–H bond energy of CH4, the activation and directional transformation of CH4 molecules have been hot topics in the field of catalysis.
On the one hand, the present literature concentrates on the high-temperature tolerance [
56] and water resistance [
91,
92] of SACs on CH
4 combustion. The activity and stability were improved several times when Pt NPs were converted into Pt atoms in situ [
56]. By combining the experiments with the DFT calculations, the volatilization of Pt at less than 600 °C is found to be thermally neutral or slightly endothermic, indicating that temperature plays a critical role in the dispersion of Pt NPs, that is, monoatomic dispersion can only be achieved at a temperature higher than 600 °C. Doping Fe
2O
3 into Al
2O
3 is beneficial to the dispersion of Pt atoms, because the reducible Fe
2O
3 displays a stronger interaction with Pt atoms than the irreducible Al
2O
3 displays with Pt atoms. In fact, a large amount of water vapor in the exhaust gas of methane combustion in actual situations will influence the performance of the Pd catalyst, even inducing deactivation.
4. Volatile Organic Compounds (VOCs) Oxidation
Given that noble metal catalysts are of high cost, SACs show great potential in many oxidation reactions, such as the oxidation of alcohol [
46,
54,
83], formaldehyde [
85,
86,
87], and benzene/toluene [
47,
80,
84]. These studies were mainly focused on Pt, Pd, Ag, and other SACs. The extensive applications of metal SACs in VOCs oxidation will be introduced in detail.
First of all, a proper reducible support is critical to successfully preparing SACs. Except for the excellent pollutant removal efficiency, maintaining stability for a long time is also important to the catalytic performance of SACs. As is well known, the metal–support interaction helps to maintain the thermodynamic stable state of SACs. For the supports with more oxygen vacancies, the oxygen vacancies strongly combine with the metal atoms and are difficult to replenish in time, resulting in a drop in catalytic activity. For the supports with a low number of oxygen vacancies, the binding force to the noble metal atoms is too weak to stabilize single atoms, so supports such as CeO
2 [
83], MnO
x [
84,
85], and Co
3O
4 [
54] were rapidly developed for the thermocatalytic oxidation of VOCs. For instance, Pt atoms are attached to the Co
3O
4 (111) crystal plane and occupy part of the Co
2+ atomic sites. The electrons are transferred from Pt to Co sites, thus increasing the proportion of oxygen vacancies on the Pt
1-Co
3O
4 surface. The regeneration of these oxygen vacancies reduces the adsorption energy of methanol and significantly promotes the dissociation of the C−H bond in methanol oxidation [
54]. Furthermore, the majority SACs showed a better performance than their NPs counterparts in many VOCs oxidation reactions. For example, the reaction rate of 0.25Pt
1/meso-Fe
2O
3 for benzene oxidation was seven times higher than that of the NPs sample, which was undoubtedly related to the good utilization of Pt atoms [
47]. The 0.03 wt% Pd/Al
2O
3 SAC was the most active cinnamyl alcohol oxidation catalyst reported so far; the activity of each Pd atom increased by 30 times if the 4.7 wt% Pd cluster catalyst was prepared to the 0.03 wt% Pd SAC catalyst [
46]. The above-mentioned cases indicate that the metal–support interaction in SACs highlights the advantages in the oxidation reaction. Interestingly, Jiang et al. further discovered that the Pt−O−Ce interface in Pt
1/CeO
2 SAC could spontaneously deform to balance the Fermi energy and charge density between Pt and the support, and the changes in the valence state and electronic structure increased the adsorption of oxygen and methanol, which was the essential reason for the excellent catalytic activity and thermal stability of the Pt
1/CeO
2 catalyst in the oxidation of oxygenated hydrocarbons [
83].
Secondly, modifying the catalyst to form hydroxyl radicals can promote the oxidative activity for pollutant removal. Over the Pt/MnO
2 SAC, a 100% conversion of toluene (0.42 ppm) was obtained at room temperature, and the oxygen vacancies and strong oxidizing hydroxyl radicals were considered to be the main reasons for its outstanding stability and excellent activity [
84]. Moreover, introducing alkali metal ions (e.g., Li
+, Na
+, and K
+) to Pt/TiO
2 SAC promoted the highly efficient removal of formaldehyde. The formed PtO(OH)
x-alkali metal species, as a promoter for the dispersion of Pt atoms, remarkably promoted the activity of formaldehyde oxidation by activating the simple reaction between hydroxyl and formate species on the surface [
86].
In addition, the loading of metal or metal oxides, the addition of water vapor, and the special structure of the catalyst also seriously affect the performance of the catalyst. Pt/Mn−TiO
2, with excellent activity and an acceptable cost, was screened as the best catalyst for formaldehyde oxidation. The loading of Pt enhanced the reactivity of surface lattice oxygen [
87]. However, it is worth noting that only 0.5 wt% Pt loading can completely oxidize formaldehyde at low temperatures and achieve long-term stability under simulated conditions. The addition of water vapor can suppress the side reaction and favor the deep oxidation of formaldehyde. The MnO
x nanorods-supported monoatomic Ag chain catalyst possessed a good activation ability for molecular and lattice oxygen at low temperatures [
85], in which the Ag atoms were initially uniformly dispersed and then thermally migrated to the end of the support to generate a single-atom Ag chain. In general, SACs have shown promising application prospects in many fields. A number of targeted strategies have been developed to improve the efficiency of SACs, especially those aiming at multicomponent VOCs, which are still under development.
5. NOx Reduction
Nitrogen oxides (NOx, x = 1 or 2), derived from the exhaust emissions of diesel vehicles, are one of the important precursors of the atmospheric pollutants PM2.5 and the ozone. Their large amount of emissions lead to environmental pollution (e.g., haze, acid rain, and photochemical smog). The most effective NOx removal technology is using NH3 for the selective catalytic reduction of NOx (NH3-SCR) to generate environmentally friendly N2 and H2O. The key issue is the successful preparation of high-performance SCR catalysts with a high N2 selectivity.
The Pt group catalysts have a great potential for NO
x emission control [
94,
105]. The 0.06 wt% Pt single-atom catalyst (Pt-SAC) could efficiently eliminate NO in a wide temperature range and showed a high N
2 selectivity. Compared with the NPs catalyst, Pt-SAC possessed more oxygen vacancies for NO adsorption and the breaking ability of the N−O bond [
105]. Pd SACs exhibited a high selectivity for converting NO into nitrate and an excellent resistance to nitrate poisoning [
94]. However, when the Pd loading increased to more than 0.055 wt%, Pd atom clusters or particles will form and significantly decrease the activity. This indicates that the catalytic performance of SACs is governed by the content of noble metals.
Transition-metal SACs have also received much attention [
95,
106] in NO
x reduction. Acidic metal oxides (e.g., Nb
2O
5, WO
3, and MoO
3)-modified CeO
2 catalysts show excellent NH
3-SCR performance at medium and high temperatures, especially the Nb
2O
5−CeO
2 catalyst. Ulteriorly, Xie et al. prepared a Cu atom-promoted Nb
2O
5/CeO
2 catalyst (NbCuCe), and the formed strong interaction of Nb−O−Cu and Cu−O−Ce effectively promoted the activation of reactant molecules [
95]. After aging under actual reaction conditions, the NbCuCe catalyst showed better activity than the commercial Cu-CHA catalyst. Such a catalyst shows good application prospects in diesel vehicle exhaust low-temperature denitrification.
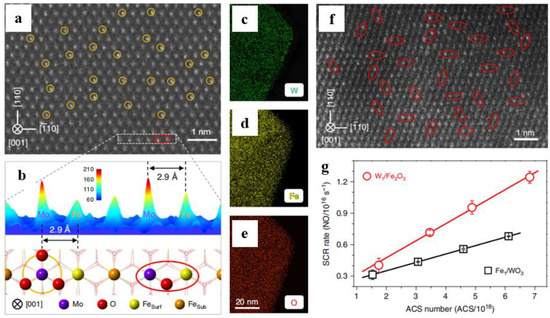
The Mo
1/Fe
2O
3 catalyst has been used to study the active sites and reaction mechanisms of NO
x reduction [
96,
106]. The formation of acidic-redox double active sites was caused by the Mo atoms and the surrounding Fe atoms (
Figure 4a,b). The SCR performance was linearly related to the number of active catalytic sites (ACS) (
Figure 4c−g). The redox ability of the binuclear W
1−Fe
1 site in Fe
1/WO
3 was much weaker than that in W
1/Fe
2O
3, which mainly led to an SCR performance of Fe
1/WO
3 that was inferior to that of W
1/Fe
2O
3 (
Figure 4g). Thus, modulating the acidity or redox properties of W
1/Fe
2O
3 and Fe
1/WO
3 SACs can improve the SCR activity [
106]. In addition, commercial catalysts are often inactivated due to the occupation of the active sites by the formed NH
4HSO
4 in the presence of SO
2. The acidic (Mo) sites and basic (Ti) sites in the Mo
1/TiO
2 catalyst adsorbed NH
4+ and HSO
42− in NH
4HSO
4, respectively, thus decomposing NH
4HSO
4 at 225 °C and releasing SO
2 at low temperatures without causing the poisoning of the catalyst [
96]. The exploration of the active sites for NO
x reduction and the reaction mechanisms under complex industrial conditions will help to develop high-performance catalysts.
Figure 4. (
a) AC-STEM image of Mo
1/Fe
2O
3, (
b) intensity surface plot and the corresponding structural model of the selected area, (
c–
e) EDX mappings and (
f) AC-STEM image of W
1/Fe
2O
3, (
g) SCR rates of W
1/Fe
2O
3 and Fe
1/WO
3 with different ACS numbers at 270 °C [
96].
This entry is adapted from the peer-reviewed paper 10.3390/catal12101239