Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Bismuth is mainly produced as a side product of lead streams and could be isolated through the Betterton–Kroll process or through an electrochemical procedure known as Betts electrolytic process. It is obtained in a highly purified form for those applications where it is used as a replacement for lead. Commonly, bismuth is used in form of halide, oxo-halide, nitrate and oxides derivatives. Bismuth use for biomedical applications is a very intriguing route to exploite the unique features of bimsmuth nanomaterials.
- bismuth oxide
- nanoparticles
- radiopacity
- chemotherapy
- theragnostic
1. Bismuth Based Nanomaterials as Biological Active Drugs
The first and main point to clarify is about the interaction between bismuth-based materials and living organisms. In 1989, Slikkerveer et al. [1] reported a very comprehensive overview of the toxicity of bismuth species. As clearly emerged, the oral intake of bismuth compounds leads to a significant increase in blood concentrations of bismuth [2] and the amount rose rapidly up to 380 µmL/g [3]. Gavey et al. [4] show how the uptake could be magnified by bismuth citrate soluble species or by the simultaneous administration of cysteine [5]. Lechat et al. [6] reported a study about the administration of bismuth subnitrate showing how poorly or watery insoluble bismuth species decrement the organism uptake. As reported by several studies run on rats using BiCl3 [7][8], bismuth binds to high molecular weight metallothionein protein close to those that bind copper cations [9]. Bismuth is excreted by both urine and feces but rats retain up to 10 wt.% of the dose administrated even after 90 days [10].
The in vivo tests suggest that bismuth salts or organometallic derivatives could lead to bioaccumulation and encephalopathy [11]. Stephens et al. [12] used homo- and heteroleptic bismuth(III) thiolates to prove that the bismuth complex surrounding drives the antimicrobial activity of the bismuth species.
Abudayyak et al. [13] studied bismuth oxide nanoparticles like the ones shown in Figure 1 regarding their cytotoxicity, genotoxicity, oxidative damage and ability to induce apoptosis in multiple tumoral cell lines (HepG2, NRK, Caco-2, A549).
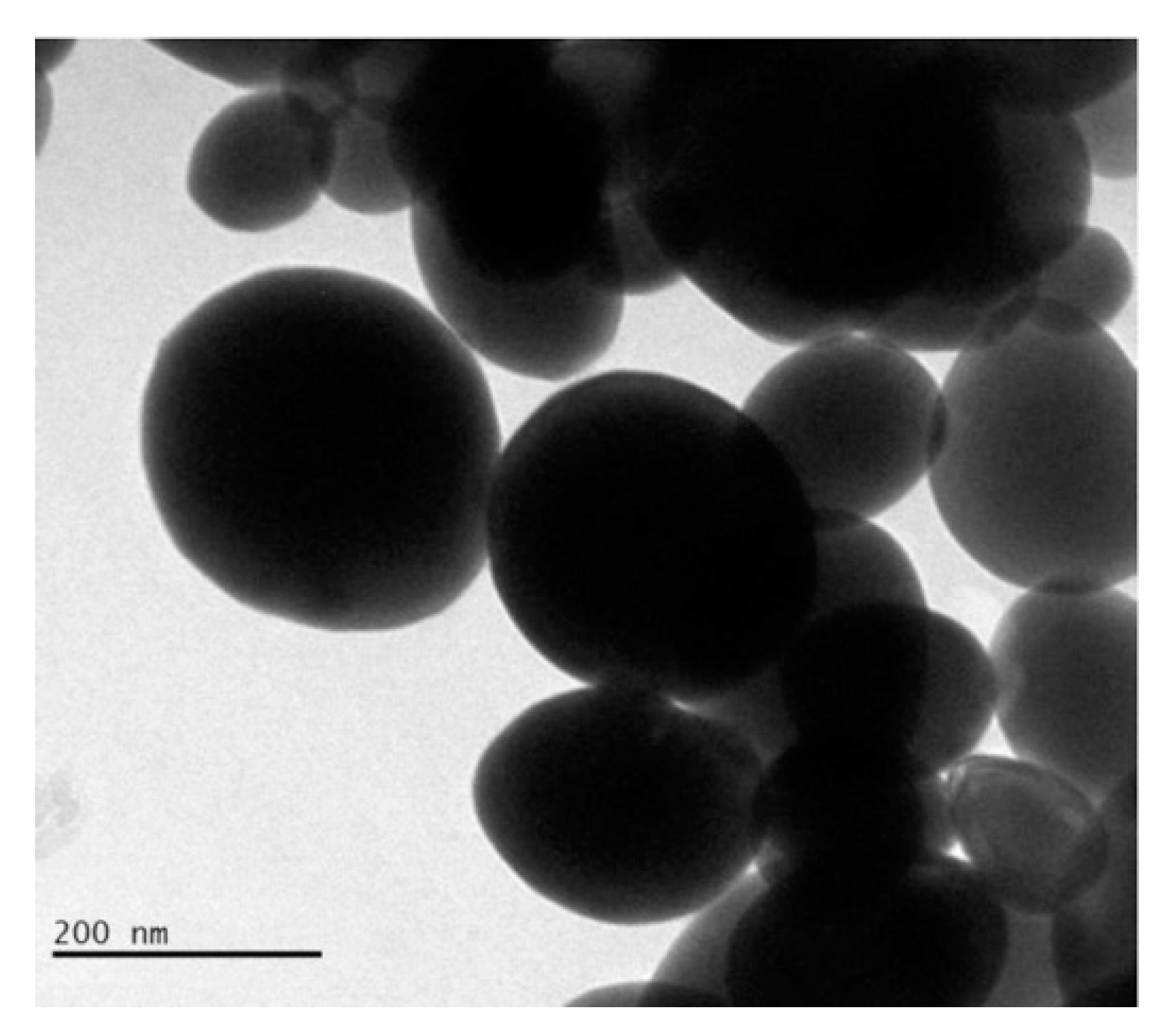
Figure 1. Transmission electronic microscopic capture of bismuth oxide nanoparticles with average diameter ranging from 150 nm to 200 nm. Picture is reprinted with permission from Abudayyak et al. [13].
Authors proved that bismuth oxide nanoparticles differently interacted with different cell lines inducing death through apoptosis in HepG2 and NRK-52E cells and through necrosis in A549 and Caco-2 cells. Among all morphologies, spherical nanoparticles are the most investigated but several studies [14][15][16] have proved that rod-like particles have a higher cellular uptake and transport across intestinal cells. As reported by Truong et al. [17], morphology is a key point to the rational design of biologically active species with cylindrical particles that are the most suitable for tumor accumulation [18]. Among bismuth species, spherical and sponge-like [19] shapes are the most common morphologies but BiONO3 [20] could be produced as road-like nanosized particles. Even if this material has been used only as precursors in inorganic synthesis [21] and for the realization of biosensors [20], it could represent an interesting material to improve the bismuth oxide material cellular uptaking.
Ahamed et al. [22] evaluated the effect of Bi2O3 accordingly to the scheme summarized in Figure 2 by using the MCF-7 cell line.
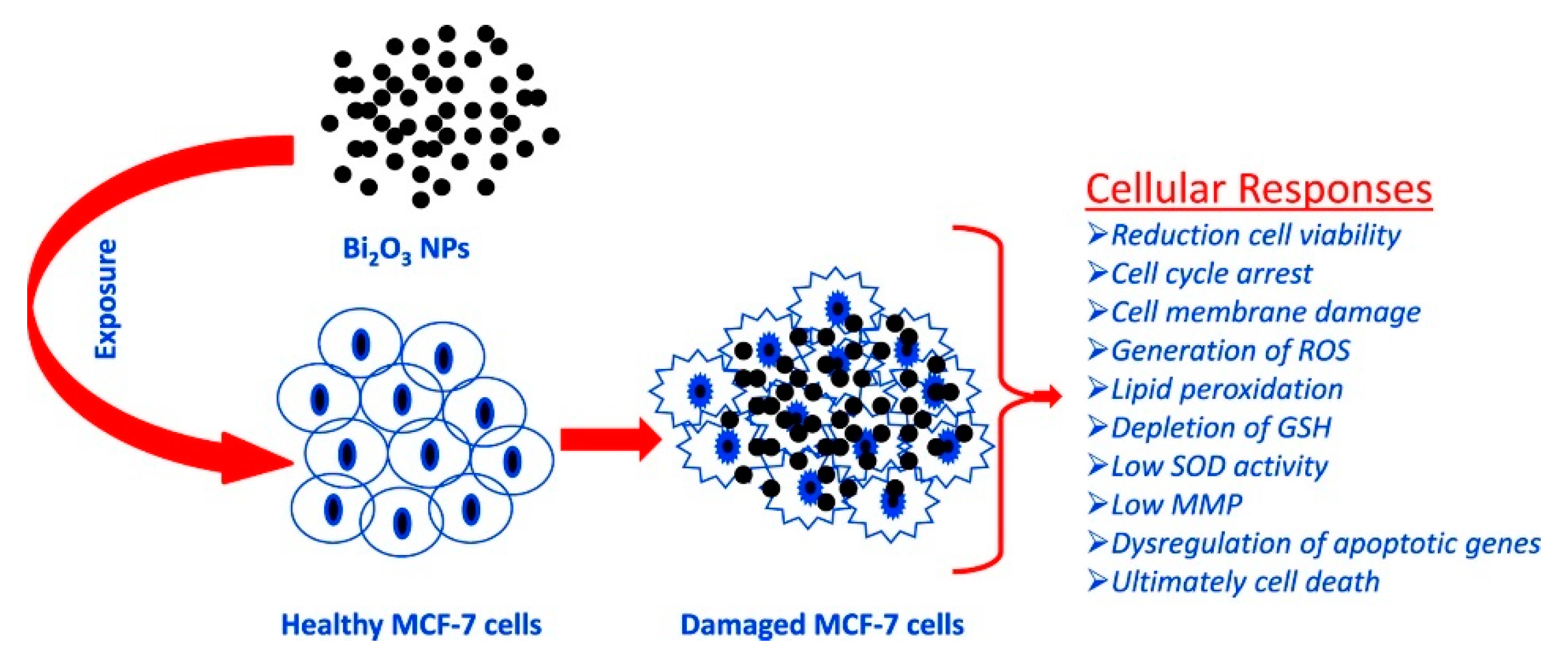
Figure 2. Summary of the process of oxidative stress induced by Bi2O3 in MCF-7. Picture is reprinted with permission from Ahamed et al. [22].
The bismuth oxide nanoparticles induced apoptotic response in MCF-7cells and suggested this occurs by undermining the regulation of Bcl-2, Bax and caspase-3 genes. Curiously, the authors observed that with the addition of the external antioxidant N-acetyl-cysteine, the bismuth cytotoxicity was almost inhibited. This suggests that the toxicity of bismuth could be tuned by tailoring the composition of the administered formulation.
Genotoxicity of Bi2O3 was also investigated by Liman [23] showing an unneglectable effect on root cells of Allium cepa. Even in combination with Portland cement [24] or other minerals [25], bismuth oxide shows a proved citoxicity and antimicrobial effects during in vivo tests.
Li et al. [26] studied the action mechanism of bismuth-based drugs for treating the Helicobacter Pylori infection by using pharmacology and metalloproteomics approaches. The authors described the efficacy of bismuth-based drugs as a consequence of bismuth ability to interrupt several biological pathways by perturbing the activity of key enzymes as shown in Figure 3.
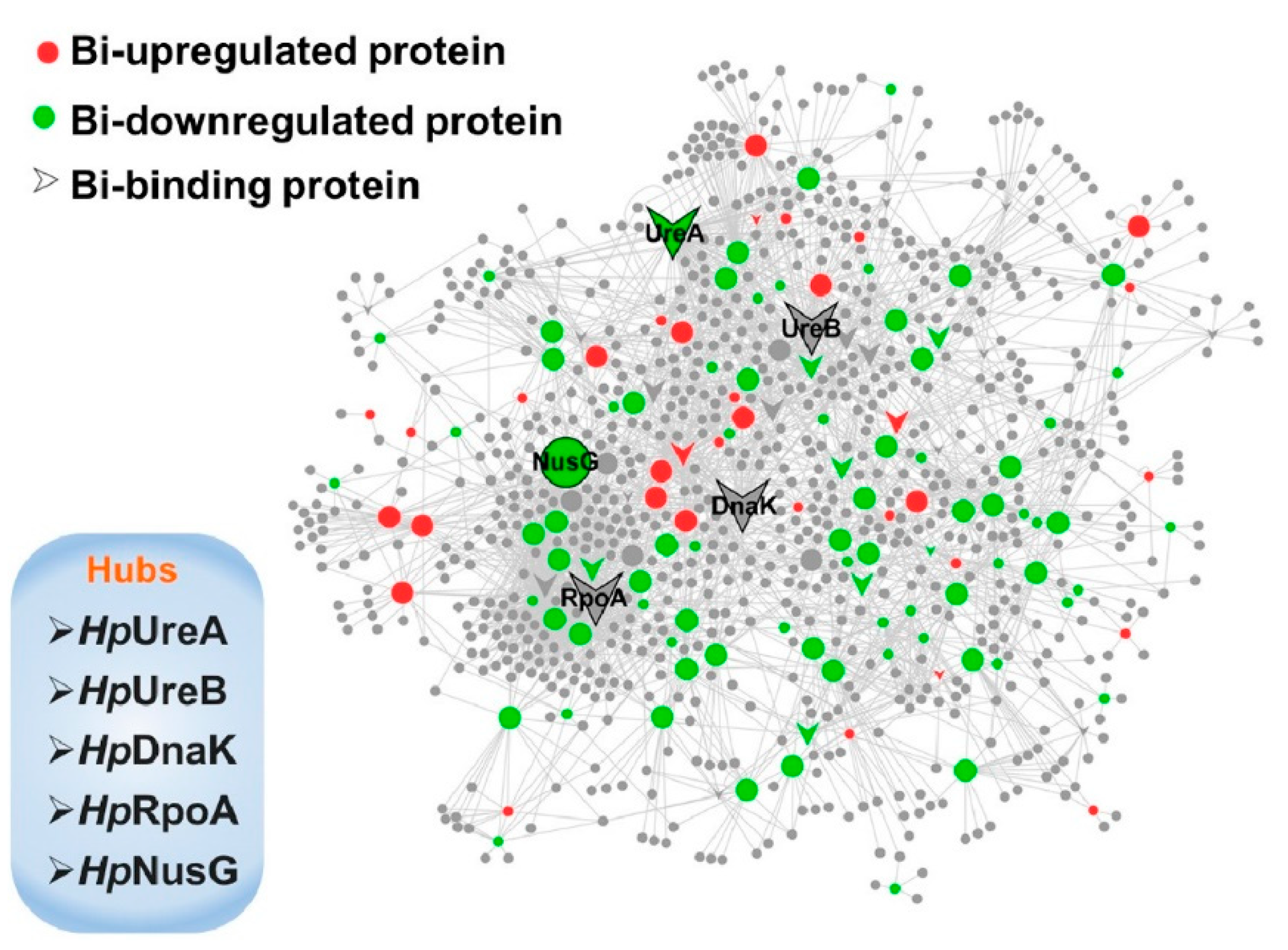
Figure 3. Schematic network depicting bismuth interaction with proteins in H. pylori. Proteins are colored and shaped according to their different properties in the network. Adapted with permission from Li et al. [26]. Copyright 2019 American Chemical Society.
Moving on from neat bismuth oxides, another interesting bioactive bismuth species is represented by bismuth oxohalides. Gao et al. [27] reported an in vitro study on the cytotoxicity of BiOCl nanosheets in human HaCaT keratinocytes. The authors reported negligible BiOCl cytotoxicity for concentrations lower than 0.5 µg/mL but the appreciable effect on cancerous cells for concentrations ranging from 5 µg/mL of up to 100 µg/mL. The authors related the cytotoxicity of BiOCl with changes in cell morphology and impairment of intracellular organules. Furthermore, BiOCl induced apoptosis through oxidative stress and eventually cells cycle arrest in G0/G1 phase.
Several proves have been reported on the combination of BiOI photocatalytic activity and antimicrobial effect as described by Jamil et al. [28] and outlined in Figure 4 for the inhibition of Escherichia coli.
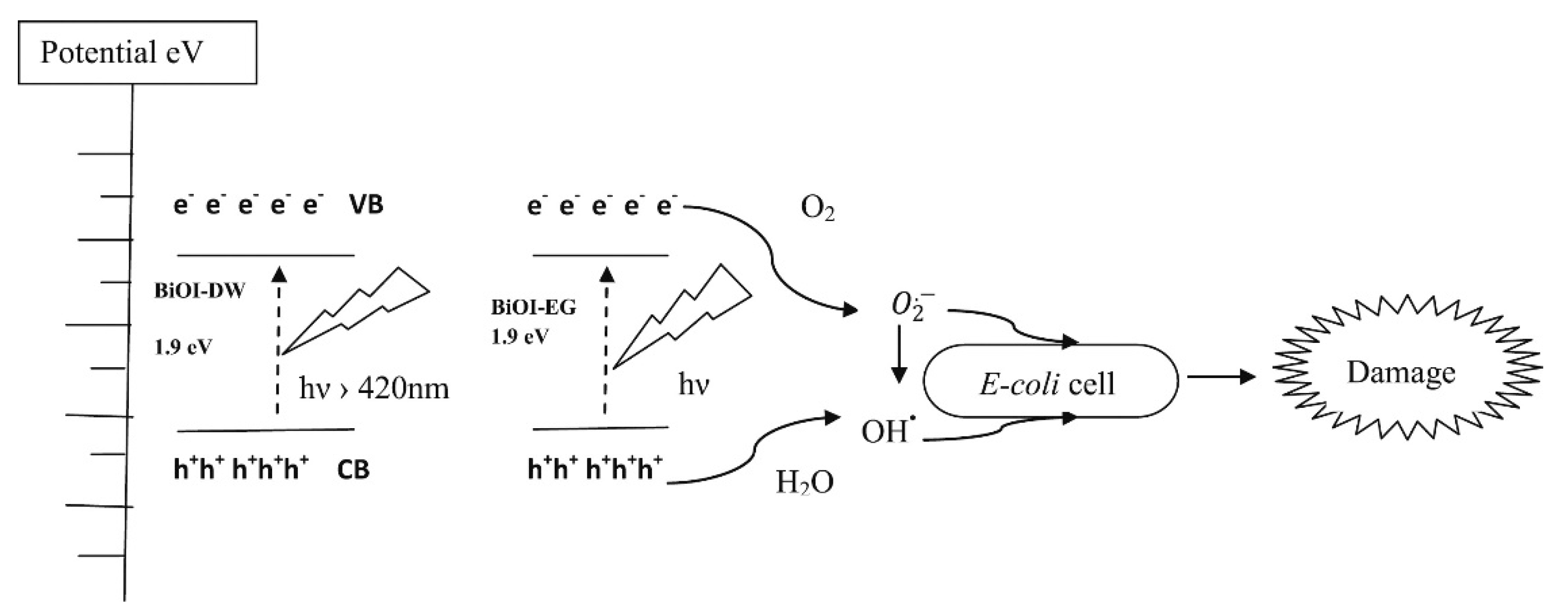
Figure 4. Schematic illustration of energy bands, electron–hole separation and damage mechanism for E. coli. Adapted with permission from Jamil et al. [28].
Direct effects of bismuth oxide and related materials on cell viability are not the only appreciable strategies that could base on these materials. Bismuth oxides could be used as effective radiosensitizers species. A radiosensitizer is a chemical that increases the radiation effect on cell viability. These chemotherapy agents are used during radiotherapy in combination with harmful radiation to damage the DNA of cells. As reported by Lawrence [29], radiosensitizing represents the greatest step forward in anticancer treatment and nanoparticle species are one of the most interesting materials for such aim [30]. In 2016, Stewart and co-workers [31] reported the first case of study of bismuth oxide nanoparticles as efficient radiosensitizers on highly radioresistant 9L gliosarcoma cell line. The authors exposed 9 L cells to a bismuth oxide nanoparticle concentration of up to 50 μg/mL achieving a sensitization enhancement of up to 1.5 and 1.3 by using an energy of 125 kV and 10 MV, respectively. Similarly, Liu et al. [32] combined radiotherapy and chemotherapy treatments by administration of mesoporous bismuth litchi-shaped Na0.2Bi0.8O0.35F1.91 as both radiosensitizer and as a nanovehicle for loading and slow-releasing doxorubicin. This bismuth oxide material combined with radiation and doxorubicin showed a remarkable synergistic ability for tumor elimination ability. Farahani et al. [33] combined the bismuth nanoparticles with polymer gel dosimetry technique testing their effect in kilovolt and Megavolt radiation therapy proving the strong energy dependence of dose enhancement.
2. Bismuth Based Nanomaterials as Additives for the Production of Biomaterials
Bismuth oxides and related materials are quite interesting for all the applications where a high radiopacity together with a good value of biocompatibility is required. Radiopacity is simply defined according to the following equation [34]:
where I(x) is the intensity of the attenuated radiation, I0 is the original radiation intensity, ρ is the mass density of the material, µ(ν) is the attenuation coefficient for a fixed radiation frequency and x is the length of the travelled path through the material.
For biological applications, radiopacity is measured by using the Hounsfield scale [35] according to the following equation
where µw is the attenuation coefficient of water and µa is the attenuation coefficient of air.
Bismuth based materials have raised great interest in the production of orthodontic cement due to a combination of biocompatibility, radiopacity and antimicrobial effects [36][37].
Similarly, several authors reported analogous results for dental repairing applications performed by using Portland and bismuth oxide composites proofing their reliability [38][39].
Furthermore, bismuth could be used for a tissue engineering application as reported by Pazarçeviren et al. [40]. The authors doped a composite made of 45S5 nanobioactive bioglass and graphene oxide with bismuth nanoparticles through a sol–gel methodology. By adding bismuth, authors increased both the composite density and the diametral tensile strength of up to 2.5% retaining cell viability. Additionally, bismuth oxides and related materials could be dispersed into a polymeric matrix to mitigate the effect of harmful radiations during the diagnostic procedures [41][42].
3. Bismuth Based Nanomaterials as Diagnostic Agents
Bismuth oxides and related materials are also used as contrast agents due to their radiopacity. Bi et al. [43] used poly(ethylenglycol) modified bismuth nanoparticles for applications as multifunctional probes during X-ray computed tomography (CT) and fluorescence imaging. The authors tested the in vivo circulation time and specific accumulation behavior in the liver and intestines by using a CT scan as shown in Figure 5.
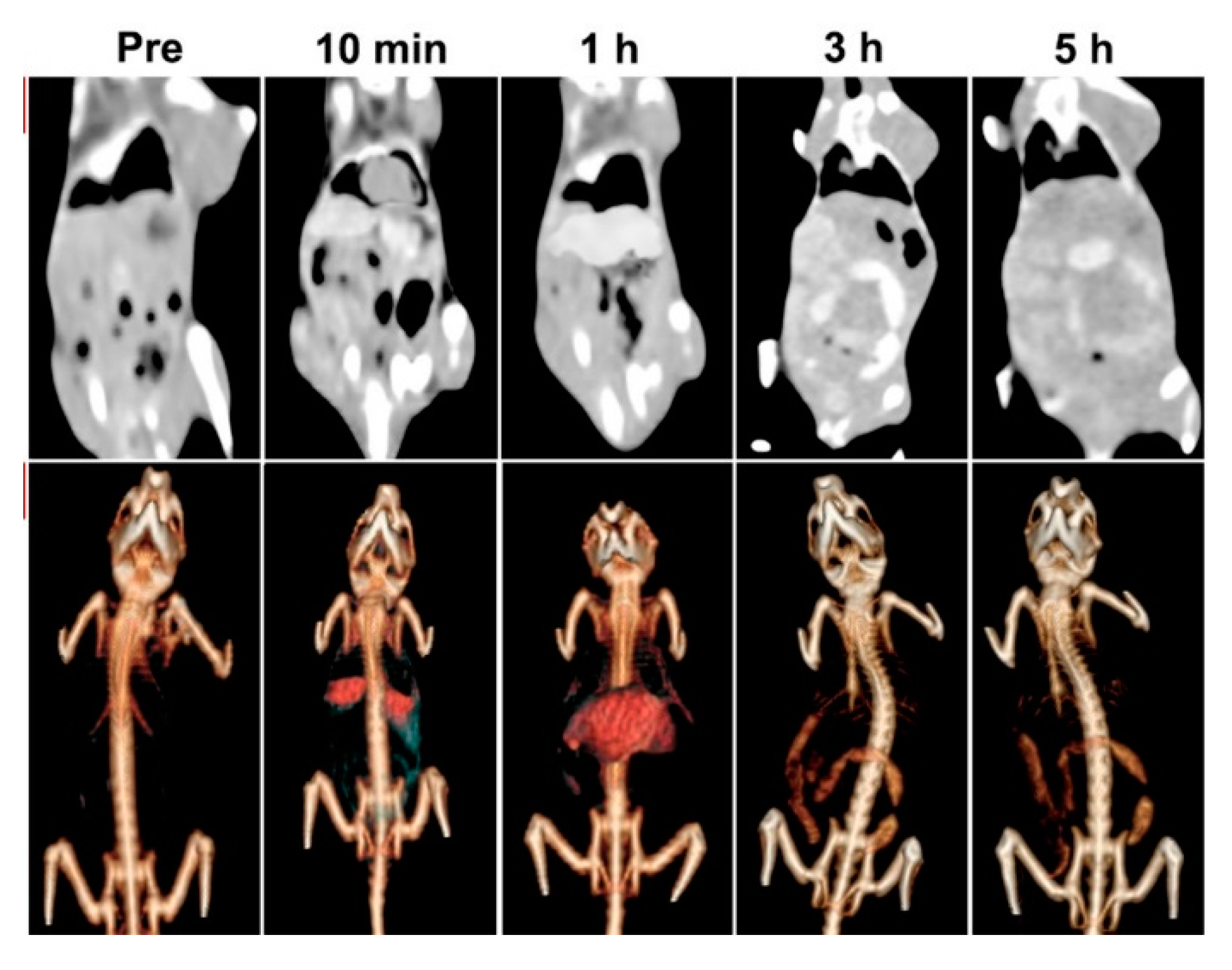
Figure 5. CT images and renderings of CT images of rat after the administration of bismuth modified nanoparticles after different times. Adapted with permission from Bi et al. [43]. Copyright 2018 American Chemical Society.
Results showed the possible applications of these formulations for target imaging and tracing of the specific areas where bismuth was preferentially accumulat
Several studies have proved the reliability of bismuth oxide as a CT contrast agent with similar or better performances compared with other oxides [44]. Brown et al. [45] developed an ultra-high payload metallic bismuth nanoparticle used as X-ray contrast agents. The authors showed that metallic bismuth nanoparticles will oxidatively decompose to biocompatible Bi(III) based species that are renal excreted after the CT analysis. Dadashi and co-workers [46] combined bismuth nanoparticles together with gold species producing aggregates of up to 40 nm in diameter demonstrating a higher X-ray attenuation in comparison with commercial iodine-based molecules.
Hu et al. [47] synthesized a nanostructured (BiO)2CO3 rod-like material through a solvothermal route and used it as a renal clearable CT contrast agent as shown in Figure 6.
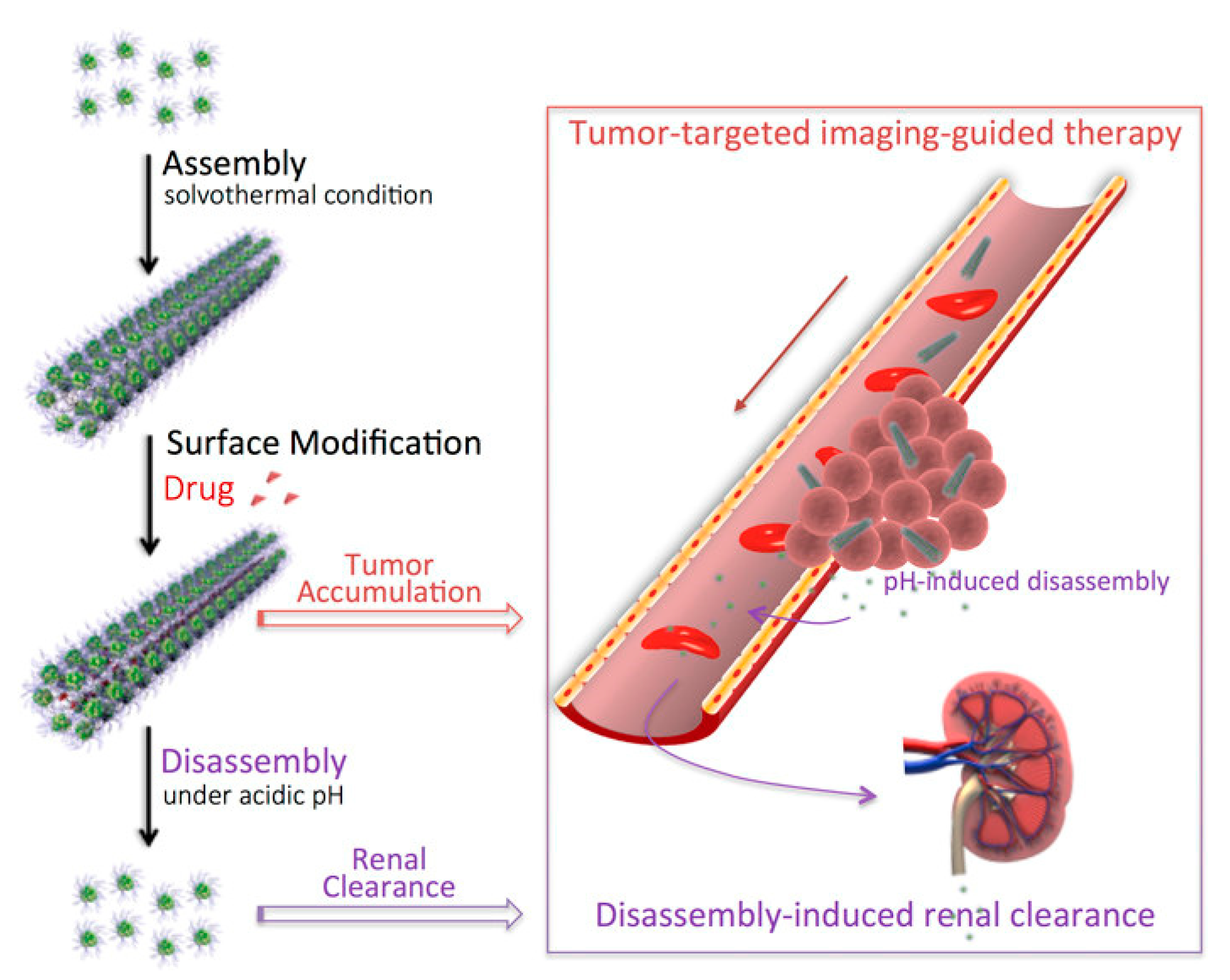
Figure 6. Production and biological pathway of bismuth subcarbonates rods as reported by [47]. Copyright 2018 American Chemical Society.
The authors efficiently used the bismuth subcarbonate as a high-resolution CT contrast agent proving that its high aspect ratio actively promoted take-up and retention in the rat tumors tested. The authors also reported the disassembling of the bismuth rods in the acidic microenvironment of tumors enhancing the renal clearance.
Naha et al. [48] reported the production of dextran-coated bismuth/iron oxide nanostructures for magnetic resonance (MR) applications. Results showed a decrement in T2-weighted MR contrast with increasing bismuth content in liver cells. The authors did not observe any cytotoxicity on Hep G2 and BJ5ta cell lines after 24 h incubation with the nanohybrids. Furthermore, the authors ran an in vivo test using mice observing a 2 h circulation time in heart and blood vessels of the bismuth contrast agent. Additionally, this bismuth-based contrast agent was rapidly excreted with urine.
Rivera et al. [49] encapsulated BiOCl into carbon nanostructures and tested it as an agent for X-ray imaging. The authors achieved a high contrast by using a low bismuth loading on nanocarbon (up to 2.7 wt.%) without compromising cell viability. Data enlightened a magnification of up to 500 times of CT resolution compared with traditional iodine-based agents.
BiOCl could be also used as support for the immobilization of aptameric tailored gold nanoparticles as reported by Hsu et al. [50]. This hybrid material showed high peroxidase-like activity and was used for the conversion of Amplex Red proteinic complex to resorufin. According to the authors, this was a very remarkable achievement that proved the robustness of bismuth bioconjugate in proteomic applications.
4. Bismuth Based Nanomaterials as Active Agents in Theragnostic Platforms
The combination of diagnostic procedures together with a therapeutic protocol is defined as theragnostic and represents the last frontier in advanced treatments [51]. Nanoscale theragnostic is a fast-growing branch of medicinal chemistry for simultaneously monitoring drug release and its distribution, and to evaluate the real-time therapeutic efficacy through a single nanoscale product for both treatment and diagnosis. As reported in the previous sections, bismuth materials are good and efficient contrast agents but could also be exploited for targeted cytotoxicity in vivo. The simultaneous effects herein mentioned led to the development of theragnostic platforms based on bismuth oxides and related materials.
Li et al. [52] developed a bovine serum albumin modified bismuth oxides nanoraspberries for multimodal imaging and chemo-photothermal combination therapy as summarized in Figure 7.
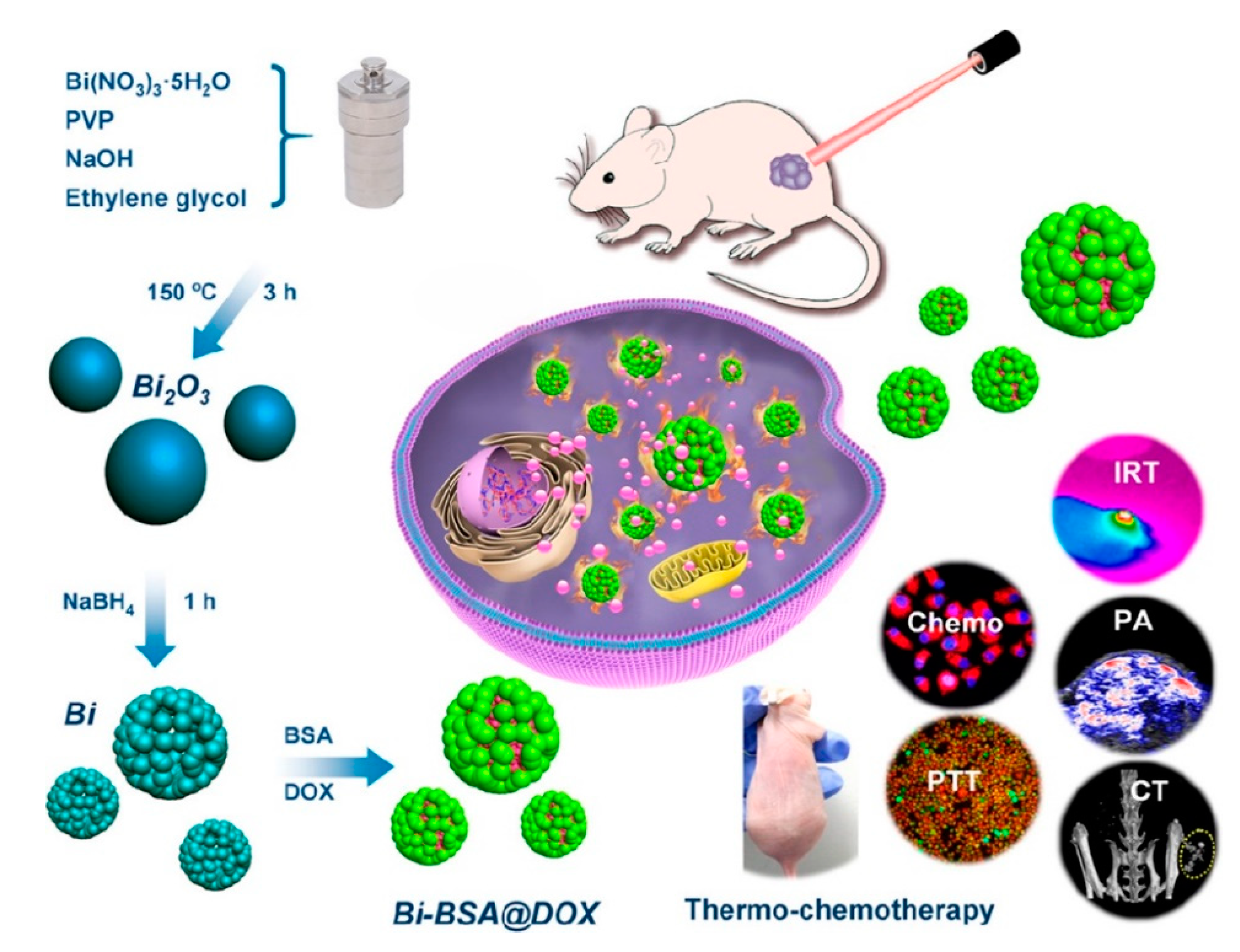
Figure 7. Production and biological action of bismuth oxide nanoraspberries species as reported by Li et al. [52]. Copyright 2018 American Chemical Society.
The authors synthesized the nanoparticles through a watery reduction by using sodium borhydride under pressure at 150 °C for 3 h. The synthesized material showed a surface area of up to 53 m2/g and a DOX drug loading of up to 69 wt.% with release occurring upon pH variations. The authors reported the bismuth-based theragnostic agent’s ability to efficiently convert near-infrared light to thermal energy for photothermal ablation of cancer cells. The toxicity studies proved the high biocompatibility without any appreciable toxicity to the mice tested. Additionally, the high radiopacity of bismuth raspberries allows the use of this formulation also during CT analysis. Lu et al. using a similar approach combined the radiopacity of bismuth nanoparticles with photothermal therapy. The authors were able to reach up to 70 °C after 4 min of infrared irradiation showing an enhancement in both CT imaging and in vitro suppression of glioma growth. Xuan et al. [53] prepared bismuth nanoparticles embedded into a nanohydrogel by ultraviolet light-mediated synthesis. The produced materials were combined with DOX and used simultaneously as a contrast agent, as a nanocarrier for drugs and for inducing cell death by thermal ablation. Analogously, Yang et al. [54] produced a bismuth-based CT contrast agent used in photothermal therapy and in ultrasound imaging. They used also several tailored approaches aimed to enhance the theragnostic effects of bismuth preparations. Yu et al. [55] described a thiol capping of bismuth nanoparticles that prevents the unwanted release of bismuth in the organism.
Bismuth oxides and related materials could be also combined with other species. Detappe et al. [56] produced a hybrid material by using ultrasmall silica-based bismuth and gadolinium nanoparticles for dual magnetic resonance and CT imaging while Badrigilan et al. [57] conjugated Bi2O3 with iron oxides to improve the photothermal behaviour leaving untouched the high bismuth radiopacity.
This entry is adapted from the peer-reviewed paper 10.3390/ma13225234
References
- Slikkerveer, A.; de Wolff, F.A. Pharmacokinetics and toxicity of bismuth compounds. Med. Toxicol. Advers. Drug Exp. 1989, 4, 303–323.
- Nwokolo, C.U.; Gavey, C.J.; Smith, J.T.L.; Pounder, R.E. The absorption of bismuth from oral doses of tripotassium dicitrato bismuthate. Aliment. Pharm. Ther. 1989, 3, 29–39.
- Conso, F. Bismuth Sanguin Et Urinaire Apres Traitement Bref Par Differents Sels Insolubles De Bismuth. Eur. J. Toxicol. 1975, 8, 137–141.
- Gavey, C.; Szeto, M.L.; Nwokolo, C.; Sercombe, J.; Pounder, R. Bismuth accumulates in the body during treatment with tripotassium dicitrato bismuthate. Aliment. Pharm. Ther. 1989, 3, 21–28.
- Chaleil, D. Augmentation des concentrations sanguines de bismuth par la cystéine chez le rat. Thérapie 1979, 34, 397–399.
- Lechat, P.; Majoie, B.; Levillain, R.; Cluzan, R.; Deleau, D. Étude de la toxicité à court terme de l’association sous-nitrate de bismuth et sorbitol. Therapie 1964, 19, 551–556.
- Szymanska, J.A.; Mogilnicka, E.M.; Kaszper, B.W. Binding of bismuth in the kidney of the rat. The role of metallothionein-like proteins. Biochem. Pharmacol. 1977, 26, 257–258.
- Szymanska, J.A.; Piotrowski, J.K. Studies to identify the low molecular weight bismuth-binding proteins in rat kidney. Biochem. Pharm. 1980, 29, 2913–2918.
- Żelazowski, A.J.; Piotrowski, J.K. Mercury-binding, copper-zinc proteins from rat kidney. Amino acid composition, molecular weight and metal content. Biochim. Et Biophys. Acta (Bba)-Protein Struct. 1980, 625, 89–99.
- Chaleil, D.; Regnault, J.; Allain, P.; Motta, R.; Raynaud, G. Action d’une flore microbienne méthanogène d’origine humaine sur l’absorption et la fixation du bismuth chez le rat. Ann. Pharm. Fr. 1988, 46, 133–137.
- Buge, A. 20 observations d’encéphalopathies aiguës avec myoclonies au cours de traitements oraux par les sels de bismuth. Ann. Méd. Interne 1974, 125, 877–888.
- Stephens, L.J.; Munuganti, S.; Duffin, R.N.; Werrett, M.V.; Andrews, P.C. Is Bismuth Really the “Green” Metal? Exploring the Antimicrobial Activity and Cytotoxicity of Organobismuth Thiolate Complexes. Inorg. Chem. 2020, 59, 3494–3508.
- Abudayyak, M.; Öztaş, E.; Arici, M.; Özhan, G. Investigation of the toxicity of bismuth oxide nanoparticles in various cell lines. Chemosphere 2017, 169, 117–123.
- Banerjee, A.; Qi, J.; Gogoi, R.; Wong, J.; Mitragotri, S. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J. Control. Release 2016, 238, 176–185.
- Mitragotri, S. In drug delivery, shape does matter. Pharm. Res. 2009, 26, 232–234.
- Christian, D.A.; Cai, S.; Garbuzenko, O.B.; Harada, T.; Zajac, A.L.; Minko, T.; Discher, D.E. Flexible filaments for in vivo imaging and delivery: Persistent circulation of filomicelles opens the dosage window for sustained tumor shrinkage. Mol. Pharm. 2009, 6, 1343–1352.
- Truong, N.P.; Whittaker, M.R.; Mak, C.W.; Davis, T.P. The importance of nanoparticle shape in cancer drug delivery. Expert Opin. Drug Deliv. 2015, 12, 129–142.
- Dickerson, E.B.; Dreaden, E.C.; Huang, X.; El-Sayed, I.H.; Chu, H.; Pushpanketh, S.; McDonald, J.F.; El-Sayed, M.A. Gold nanorod assisted near-infrared plasmonic photothermal therapy (PPTT) of squamous cell carcinoma in mice. Cancer Lett. 2008, 269, 57–66.
- Gadhi, T.A.; Hernández, S.; Castellino, M.; Jagdale, P.; Husak, T.; Hernández-Gordillo, A.; Tagliaferro, A.; Russo, N. Insights on the role of β-Bi2O3/Bi5O7NO3 heterostructures synthesized by a scalable solid-state method for the sunlight-driven photocatalytic degradation of dyes. Catal. Today 2019, 321–322, 135–145.
- Liu, G.-Q.; Zhong, H.; Li, X.-R.; Yang, K.; Jia, F.-F.; Cheng, Z.-P.; Zhang, L.-L.; Yin, J.-Z.; Guo, L.-P.; Qian, H.-Y. Research on nonenzymatic electrochemical sensor using HO-BiONO3 nanocomposites for glucose detection. Sens. Actuators B 2017, 242, 484–491.
- Zhou, C.; Cao, J.; Lin, H.; Xu, B.; Huang, B.; Chen, S. Controllable synthesis and photocatalytic activity of Ag/BiOI based on the morphology effect of BiOI substrate. Surf. Coat. Technol. 2015, 272, 213–220.
- Ahamed, M.; Akhtar, M.J.; Khan, M.A.M.; Alrokayan, S.A.; Alhadlaq, H.A. Oxidative stress mediated cytotoxicity and apoptosis response of bismuth oxide (Bi2O3) nanoparticles in human breast cancer (MCF-7) cells. Chemosphere 2019, 216, 823–831.
- Liman, R. Genotoxic effects of Bismuth (III) oxide nanoparticles by Allium and Comet assay. Chemosphere 2013, 93, 269–273.
- Zeferino, E.; Bueno, C.S.; Oyama, L.; Ribeiro, D. Ex vivo assessment of genotoxicity and cytotoxicity in murine fibroblasts exposed to white MTA or white Portland cement with 15% bismuth oxide. Int. Endod. J. 2010, 43, 843–848.
- Camilleri, J.; Montesin, F.E.; Papaioannou, S.; McDonald, F.; Pitt Ford, T.R. Biocompatibility of two commercial forms of mineral trioxide aggregate. Int. Endod. J. 2004, 37, 699–704.
- Li, H.; Wang, R.; Sun, H. Systems Approaches for Unveiling the Mechanism of Action of Bismuth Drugs: New Medicinal Applications beyond Helicobacter Pylori Infection. Acc. Chem. Res. 2019, 52, 216–227.
- Gao, X.; Zhang, X.; Wang, Y.; Wang, Y.; Peng, S.; Fan, C. An in vitro study on the cytotoxicity of bismuth oxychloride nanosheets in human HaCaT keratinocytes. Food Chem. Toxicol. 2015, 80, 52–61.
- Jamil, T.S.; Mansor, E.S.; Azab El-Liethy, M. Photocatalytic inactivation of E. coli using nano-size bismuth oxyiodide photocatalysts under visible light. J. Environ. Chem. Eng. 2015, 3, 2463–2471.
- Lawrence, T.S.; Blackstock, A.W.; McGinn, C. The mechanism of action of radiosensitization of conventional chemotherapeutic agents. Semin. Radiat. Oncol. 2003, 13, 13–21.
- Brun, E.; Sicard-Roselli, C. Actual questions raised by nanoparticle radiosensitization. Radiat. Phys. Chem. 2016, 128, 134–142.
- Stewart, C.; Konstantinov, K.; McKinnon, S.; Guatelli, S.; Lerch, M.; Rosenfeld, A.; Tehei, M.; Corde, S. First proof of bismuth oxide nanoparticles as efficient radiosensitisers on highly radioresistant cancer cells. Phys. Med. 2016, 32, 1444–1452.
- Liu, J.; Deng, Y.; Qin, X.; Li, B.; Zhang, J.; Xu, Y.; Ouyang, R.; Li, Y.; Miao, Y.; Sun, Y. Ultrafast Synthesizing Bismuth Mesoporous Nanolitchi Radiosensitizer Loading High Dose DOX for CT-Guided Enhanced Chemoradiotherapy. Acs Appl. Mater. Interfaces 2019, 11, 42932–42942.
- Farahani, S.; Riyahi Alam, N.; Haghgoo, S.; Shirazi, A.; Geraily, G.; Gorji, E.; Kavousi, N. The effect of bismuth nanoparticles in kilovoltage and megavoltage radiation therapy using magnetic resonance imaging polymer gel dosimetry. Radiat. Phys. Chem. 2020, 170, 108573.
- McNaught, A.D.; Wilkinson, A. Attenuation Coefficient; Blackwell Science Oxford: Oxford, UK, 1997; Volume 1669.
- DenOtter, T.D.; Schubert, J. Hounsfield Unit. In StatPearls ; StatPearls Publishing: Treasure Island, FL, USA, 2019.
- Deb, S.; Abdulghani, S.; Behiri, J. Radiopacity in bone cements using an organo-bismuth compound. Biomaterials 2002, 23, 3387–3393.
- Chen, F.; Liu, C.; Mao, Y. Bismuth-doped injectable calcium phosphate cement with improved radiopacity and potent antimicrobial activity for root canal filling. Acta Biomater. 2010, 6, 3199–3207.
- Hwang, Y.-C.; Lee, S.-H.; Hwang, I.-N.; Kang, I.-C.; Kim, M.-S.; Kim, S.-H.; Son, H.-H.; Oh, W.-M. Chemical composition, radiopacity, and biocompatibility of Portland cement with bismuth oxide. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, e96–e102.
- Kim, E.-C.; Lee, B.-C.; Chang, H.-S.; Lee, W.; Hong, C.-U.; Min, K.-S. Evaluation of the radiopacity and cytotoxicity of Portland cements containing bismuth oxide. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 105, e54–e57.
- Pazarçeviren, A.E.; Tahmasebifar, A.; Tezcaner, A.; Keskin, D.; Evis, Z. Investigation of bismuth doped bioglass/graphene oxide nanocomposites for bone tissue engineering. Ceram. Int. 2018, 44, 3791–3799.
- Jagdale, P.; Rovere, M.; Ronca, R.; Vigneri, C.; Bernardini, F.; Calzetta, G.; Tagliaferro, A. Determination of the X-ray attenuation coefficient of bismuth oxychloride nanoplates in polydimethylsiloxane. J. Mater. Sci. 2020, 55, 7095–7105.
- Mehnati, P.; Arash, M.; Akhlaghi, P. Bismuth-silicon and bismuth-polyurethane composite shields for breast protection in chest computed tomography examinations. J. Med. Phys. 2018, 43, 61.
- Bi, H.; He, F.; Dong, Y.; Yang, D.; Dai, Y.; Xu, L.; Lv, R.; Gai, S.; Yang, P.; Lin, J. Bismuth Nanoparticles with “Light” Property Served as a Multifunctional Probe for X-ray Computed Tomography and Fluorescence Imaging. Chem. Mater. 2018, 30, 3301–3307.
- Ghazanfari, A.; Marasini, S.; Miao, X.; Park, J.A.; Jung, K.-H.; Ahmad, M.Y.; Yue, H.; Ho, S.L.; Liu, S.; Jang, Y.J.; et al. Synthesis, characterization, and X-ray attenuation properties of polyacrylic acid-coated ultrasmall heavy metal oxide (Bi2O3, Yb2O3, NaTaO3, Dy2O3, and Gd2O3) nanoparticles as potential CT contrast agents. Colloids Surf. A 2019, 576, 73–81.
- Brown, A.L.; Naha, P.C.; Benavides-Montes, V.; Litt, H.I.; Goforth, A.M.; Cormode, D.P. Synthesis, X-ray Opacity, and Biological Compatibility of Ultra-High Payload Elemental Bismuth Nanoparticle X-ray Contrast Agents. Chem. Mater. 2014, 26, 2266–2274.
- Dadashi, S.; Poursalehi, R.; Delavari, H. Optical and structural properties of oxidation resistant colloidal bismuth/gold nanocomposite: An efficient nanoparticles based contrast agent for X-ray computed tomography. J. Mol. Liq. 2018, 254, 12–19.
- Hu, X.; Sun, J.; Li, F.; Li, R.; Wu, J.; He, J.; Wang, N.; Liu, J.; Wang, S.; Zhou, F.; et al. Renal-Clearable Hollow Bismuth Subcarbonate Nanotubes for Tumor Targeted Computed Tomography Imaging and Chemoradiotherapy. Nano Lett. 2018, 18, 1196–1204.
- Naha, P.C.; Al Zaki, A.; Hecht, E.; Chorny, M.; Chhour, P.; Blankemeyer, E.; Yates, D.M.; Witschey, W.R.T.; Litt, H.I.; Tsourkas, A.; et al. Dextran coated bismuth–iron oxide nanohybrid contrast agents for computed tomography and magnetic resonance imaging. J. Mater. Chem. B 2014, 2, 8239–8248.
- Rivera, E.J.; Tran, L.A.; Hernández-Rivera, M.; Yoon, D.; Mikos, A.G.; Rusakova, I.A.; Cheong, B.Y.; Cabreira-Hansen, M.d.G.; Willerson, J.T.; Perin, E.C.; et al. as a potential contrast agent for X-ray imaging applications. J. Mater. Chem. B 2013, 1, 4792–4800.
- Hsu, C.-L.; Lien, C.-W.; Wang, C.-W.; Harroun, S.G.; Huang, C.-C.; Chang, H.-T. Immobilization of aptamer-modified gold nanoparticles on BiOCl nanosheets: Tunable peroxidase-like activity by protein recognition. Biosens. Bioelectron. 2016, 75, 181–187.
- Pene, F.; Courtine, E.; Cariou, A.; Mira, J.-P. Toward theragnostics. Crit. Care Med. 2009, 37, S50–S58.
- Li, Z.; Hu, Y.; Miao, Z.; Xu, H.; Li, C.; Zhao, Y.; Li, Z.; Chang, M.; Ma, Z.; Sun, Y. Dual-stimuli responsive bismuth nanoraspberries for multimodal imaging and combined cancer therapy. Nano Lett. 2018, 18, 6778–6788.
- Xuan, Y.; Song, X.-L.; Yang, X.-Q.; Zhang, R.-Y.; Song, Z.-Y.; Zhao, D.-H.; Hou, X.-L.; An, J.; Zhang, X.-S.; Zhao, Y.-D. Bismuth particles imbedded degradable nanohydrogel prepared by one-step method for tumor dual-mode imaging and chemo-photothermal combined therapy. Chem. Eng. J. 2019, 375, 122000.
- Yang, C.; Guo, C.; Guo, W.; Zhao, X.; Liu, S.; Han, X. Multifunctional Bismuth Nanoparticles as Theranostic Agent for PA/CT Imaging and NIR Laser-Driven Photothermal Therapy. ACS Appl. Nano Mater. 2018, 1, 820–830.
- Yu, N.; Wang, Z.; Zhang, J.; Liu, Z.; Zhu, B.; Yu, J.; Zhu, M.; Peng, C.; Chen, Z. Thiol-capped Bi nanoparticles as stable and all-in-one type theranostic nanoagents for tumor imaging and thermoradiotherapy. Biomaterials 2018, 161, 279–291.
- Detappe, A.; Thomas, E.; Tibbitt, M.W.; Kunjachan, S.; Zavidij, O.; Parnandi, N.; Reznichenko, E.; Lux, F.; Tillement, O.; Berbeco, R. Ultrasmall Silica-Based Bismuth Gadolinium Nanoparticles for Dual Magnetic Resonance–Computed Tomography Image Guided Radiation Therapy. Nano Lett. 2017, 17, 1733–1740.
- Badrigilan, S.; Shaabani, B.; Gharehaghaji, N.; Mesbahi, A. Iron oxide/bismuth oxide nanocomposites coated by graphene quantum dots: “Three-in-one” theranostic agents for simultaneous CT/MR imaging-guided in vitro photothermal therapy. Photodiagn. Photodyn. Ther. 2019, 25, 504–514.
This entry is offline, you can click here to edit this entry!
