Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Chemistry, Applied
Boron and boron compounds have been extensively studied together in the history and development of lithium batteries, which are crucial to decarbonization in the automotive industry and beyond. As early as the year 2000, lithium bis(oxalato)borate, also known as LiBOB, was first reported for synthesis and use in electrolytes of LIBs. Because of the merits of being halide-free and more thermally stable than LiPF6, LiBOB has been proven beneficial either as a direct replacement for LiPF6 or as an electrolyte additive (2 wt%) in a nickel cobalt aluminum (NCA)—graphite system.
- boron
- boron compounds
- boric acid
- lithium battery
- lithium-ion batteries
1. Introduction to Boron and Lithium
During decarbonization, the trend of electrification continues to intensify and will drive the demand for batteries, especially for electric vehicles (EVs), where lithium plays a critical role. The challenge to improve battery performance has made boron, in various forms of compounds, a research topic in relation to lithium-ion batteries (LIBs) for decades.
Boron and lithium are similar elements in some ways. They are both considered light elements and less abundant in both present crustal concentrations and, indeed, in the universe. Their formation requires a further enrichment process that involves continental crust growth [1,2]. Furthermore, the extraction of lithium and boron together is common in existing mining operations and some prospective greenfield projects. Application wise, both elements could be used as flux in the making of vitreous materials, such as glass.
Why is boron used in batteries? Boron is a unique element in many respects. Firstly, boron is lightest element of the “metalloids”, which separates metals and non-metals in the periodic table. The fact that many boron compounds are electron deficient means that they have Lewis acidic characteristics. The four covalent bonds and the variety in the molecular structures of its compounds result in a wide range of chemical properties [3]. Consequently, its applications in urbanization are extremely diverse: glass and ceramics, oil and gases, polymer fire retardants, wood preservatives, fertilizers in agriculture, and many more. The benefit of using boron is often multifaceted. For instance, when sodium borate pentahydrate is used in fiberglass, it brings a unique combination of benefits to both the melting process and fiber properties; when disodium octaborate tetrahydrate (DOT) is used as an indoor wood preservative, it offers deep penetration into wood and residential benefits (such as low mammal toxicity).
2. Boron Used in Electrolytes
Battery mainly consists of four major components, which are cathodes, anodes, the electrolyte, and separators. Figure 1 shows the schematics of these components in conventional Li ion batteries (LIBs) and the movement of electrons, ions, and current flow under charging and discharging condition.
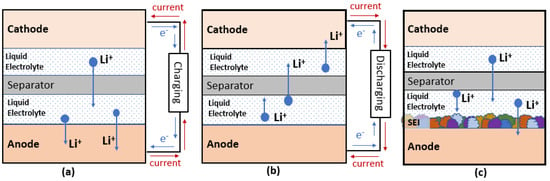
Figure 1. Schematic diagram of LIBs for (a) charging (b) discharging and (c) formation of SEI.
2.1. Electrolyte Additives—LiBOB
As early as the year 2000, lithium bis(oxalato)borate, also known as LiBOB, was first reported for synthesis and use in electrolytes of LIBs [4]. The presence of bis(oxalate)borate (BOB) anions helps the formation of a solid electrolyte interface (SEI) (Figure 1c), which is critical for the stability and long-term cyclability of graphitic anodes [5]. Because of the merits of being halide-free and more thermally stable than LiPF6, LiBOB has been proven beneficial either as a direct replacement for LiPF6 or as an electrolyte additive (2 wt%) in a nickel cobalt aluminum (NCA)—graphite system [4,6]. Moreover, LiBOB was studied as an enabler for using propylene carbonate (PC) as a solvent [6]. Although it was beneficial to the NCA cathode system, research revealed that Co could play a role in catalyzing the decompositions of BOB anions on cathode surfaces, which explained the inferior performance of LiBOB in lithium cobalt oxide (LCO) cathodes and nickel manganese cobalt (NMC) cathodes. Another study found that one disadvantage of using LiBOB was that SEI could become too resistive to perform well at sub-zero temperatures [7].
2.2. Electrolyte Additives—TPFPB and TB
Regarding the interface on the cathode side, tris(pentafluorophenyl)borane (TPFPB) can be added into the electrolyte as a film stabilizer on the cathode, which improves the power capability in lithium manganese nickel (LMN), NMC333, and LiFePO4 (LFP) cathodes [8,9,10]. Similarly, adding <0.1 M of trimethyl borate (TB) or 0.1 M TPFPB to LiPF6 was found to be effective at suppressing the thermal breakdown of electrolytes and promoting the establishment of a protective film on LFP, hence improving the cyclic stability at 55–60 °C [11,12]. TB with 10 wt% was also found to be beneficial to the cyclic stability and coulombic efficiency in NMC333 [13].
2.3. Electrolyte Additives—LiBF4
Around the time of the first report of LiBOB, LiBF4 was found to have lower conductivity but better low-temperature performance than LiPF6 [14]. Later in LIB development, LiBF4 salt in carbonate electrolytes was tested as a direct replacement for LiPF6, which improved capacity retention in NMC622 and NMC442 systems, due to a more stable B-F bond compared to the P-F bond [15,16,17]. When only used as an additive to LiPF6, 1 to 2 wt% of LiBF4 significantly enhanced capacity retention in the NMC532 system, suggesting the likely mechanism that LiBF4 contributed to the formation of SEI on both electrodes [18]. It is worth noting that one study suggested that boron trifluoride (BF3), which is the decomposed product from LiBF4, was a typical Lewis acid and could dissolve the decomposition product LiF from LiPF6 [19]. Harnessing the BF3 functionality mechanism, pyridine-boron trifluoride (PBF) was tested as an additive. A 3 wt% dosage was able to enhance capacity retention and preserve low impedance in NMC/graphite systems, such as NMC111 and NMC442 [19]. Similar effectiveness was also found in a pouch cell experiment on NMC532 and NMC622 [20]. A combination of using PBF as additives and LiBF4 as electrolytes for NMC442 provided incremental benefits compared to using LiBF4 as electrolytes [15].
2.4. Solid-State Electrolyte—Boron Nitride & Boric Acid
To advance the safety aspect of LIBs, solid electrolytes were also extensively studied for their mechanical stability and characteristic of lower flammability. Several combinations for solid electrolytes exist, but they usually contain inorganic and polymer materials. The typical challenge of using solid electrolytes, however, relates to the relatively inferior ionic conductivity and high interface resistance with electrodes [21]. Boron nitride has been commonly studied because of its physical, thermal, and electrical properties [22].
Studies revealed that boron nitride (BN) could be made into a sheet-form hosting structure for ionic liquids (ILs) containing solid electrolytes; as a result, low-temperature conductivity was increased [21]. Other studies showed that adding 1% BN as additives could improve the cycling performance in polymer/salt hybrid electrolytes [23]. Hexagonal boron nitride (h-BN) was also studied to improve cycling performance at a temperature of up to 175 °C [24]. It is also worth noting that, even in non-solid-state electrolytes, adding h-BN was proven to be effective at improving thermal stability and cycling performance [25]. It is also interesting to note that light elements similar to boron, such as fluorine, were also commonly studied as doping elements in solid state batteries to improve stability [26,27].
Li metal batteries (LMBs), that utilize lithium as anodes, have the advantage of a higher energy density. Their use has been commonly studied together with solid-state electrolytes. The challenges of using Li metal concern Li reactivity and the formation of Li dendrites [28]. A study showed that BN could be used as a coating on solid-state electrolytes of LMBs to stabilize the electrolyte–anode interface and hence improve cycling performance in LFP systems [29]. Other studies showed that cycling performance could be improved by adding 1% BN to ceramic electrolytes [30]; adding hexagonal boron nitride (h-BN) to polymer composite electrolytes of LFP [31]; adding amine-functionalized boron nitride nanosheets to gel polymer electrolytes of LFP and LCO [28]; or adding hexagonal boron nitride nanosheets (h-BNNS) to a composite polymer on LFP [32]. An alternative route to electrolyte additives was found to use a boric acid treatment on a Li anode to form a lithium borate layer that suppressed dendrite growth and enhanced cycling performance in LMO/Li systems [33]. Using boric acids as additives was also found to successfully suppress dendrite growth, stabilizing interfacial film on the Li-metal anode in various LMBs systems [34,35].
This entry is adapted from the peer-reviewed paper 10.3390/batteries8100187
This entry is offline, you can click here to edit this entry!
