Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Tetrodotoxin (TTX), one of the deadliest natural toxins, has attracted the interest of researchers from various fields for decades. This non-protein, weakly basic, heat-resistant low-molecular-weight toxin selectively blocks voltage-gated sodium channels along the muscle and nerve cells.
- biotoxin
- tetrodotoxin
- TTX
- TTX-bearing animals
1. Actinopterygii
The first experiments that led to the discovery of the toxins in the ovaries of fish belonging to the order Tetraodontiformes were conducted from the end of the 19th century to the middle of the 20th century [1]. The isolation of TTX and elucidation of its chemical structure led to the first large-scale toxicity studies of pufferfish inhabiting Japan [2][3]. Species of the genus Takifugu, particularly Takifugu pardalis, Takifugu flavipterus (Takifugu poecilonotus), and Takifugu alboplumbeus (Takifugu niphobles), are highly toxic. In subsequent years, TTX was found among most genera of the family Tetraodontidae dwelling in different countries and regions. High levels of toxicity have been observed in species of the genera Arothron, Amblyrhynchotes, Chelonodon, Dichotomyctere, Lagocephalus, and Sphoeroides [4][5][6][7][8][9]. TTX is also found in the species Yongeichthys criniger (Gobius criniger) of the order Gobiiformes [10][11][12][13].
In previous studies, the authors reported either the general toxicity of the animal [14][15] or the toxicity of its ovaries [16]. Since the 1980s, the toxicity to individual organs and tissues has been studied. Researchers have mainly focused on the skin, muscles, intestines, gonads, and liver (or hepatopancreas, according to Sato et al. [17]); the fins, spleen, blood, and gills have been studied less extensively. Many pufferfish species have high concentrations of TTX in the ovaries, followed by the liver and/or skin; the remaining organs contain small amounts of TTX [3][4][7][18][19][20][21][22][23][24][25][26][27] (Figure 1). In T. alboplumbeus, the ventral skin is more toxic than the dorsal [21]. Comparative toxicity analysis of the fins and skin of Takifugu vermicularis, Takifugu snyderi, and Takifugu porphyreus showed that these tissues in some species had similar levels of toxicity [28]. In Chelonodon patoca, the highest toxicity is found in the skin; the muscles, liver, and ovaries of fish contain lower levels of TTX [23]. The brackish and freshwater species of the genera Dichotomyctere and Pao also have high concentrations of TTX in the skin compared to that in other organs [29][30] High concentrations of TTX can be found in the skin mucus of Arothron meleagris and Sphoeroides lispus [6]. In several species of the genus Lagocephalus, the gastrointestinal tract is the most toxic organ [9][31]. Artificially reared T. rubripes contain low concentrations of TTX accumulated in the ovaries and liver, similar to the observations in wild specimens [26][32]. Sato et al. [33] reported trace amounts of TTX in the guts of cultured T. rubripes. However, some studies reported the absence of toxins in cultured pufferfish [34][35].
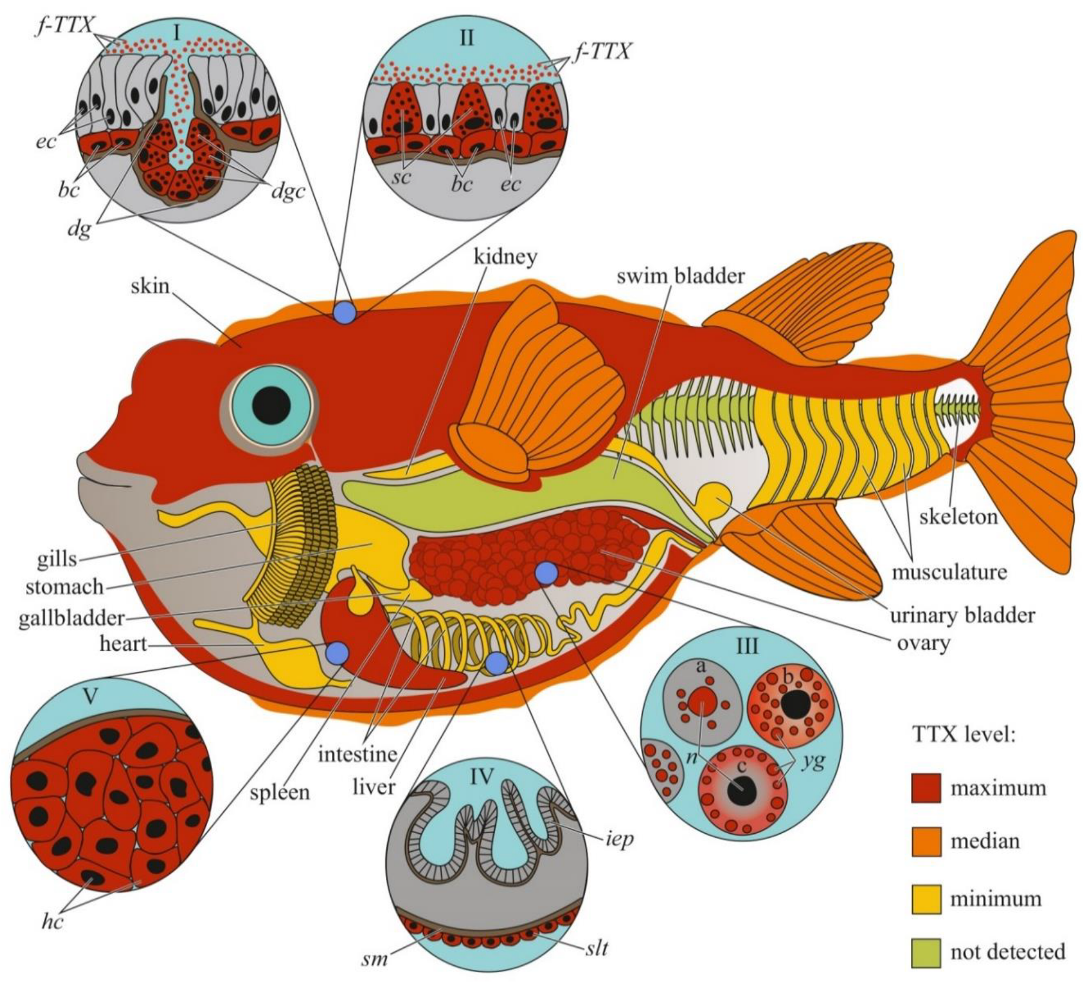
Figure 1. Schematic illustration of the levels and intra-organismal distribution of tetrodotoxin (TTX) in the adult pufferfish (family Tetraodontidae). Red color on the insets indicates TTX-positive cells. I—Skin with dermal gland (dg). II—Skin with singly scattered succiform cells (sc). III—Oocytes on different maturation stages: a—immature oocyte, b—newly mature oocyte, c—mature oocyte. IV—Intestinal epithelium (iep) and sac-like tissue (slt) outside the serous membrane (sm). V—Liver with hepatocytes (hc). Abbreviations: ec, epithelial cell; bc, basal cell; dg, dermal gland; dgc, dermal gland cell; f-TTX, free TTX; sc, succiform cell; n, nuclei; yg, yolk granules; iep, intestinal epithelium; slt, sac-like tissue; sm, serous membrane; hc, hepatocyte.
The distribution of TTX within the body can vary depending on the habitat of the pufferfish. In Lagocephalus lunaris caught near Thailand [8] and the west coast of Japan [36], TTX is mostly localized in the gonads, while other organs, including the liver, skin, digestive tract, and muscles, contain low concentrations of the toxin. L. lunaris, which inhabits Cambodia, contains substantial concentrations of TTX in the liver, ovaries, and intestines, while the muscles, testes, and skin are less toxic [37]. The TTX content in different organs can also be affected by seasonal changes. In females of several species of the genus Takifugu, Y. criniger, and introduced species of the genera Lagocephalus and Torquigener, caught in the Mediterranean Sea, most of the TTX is contained in the ovaries in the autumn–winter period, during maturation, and in the liver and/or skin in the spring-summer period, after spawning [5][38][39][40][41][42][43][44][45]. Different data were obtained for males of different species of the genus Takifugu. T. flavipterus and T. pardalis do not show seasonal variations in TTX content [38][42]. In male as well as female T. alboplumbeus, an increase in the toxicity level in the pre-spawning and spawning periods was observed, when TTX was localized in the skin and liver [40]. Lagocephalus males contain substantial TTX concentrations in the testes only in the summer; other organs contain trace amounts of the toxin [41]. The concentration of TTX in the organs of Torquigener flavimaculosus males reaches the maximum level in winter and gradually decreases in autumn; in summer, only the levels in the testes and skin increase to winter values [43].
The cellular and intracellular localizations of TTX in pufferfish has been studied in highly toxic organs, including the skin, ovaries, and liver (Figure 1). Kodama et al. [46] detected TTX in secretions collected from the skin gland of T. pardalis and stated that the toxin was produced by the secretory cells of this gland. Later, the localization of TTX in the secretory cells of pufferfish skin was confirmed by immunohistochemistry using anti-TTX antibodies [17][23][47][48]. In T. pardalis [46], T. vermicularis [23], T. flavipterus, and Canthigaster rivulata [17], TTX-secreting cells are located in the glands or gland-like structures. In C. patoca [23], Dichotomyctere ocellatus (Tetraodon steindachneri) [47], and Dichotomyctere nigroviridis (Tetraodon nigroviridis) [48], TTX is located in the so-called succiform cells evenly scattered throughout the skin of the animal. Itoi et al. [49] showed that succiform cells of T. alboplumbeus males stained for TTX more intensely than the succiform cells of the females. TTX has also been found in the undifferentiated basal cells of pufferfish skin [39][47][48][49]. According to electron microscopic studies of D. nigroviridis skin, TTX in the basal cells is associated with membrane-bound granules (presumably lysosomes) [48]. The TTX absorption ability of the basal cells of the skin was demonstrated in experiments with intramuscular, intraperitoneal, and oral administration of toxins to non-toxic cultured pufferfish [50][51][52]. Interestingly, the intensity of staining for TTX in the skin cells depends on the dose of the injected toxin: low concentrations of injected TTX in T. rubripes only resulted in basal cell staining, while an increased dose stained the entire epidermis [52]. In vitro skin slices incubated in a TTX solution showed that TTX was transferred to the basal cells from connective tissue [53]. In addition to succiform and basal cells in the skin of T. alboplumbeus, TTX is found in mucous cells and the flat epithelial cell layer [49]. In the skin of Y. criniger, all epidermal cells, including filament-containing Malpighian [54], basal, and succiform cells, stained positively for TTX [39].
After studying the distribution of TTX in the cellular and subcellular liver fractions of T. pardalis and Takifugu snyderi (Takifugu vermicularis snyderi), Nagashima et al. [55] found that TTX was predominantly associated with the cytosolic fraction of the liver cells. Subsequently, immunocytochemistry with anti-TTX antibodies has been used to detect TTX in the cytoplasm of parenchymal hepatocytes in several species of Takifugu and C. patoca [17][23][49][51][53]. An in vitro experiment with the incubation of T. rubripes liver slices in a TTX solution showed that the toxin was transferred to the parenchymal hepatocytes from the pancreatic exocrine cells [53].
In the first study on the micro distribution of TTX in pufferfish ovaries, TTX was detected in the cytoplasm and membrane-limited yolk granules of pre-ovulated oocytes and the vitellin envelope of the ovulated oocytes [56]. In T. vermicularis ovaries, TTX is found in the yolk granules, vesicles, and nuclei of mature oocytes; immature oocytes do not contain the toxin [23]. In C. patoca, TTX is localised in the connective tissues and nuclei of some oocytes [23]. Itoi et al. found TTX in the oocyte nuclei of T. alboplumbeus [49]. Gao et al. [42] traced the localization of TTX during oocyte maturation in T. pardalis; in early maturation stages, TTX was localized in the nucleus and yolk granules, and late stages, in the cytoplasm and on the periphery of the cell.
Immunohistochemical studies have shown a weak TTX-positive signal in the sac-like tissues outside the serous membrane of the T. flavipterus intestine [17] and in the brain (optic tectum, cerebellum, and medulla oblongata), optic nerve, and olfactory epithelium of T. rubripes juveniles [51].
2. Amphibia
Newts are the most popular study animals among terrestrial TTX-bearers. In 1963, a toxin was isolated from the eggs of the newt Taricha torosa (Triturus torosus) [57], which was soon identified as TTX [58][59]. This discovery led to an array of toxicity studies on various members of the Salamandridae family. The first large-scale screening by Wakely et al. [60] showed the presence of TTX in several members of the genera Taricha, Cynops, and Triturus as well as in Lissotriton vulgaris (Triturus vulgaris), Ichthyosaura alpestris (Triturus alpestris), and Notophthalmus viridescens. To date, TTX has been found in 10 genera of Salamandridae residing in different regions of the world: Taricha, Notophthalmus, Cynops, Pachytriton, Paramesotriton, Laotriton, Triturus, Lissotriton, Ichthyosaura, and Ambystoma [60][61][62][63][64]. Differences in TTX content among individuals within populations have been reported for Taricha granulosa [65][66], N. viridescens [67], and Cynops pyrrhogaster [68].
Studies on individual organs and tissues of newts have revealed that TTX is usually localized in the skin and ovaries; other organs, including the muscles, blood, viscera, liver, and testes, contain low concentrations of the toxin [17][60][61][62][69][70] (Figure 2). In some newt species, high TTX concentrations have also been found in the liver [70] and muscles [71].
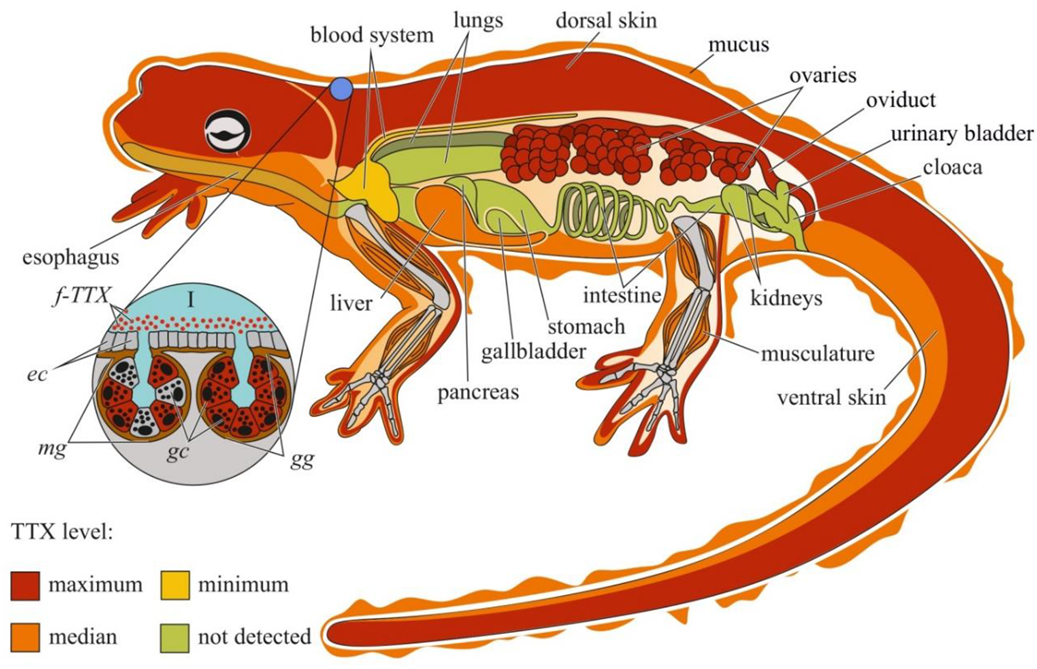
Figure 2. Schematic illustration of the levels and intra-organismal distribution of tetrodotoxin (TTX) in the adult newt (family Salamandridae). Red color on the insets indicates TTX-positive cells. I—Skin with mature granular (gg) and mixed (mg) glands. Abbreviations: ec, epithelial cell; gc, glandular cell; gg, mature granular gland; mg, mature mixed gland; f-TTX, free TTX.
TTX was first visualized in the skin of C. pyrrhogaster by immunohistochemistry with anti-TTX antibodies [72]. In the juveniles of C. pyrrhogaster, TTX is contained in the glandular cells of immature dermal glands, in adult individuals, in the glandular cells of granular and mixed dermal glands [72]. Sato et al. [17] found TTX-positive cells in the dermal glands of only adult C. pyrrhogaster; TTX-positive structures were absent in the liver, intestines, testes, and ovaries. Mailho-Fontana et al. [73] detected TTX in the dermal glands and blood plasma of dermal capillaries of T. granulosa. The authors also revealed differences in the morphology of TTX-positive glandular cells and the structure and chemical composition of their secretions between individuals from TTX-bearing and non-toxic T. granulosa populations. In N. viridescens, the dermal glands stained the most intensively for TTX; in the connective tissues, liver, intestinal epithelium, ovaries, testes, and kidneys, the TTX-positive labelling was moderate [70]. Spicer et al. [74] hypothesized that similar to other newt species, N. viridescens might possess an increased number of granular glands in brightly pigmented spots on the dorsal skin, which might be associated with increased levels of TTX. Although no differences in TTX concentration were found between the red spots and neighboring skin without spots, juveniles with more dorsal spots possessed higher TTX levels. Uniform TTX-staining was observed in the tissues of nematodes, trematodes, and cestodes parasitizing the intestinal cavity of N. viridescens [75].
In the order Anura, TTX has been found in four genera: Atelopus (family Bufonidae) [76][77][78][79][80][81][82][83], Colostethus (family Dendrobatidae) [80], Brachycephalus (family Brachycephalidae) [84][85][86], and Polypedates (family Rhacophoridae) [87]. TTX in frogs was first reported by Kim et al. [76] in 1975. The authors detected TTX in the skin of Atelopus varius and Atelopus chiriquiensis; internal organs, muscles, and bones did not contain TTX. A similar TTX distribution has been observed in Colostethus inguinalis [80] and Polypedates sp. [87]. Later, TTX was also found in the liver and ovaries of the Anura representatives. In Brachycephalus ephippium, TTX is contained in the skin, liver, and ovaries [84][86], whereas in Brachycephalus pernix, only in the skin and liver [86]. However, the TTX concentration in the ovaries of B. ephippium was three times lower than that in the skin [86]. In contrast, the ovaries of A. chiriquiensis [77] and Atelopus glyphus [82] contained more toxins than the skin. Immunohistochemical studies of Atelopus hoogmoedi showed the presence of TTX in the hepatocytes, granular skin glands, and epithelial skin cells [83].
3. Mollusca
The data on intra-organismal TTX distribution in octopuses, gastropods, and bivalves are summarized in Figure 3. TTX in molluscs was first discovered in 1978 when a toxin isolated from the posterior salivary glands of the octopus Hapalochlaena maculosa was identified [88]. Subsequently, TTX in H. maculosa was found not only in the salivary glands, but also in all body parts, including the arms, cephalothorax, and abdomen [89][90]. In Hapalochlaena fasciata, TTX has been found in the anterior and posterior salivary glands, arms, mantle, digestive gland, gonads, brachial heart, nephridia, gills, oviducal gland, and ink sac [91][92][93]. Hapalochlaena lunulate contains TTX in its salivary glands, gonads, mantle, arms, and ink [91][93][94]. However, the posterior salivary glands were the most toxic organs in both species. Immunofluorescence microscopy of the micro distribution of TTX in the tissues of H. lunulate and H. fasciata showed that TTX was concentrated in the cells lining the secretory tubules within the posterior salivary gland [93]. In the mantle and arms of H. lunulate, TTX was concentrated beneath the integumentary epidermis and in the channels of the circulatory system running through the dermis.
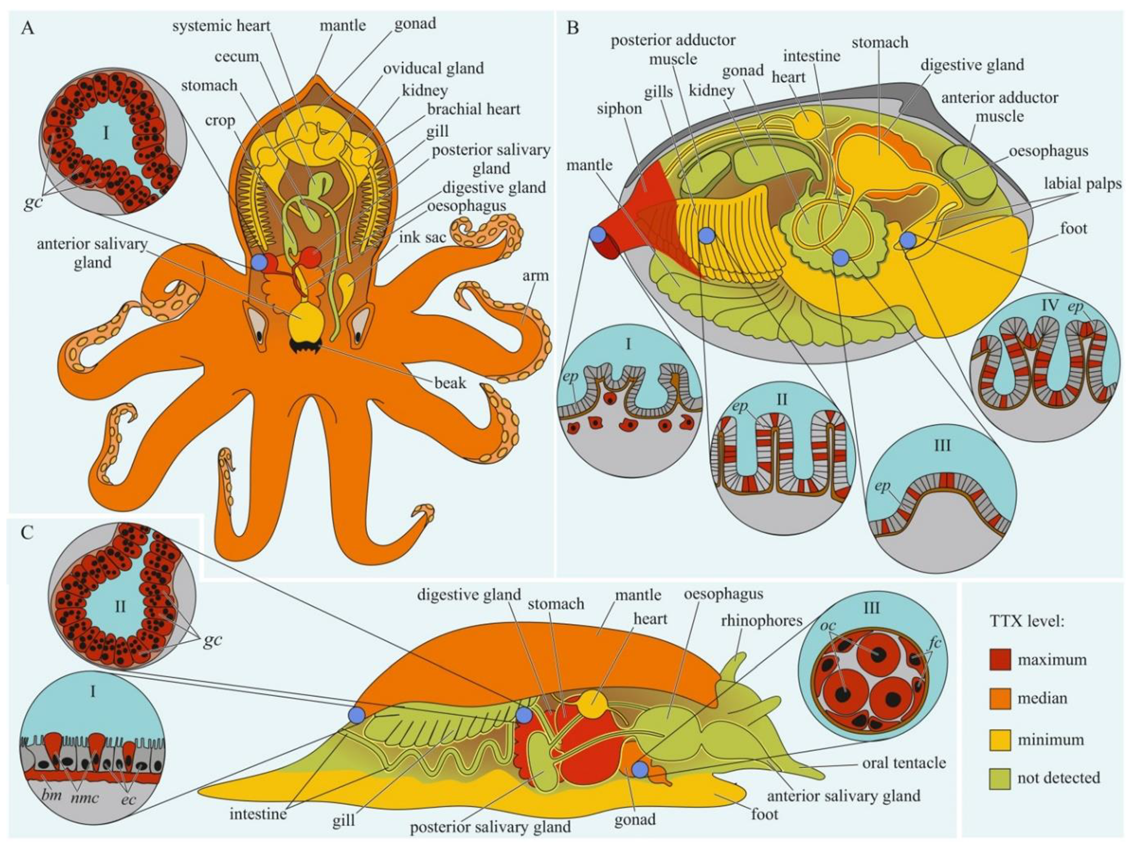
Figure 3. Schematic illustration of the levels and intra-organismal distribution of tetrodotoxin (TTX) in molluscs. Red color on the insets indicates TTX-positive cells. (A)—Blue-ringed octopus (genus Hapalochlaena). I—Posterior salivary gland with glandular cells (gc). (B)—Marine gastropod (genus Pleurobranchaea). I—The epidermis of the mantle. II—Digestive gland with glandular cells (gc). III—Oocytes (oc) surrounded by follicles (fc). (C)—Marine bivalve mollusc (genus Paphies). I—Siphon with TTX-positive cells located under the epithelium (ep). Gills (II), intestine (III), and labial palp (IV) showing epithelium (ep). Abbreviations: gc, glandular cells; bm, basement membrane; ec, epithelial cells; nmc, neutral mucin cells; oc, oocytes; fc, follicles; ep, epithelium.
In 1981, a substance with a neuroparalytic effect and physicochemical properties similar to TTX was isolated from the digestive glands of the sea snails, Charonia lampas (Charonia sauliae) and Babylonia japonica [95][96]. Further studies revealed TTX in sea snails Tutufa bufo (Tutufa lissostoma) [97], Buccinum undatum [98], Patella depressa, and Nucella lapillus [99], as well as in some members of the families Naticidae [100][101], Trochidae [102][103], Nassariidae [104][105][106][107][108][109][110][111][112][113], and Olividae [114][115]. In most studies, TTX has been detected in the digestive glands. In the lined moon snail Tanea lineata (Natica lineata), the muscles are the most toxic part of the body, followed by the remaining parts, including the salivary gland, brain, and mouth organs; the digestive gland contains the least amount of TTX [116]. Hwang et al. [101] found that when seawater was released from the mantle cavity of T. lineata, TTX was secreted into the water. In another study, Nassarius conoidalis (Niotha clathrata) released TTX into the water in response to the first electric shock treatment; repeated stimulation did not cause toxin secretion [117].
TTX was also found in sea slugs Pleurobranchaea maculata [118], Onchidella celtica, and Aplysia depilans [99]. Wood et al. [119][120] reported high TTX concentrations in the stomachs of P. maculata, while the mantle and gonads of the mollusc were less toxic. A detailed study of the tissues and cells of P. maculata revealed the presence of TTX in the neutral mucin cells and basement membrane in the mantle, oocytes, and follicles in the gonads, and digestive gland [121]. TTX was found in P. maculate egg clutches [120][121].
The well known TTX-bearing bivalve mollusc is Paphies australis [122]. In P. australis, the highest level of TTX is contained in the siphon [123]. Immunohistochemical studies have shown that TTX was localized in the cells of the inner and outer epithelia of the siphon, in the inside cells of the epithelium of the intestine and rectum, and in the cytoplasm of some epithelial cells of the labial palps and gills [124]. Since 2008, TTX has been detected in many commercial bivalves living in European waters [98][99][115][123][125][126][127][128][129][130].
4. Echinodermata
The undigested body parts of starfish, found in the contents of the digestive glands of marine TTX-bearing gastropods, served as a starting point for the search for TTX in echinoderms. TTX has been found in several starfish species of the genus Astropecten [131][132][133][134] and in Ophidiaster ophidianus as well as in sea urchins Echinus esculentus [99] and Fellaster zelandiae (Arachnoides zelandiae) [135]. Lin et al. showed that the internal organs of A. scoparius had the highest toxicity, whereas the gonads and body wall were less toxic [102][136]. The tissue and cell distribution of TTX in echinoderms has not been studied.
5. Nemertea
For the first time, TTX was detected in two species of marine ribbon worms, Lineus fuscoviridis, and Tubulanus punctatus in 1988 [137], although pyridine compounds with a neurotoxic effect were previously found in extracts of Amphiporus angulatus [138]. In the nemertean Cephalothrix simula (in the article by Cephalothrix linearis), both the body and proboscis were found to be highly toxic, with the proboscis being the most toxic [139]. In addition, TTX was released into the mucus on the surface of the animal’s body during mechanical stimulation. Subsequently, TTX and its analogs have been found in representatives of the genera Cephalothrix [140][141][142][143][144][145][146][147], Lineus [143][145][146][148][149], Ramphogordius [145], Riseriellus [145], Amphiporus [145], Yininemertes [150], Quasitetrastemma [146], and Collarenemertes [146].
The cellular and tissue localizations of TTX has been well studied in highly toxic nemerteans of the genus Cephalothrix [151][152] (Figure 4). In an early study on C. simula (Cephalothrix sp.), TTX was detected in the vesicles of the epidermal bacillary cells, basal lamina, granular cells of the proboscis epithelium, rhynchocoel epithelium, and vesicles of the intestinal epithelial cells located near the blood vessels and rhynchocoel [151]. The excretory system (protonephridia) and eggs also contain TTX. Vlasenko and Magarlamov found higher TTX content in the anterior region of the body of C. cf. simula than in the posterior one [147]. High TTX concentrations were detected in the intestine, body wall, proboscis, and mucus on the surface of the animal body. According to immunohistochemical studies, the main sites of TTX accumulation in C. cf. simula are the secretory cells of the integument, epidermal ciliary cells, mucous cells of the cephalic glands, glandular epithelium of the proboscis, enterocytes, and terminal cells of the protonephridia [152]. In the secretory and glandular cells, TTX is associated with secretory granules, in the ciliary cells, with microvilli, in the enterocytes, with phagosomes. In the protonephridial cells, TTX is located in the cytoplasm. In Dushia atra and Micrura verrilli, TTX was found in dermal glandular cells, intestinal epithelium, and outer proboscis epithelium, including pseudocnidae-containing cells [153]. In Kulikovia alborastrata (Lineus alborostratus), TTX was present in type-I subepidermal bacillary gland cells in the cutis and pseudocnidae-containing and mucoid gland cells in the proboscis [148]. Within glandular cells, TTX is associated with the nuclear envelope, the endoplasmic reticulum membrane, and secretory granules. Moreover, the glandular cells of the cutis in K. alborostrata released TTX-containing mucus in response to external stimuli [149].

Figure 4. Schematic illustration of the levels and intra-organismal distribution of tetrodotoxin (TTX) in the marine ribbon worm (genus Cephalothrix). Red color on the insets indicates TTX-positive cells. I—Glandular epithelium of proboscis. II—Intestinal epithelium. III—Protonephridium (pn) associated with the blood vessel (bv). IV—Cephalic gland. V—Integument. VI—Oocytes. Abbreviations: pgs, proboscidial gland cell; sc, supportive cell; ent, enterocyte; igs, intestinal glandular cell; bv, blood vessel; pn, protonephridium; tc, terminal cell; cc, ciliary cell; cgc, cephalic gland cell; f-TTX, free TTX; egc, epidermal gland cells; yg, yolk granules.
6. Platyhelminthes
Although the toxicity of flatworms was reported in early naturalistic works from the 18th and 19th centuries, the first scientific work noting the presence of neurotoxins in marine and terrestrial flatworms dates back to 1943 [154]. After 40 years, TTX was found in extracts of the marine flatworms Planocera multitentaculata [155][156] and Planocera reticulata (type A) [157][158]. Subsequent studies showed the presence of TTX in several marine [121][159][160] and terrestrial [161] flatworms. In P. multitentaculata, the most toxic organs are the oviducts filled with mature eggs and organs of the digestive (including the pharynx) and reproductive systems; a weak neurotoxic effect of the mucus enveloping the worm was observed [156][162]. High TTX concentrations have also been found in the pharynx and eggs of Planocerid sp. 1 [159]. Detailed immunohistochemical studies have detected TTX in the cytoplasm of the ova and epithelial cells of the pharynx of Stylochoplana sp. [163] and the ova of P. reticulata [151]. In the terrestrial flatworms Bipalium adventitium and Bipalium kewense, most of the TTX was contained in the head and eggs; the anterior and posterior parts of the body contained smaller amounts of the toxin [161]. Figure 5 summarizes the intra-organismal TTX distribution in marine flatworms.
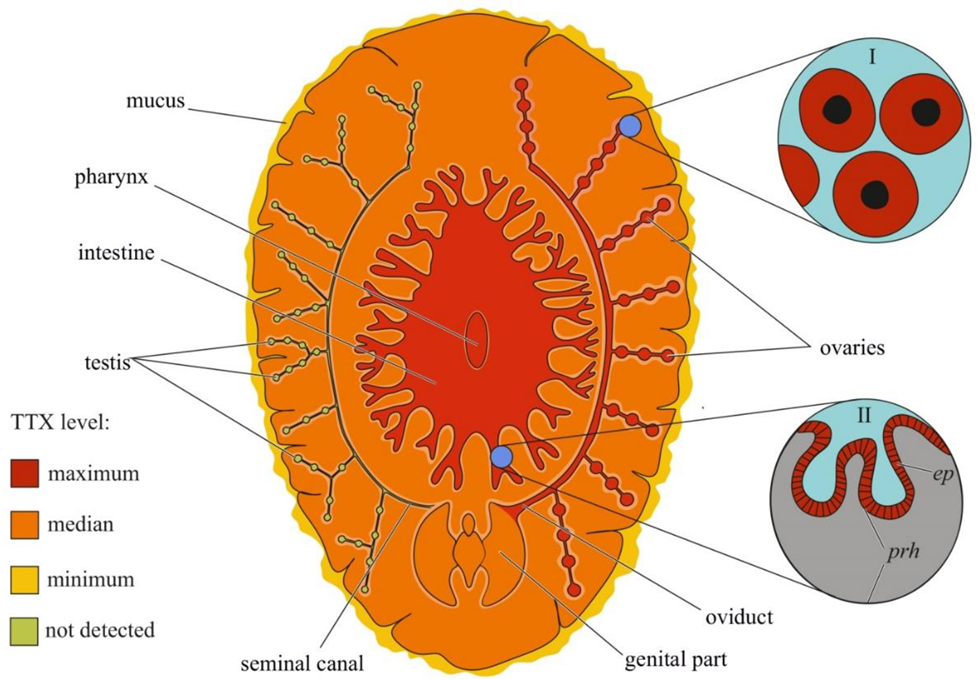
Figure 5. Schematic illustration of the levels and intra-organismal distribution of tetrodotoxin (TTX) in the marine flatworm (order Polycladida). Red color on the insets indicates TTX-positive cells. I—Oocytes. II—Pharynx showing epithelial cells (ep) and parenchyma (prh).
7. Annelida
In 1986, TTX was found in the annelid Pseudopotamilla occelata [164]. In their review, Miyazawa and Noguchi [1] mentioned that TTX was present in Lepidonotus helotypus, Halosydna brevisetosa, Hermenia acanthopeltis, and Harmothoe imbricata.
8. Chaetognatha
In 1988, TTX was found in planktonic chaetognaths Parasagitta elegans [165]; the sodium-channel-blocking activity of extracts of chaetognaths of the families Eukrohniidae (genus Eukrohnia), Sagittidae (genus Flaccisagitta), and Spadellidae (genus Spadella) was also reported; however, physicochemical methods of TTX detection were not used. Interestingly, only fractions containing chaetognath heads were toxic; the bodies did not contain TTX.
9. Arthropoda
TTX is found in three classes of arthropods: Malacostraca, Merostomata, and Copepoda. Among Malacostraca, TTX is detected in several crab species of the family Xanthidae [164][166][167][168][169][170][171][172]. Xanthid crabs contain TTX throughout their body, but the most toxic organs differ among species. In Lophozozymus pictor [167] and Demania reynaudi [170], the cephalothorax and viscera were found to be more toxic, whereas, in Atergatis floridus, the viscera and appendages were more toxic than other organs [170]. Saito et al. showed that the high toxicity of A. floridus was associated with the muscles of the chelipeds, particularly the muscles of the palm and carpus [173]. In some specimens, the muscles of the walking legs, gills, and ovaries were found to be toxic. In Demania cultripes, the viscera are more toxic than the appendages [172].
Among Merostomata, TTX is found in the horseshoe crab Carcinoscorpius rotundicauda [174]. In C. rotundicauda, TTX was first detected in the eggs [174]. Subsequent studies showed the presence of TTX in almost all organs and tissues of crabs of this species; however, most of the toxins are located in the eggs, muscles, viscera, and hepatic caecum [175][176][177].
Ikeda et al. first revealed the presence of TTX in the ectoparasitic copepod Caligus fugu (Pseudocaligus fugu) that parasitizes the pufferfish T. alboplumbeus [175]. In the same year, TTX was detected in another parasite of T. alboplumbeus, Taeniacanthus sp. [176]. Anti-TTX antibody-based immunohistochemical studies have shown that TTX in C. fugu was accumulated in all tissues of the body, intestines, and appendages except for the epicuticle and reproductive system, including the ovaries, oviduct, uterus, and egg sacs [175]. TTX appears at an early stage of chalimus development, and at chalimus stage IV and in adult copepods, it is present in all tissues and organs, except for the reproductive system [177].
10. Concluding Remarks
Data on TTX localization in animals can help elucidate the biological and physiological significance of the toxin. Most studies have focused on the anatomical distribution of TTX in animals, and only a small percentage of studies have focused on the intratissue and intracellular localization of TTX. A common tendency for TTX accumulation in the skin, ovaries, and endodermal organs (digestive system and liver) can be traced among different taxonomic groups of animals. The cellular distribution of TTX in these organs has mostly been studied in pufferfish (Figure 1) and nemerteans (Figure 4). Data on the cellular localization of TTX in other TTX-bearing animals are either limited or absent. More studies implementing high-resolution microscopy, such as laser scanning microscopy and electron microscopic immunocytochemistry, are required to trace the cellular and subcellular TTX distribution. These studies allow significantly expand the understanding of the cellular mechanisms of TTX sorption and migration within the body of a TTX-bearing animal. However, molecular mechanisms involved in TTX retention are yet to be explored. Studying the resistance of toxic pufferfish and arthropods to TTX, TTX-binding molecules in these animals were supposed [21][178][179][180]. TTX-binding proteins and high-molecular weight substances were isolated from the plasma of some pufferfish species, shore crab, and toxic gastropods [181][182][183][184][185] Nevertheless, the manner in which these molecules bind and transport TTX remains unclear. Future studies of the transcription factors involved in the regulation of TTX transport and accumulation in TTX-bearing animals are needed.
This entry is adapted from the peer-reviewed paper 10.3390/toxins14080576
References
- Miyazawa, K.; Noguchi, T. Distribution and origin of tetrodotoxin. J. Toxicol.-Toxin Rev. 2001, 20, 11–33.
- Tani, I. Nihonsan Fugu No Chudokugakuteki Kenkyu (Toxicological Studies on Japanese Puffers); Teikoku Tosho: Tokyo, Japan, 1945. (In Japanese)
- Endo, R. Toxicological studies on puffer fishes:—Comparison of toxicities on the various species. J. Toxicol. Sci. 1984, 9, 1–11.
- Khora, S.S.; Isa, J.; Yasumoto, T. Toxicity of puffers from Okinawa, Japan. Nippon Suisan Gakkaishi 1991, 57, 163–167.
- Hwang, D.F.; Kao, C.-Y.; Yang, H.-C.; Jeng, S.-S.; Noguchi, T.; Hashimoto, K. Toxicity of puffer in Taiwan. Nippon Suisan Gakkaishi 1992, 58, 1541–1547.
- Nuñez-Vázquez, E.J.; Yotsu-Yamashita, M.; Sierra-Beltrán, A.P.; Yasumoto, T.; Ochoa, J.L. Toxicities and distribution of tetrodotoxin in the tissues of puffer fish found in the coast of the Baja California Peninsula, Mexico. Toxicon 2000, 38, 729–734.
- Tao, J.; Wei, W.J.; Nan, L.; Lei, L.H.; Hui, H.C.; Fen, G.X.; Jun, L.Y.; Jing, Z.; Rong, J. Development of competitive indirect ELISA for the detection of tetrodotoxin and a survey of the distribution of tetrodotoxin in the tissues of wild puffer fish in the waters of south-east China. Food Addit. Contam.-Part A Chem. Anal. Control. Expo. Risk Assess. 2010, 27, 1589–1597.
- Chulanetra, M.; Sookrung, N.; Srimanote, P.; Indrawattana, N.; Thanongsaksrikul, J.; Sakolvaree, Y.; Chongsa-Nguan, M.; Kurazono, H.; Chaicumpa, W. Toxic marine puffer fish in Thailand seas and tetrodotoxin they contained. Toxins 2011, 3, 1249–1262.
- Thuy, L.V.; Yamamoto, S.; Kawaura, R.; Takemura, N.; Yamaki, K.; Yasumoto, K.; Takada, K.; Watabe, S.; Sato, S. Tissue distribution of tetrodotoxin and its analogs in Lagocephalus pufferfish collected in Vietnam. Fish. Sci. 2020, 86, 1101–1110.
- Hashimoto, Y.; Noguchi, T. Occurrence of a tetrodotoxin-like substance in a goby Gobius criniger. Toxicon 1971, 9, 79–84.
- Noguchi, T.; Hashimoto, Y. Isolation of tetrodotoxin from a goby Gobius criniger. Toxicon 1973, 11, 305–307.
- Itoi, S.; Sato, T.; Takei, M.; Yamada, R.; Ogata, R.; Oyama, H.; Teranishi, S.; Kishiki, A.; Wada, T.; Noguchi, K.; et al. The planocerid flatworm is a main supplier of toxin to tetrodotoxin-bearing fish juveniles. Chemosphere 2020, 249, 126217.
- Ito, M.; Furukawa, R.; Shino, Y.; Sato, M.; Oyama, H.; Okabe, T.; Suo, R.; Sugita, H.; Takatani, T.; Arakawa, O.; et al. Local differences in the toxin amount and composition of tetrodotoxin and related compounds in pufferfish. Toxins 2022, 14, 150.
- Heiser, V.G. Poisonous Fish. In Annual Report of the Bureau of Health for the Philippine Islands; Ulan Press: Washington, DC, USA, 1906; p. 70.
- Herre, A.W. Gobies of the Philippines and the China Sea. In Monographs of the Bureau of Science, Manila, Philippine Islands, 23; Bureau of Printing: Hikone, Japan, 1927; p. 352.
- Takahashi, D.; Inoko, Y. Unter suchungen über das Fugugift, Centralbl. Meb. Wiss. 1889, 27, 529–530.
- Sato, S.; Kawaura, R.; Togashi, K.; Mizusawa, N.; Yasumoto, K.; Takada, K.; Amano, M.; Watabe, S. De novo accumulation of tetrodotoxin and its analogs in pufferfish and newt and dosage-driven accumulation of toxins in newt: Tissue distribution and anatomical localization. J. Mar. Sci. Eng. 2021, 9, 1004.
- Saito, T.; Noguchi, T.; Harada, T.; Murata, O.; Hashimoto, K. Tetrodotoxin as a biological defense agent for puffers. Bull. Jpn. Soc. Sci. Fish. 1985, 51, 1175–1180.
- Fuchi, U.; Narimatsu, H.; Nakama, S.; Kotobuki, H.; Hirakawa, H.; Torishima, Y.; Noguchi, T.; Ohtom, N. Tissue distribution of toxicity in a puferfish, Arothron firmamentum (“Hishifugu”). Food Health 1991, 32, 520–524.
- Mahmud, Y.; Okada, K.; Takatani, T.; Kawatsu, K.; Hamano, Y.; Arakawa, O.; Noguchi, T. Intra-tissue distribution of tetrodotoxin in two marine puffers Takifugu vermicularis and Chelonodon patoca. Toxicon 2003, 41, 13–18.
- Jang, J.; Yotsu-Yamashita, M. Distribution of tetrodotoxin, saxitoxin, and their analogs among tissues of the puffer fish Fugu pardalis. Toxicon 2006, 48, 980–987.
- Diener, M.; Christian, B.; Ahmed, M.S.; Luckas, B. Determination of tetrodotoxin and its analogs in the puffer fish Takifugu oblongus from Bangladesh by hydrophilic interaction chromatography and mass-spectrometric detection. Anal. Bioanal. Chem. 2007, 389, 1997–2002.
- Sasaki, K.; Takayama, Y.; Tahara, T.; Anraku, K.; Ito, Y.; Akaike, N. Quantitative analysis of toxin extracts from various tissues of wild and cultured puffer fish by an electrophysiological method. Toxicon 2008, 51, 606–614.
- Indumathi, S.M.; Khora, S.S. Toxicity assessment and screening of tetrodotoxin in the oblong blowfish (Takifugu oblongus) from the Tamil Nadu Coast of Bay of Bengal, India. Asian Pac. J. Trop. Med. 2017, 10, 278–284.
- Rambla-Alegre, M.; Reverté, L.; del Río, V.; de la Iglesia, P.; Palacios, O.; Flores, C.; Caixach, J.; Campbell, K.; Elliott, C.T.; Izquierdo-Muñoz, A.; et al. Evaluation of tetrodotoxins in puffer fish caught along the Mediterranean coast of Spain. Toxin profile of Lagocephalus sceleratus. Environ. Res. 2017, 158, 1–6.
- Tatsuno, R.; Umeeda, M.; Miyata, Y.; Ideguchi, R.; Fukuda, T.; Furushita, M.; Ino, Y.; Yoshikawa, H.; Takahashi, H.; Nagashima, Y. Toxicity of Takifugu exascurus collected from the Sea of Kumano. Shokuhin Eiseigaku Zasshi 2021, 62, 28–32.
- Hassoun, A.E.R.; Ujević, I.; Jemaa, S.; Roje-Busatto, R.; Mahfouz, C.; Fakhri, M.; Nazlić, N. Concentrations of tetrodotoxin (TTX) and its analogue 4,9-anhydro TTX in different tissues of the silver-cheeked pufferfish (Lagocephalus sceleratus, Gmelin, 1789) caught in the South-Eastern Mediterranean Sea, Lebanon. Toxins 2022, 14, 123.
- Honda, S.; Ichimaru, S.; Arakawa, O.; Takatani, T.; Noguchi, T.; Ishizaki, S.; Nagashima, Y. Toxicity of puffer fish fins. Shokuhin Eiseigaku Zasshi 2007, 48, 159–162.
- Mahmud, Y.; Yamamori, K.; Noguchi, T. Toxicity and tetrodotoxin as the toxic principle of a brackish water puffer, Tetraodon steindachneri, collected from Thailand. J. Food Hyg. Soc. Jpn. 1999, 40, 391–395.
- Saitanu, K.; Laobhripatr, S.; Limpakarnjanarat, K.; Sangwanloy, O.; Sudhasaneya, S.; Anuchatvorakul, B.; Leelasitorn, S. Toxicity of the freshwater puffer fish Tetraodon fangi and T. palembangensis from Thailand. Toxicon 1991, 29, 895–897.
- Katikou, P.; Georgantelis, D.; Sinouris, N.; Petsi, A.; Fotaras, T. First report on toxicity assessment of the Lessepsian migrant pufferfish Lagocephalus sceleratus (Gmelin, 1789) from European waters (Aegean Sea, Greece). Toxicon 2009, 54, 50–55.
- Lin, S.J.; Chai, T.J.; Jeng, S.S.; Hwang, D.F. Toxicity of the puffer Takifugu rubripes cultured in northern Taiwan. Fish. Sci. 1998, 64, 766–770.
- Sato, S.; Komaru, K.; Ogata, T.; Kodama, M. Occurence of tetrodotoxin in cultered pufer. Nippon Suisan Gakkaishi 1990, 56, 1129–1131.
- Noguchi, T.; Arakawa, O.; Takatani, T. Toxicity of pufferfish Takifugu rubripes cultured in netcages at sea or aquaria on land. Comp. Biochem. Physiol.-Part D Genom. Proteom. 2006, 1, 153–157.
- Noguchi, T.; Takatani, T.; Arakawa, O. Toxicity of puffer fish cultured in netcages. Shokuhin Eiseigaku Zasshi 2004, 45, 146–149.
- Nagashima, Y.; Matsumoto, T.; Kadoyama, K.; Ishizaki, S.; Terayama, M. Toxicity and molecular identification of green toadfish Lagocephalus lunaris collected from Kyushu coast, Japan. J. Toxicol. 2011, 2011, 801285.
- Ngy, L.; Taniyama, S.; Shibano, K.; Yu, C.F.; Takatani, T.; Arakawa, O. Distribution of tetrodotoxin in pufferfish collected from coastal waters of Sihanouk Ville, Cambodia. J. Food Hyg. Soc. Jpn. 2008, 49, 361–365.
- Ikeda, K.; Emoto, Y.; Tatsuno, R.; Wang, J.J.; Ngy, L.; Taniyama, S.; Takatani, T.; Arakawa, O. Maturation-associated changes in toxicity of the pufferfish Takifugu poecilonotus. Toxicon 2010, 55, 289–297.
- Tatsuno, R.; Shikina, M.; Soyano, K.; Ikeda, K.; Takatani, T.; Arakawa, O. Maturation-associated changes in the internal distribution of tetrodotoxin in the female goby Yongeichthys criniger. Toxicon 2013, 63, 64–69.
- Itoi, S.; Ishizuka, K.; Mitsuoka, R.; Takimoto, N.; Yokoyama, N.; Detake, A.; Takayanagi, C.; Yoshikawa, S.; Sugita, H. Seasonal changes in the tetrodotoxin content of the pufferfish Takifugu niphobles. Toxicon 2016, 114, 53–58.
- Kosker, A.R.; Özogul, F.; Durmus, M.; Ucar, Y.; Ayas, D.; Regenstein, J.M.; Özogul, Y. Tetrodotoxin levels in pufferfish (Lagocephalus sceleratus) caught in the Northeastern Mediterranean Sea. Food Chem. 2016, 210, 332–337.
- Gao, W.; Kanahara, Y.; Tatsuno, R.; Soyano, K.; Nishihara, G.N.; Urata, C.; Takatani, T.; Arakawa, O. Maturation-associated changes in internal distribution and intra-ovarian microdistribution of tetrodotoxin in the pufferfish Takifugu pardalis. Fish. Sci. 2018, 84, 723–732.
- Kosker, A.R.; Özogul, F.; Durmus, M.; Ucar, Y.; Ayas, D.; Šimat, V.; Özogul, Y. First report on TTX levels of the yellow spotted pufferfish (Torquigener flavimaculosus) in the Mediterranean Sea. Toxicon 2018, 148, 101–106.
- Kosker, A.R.; Özogul, F.; Ayas, D.; Durmus, M.; Ucar, Y.; Regenstein, J.M.; Özogul, Y. Tetrodotoxin levels of three pufferfish species (Lagocephalus sp.) caught in the North-Eastern Mediterranean sea. Chemosphere 2019, 219, 95–99.
- Akbora, H.D.; Kunter, İ.; Erçetin, T.; Elagöz, A.M.; Çiçek, B.A. Determination of tetrodotoxin (TTX) levels in various tissues of the silver cheeked puffer fish (Lagocephalus sceleratus (Gmelin, 1789)) in Northern Cyprus Sea (Eastern Mediterranean). Toxicon 2020, 175, 1–6.
- Kodama, M.; Sato, S.; Ogata, T.; Suzuki, Y.; Kaneko, T.; Aida, K. Tetrodotoxin secreting glands in the skin of puffer fishes. Toxicon 1986, 24, 819–829.
- Tanu, M.B.; Mahmud, Y.; Takatani, T.; Kawatsu, K.; Hamano, Y.; Arakawa, O.; Noguchi, T. Localization of tetrodotoxin in the skin of a brackishwater puffer Tetraodon steindachneri on the basis of immunohistological study. Toxicon 2002, 40, 103–106.
- Mahmud, Y.; Arakawa, O.; Ichinose, A.; Tanu, M.B.; Takatani, T.; Tsuruda, K.; Kawatsu, K.; Hamano, Y.; Noguchi, T. Intracellular visualization of tetrodotoxin (TTX) in the skin of a puffer Tetraodon nigroviridis by immunoenzymatic technique. Toxicon 2003, 41, 605–611.
- Itoi, S.; Yoshikawa, S.; Tatsuno, R.; Suzuki, M.; Asahina, K.; Yamamoto, S.; Takanashi, S.; Takatani, T.; Arakawa, O.; Sakakura, Y.; et al. Difference in the localization of tetrodotoxin between the female and male pufferfish Takifugu niphobles, during spawning. Toxicon 2012, 60, 1000–1004.
- Ikeda, K.; Murakami, Y.; Emoto, Y.; Ngy, L.; Taniyama, S.; Yagi, M.; Takatani, T.; Arakawa, O. Transfer profile of intramuscularly administered tetrodotoxin to non-toxic cultured specimens of the pufferfish Takifugu rubripes. Toxicon 2009, 53, 99–103.
- Okita, K.; Takatani, T.; Nakayasu, J.; Yamazaki, H.; Sakiyama, K.; Ikeda, K.; Arakawa, O.; Sakakura, Y. Comparison of the localization of tetrodotoxin between wild pufferfish Takifugu rubripes juveniles and hatchery-reared juveniles with tetrodotoxin administration. Toxicon 2013, 71, 128–133.
- Tatsuno, R.; Gao, W.; Ibi, K.; Mine, T.; Okita, K.; Nishihara, G.N.; Takatani, T.; Arakawa, O. Profile differences in tetrodotoxin transfer to skin and liver in the pufferfish Takifugu rubripes. Toxicon 2017, 130, 73–78.
- Gao, W.; Yamada, M.; Ohki, R.; Nagashima, Y.; Tatsuno, R.; Ikeda, K.; Kawatsu, K.; Takatani, T.; Arakawa, O. Evaluation of the tetrodotoxin uptake ability of pufferfish Takifugu rubripes tissues according to age using an in vitro tissue slice incubation method. Toxicon 2020, 174, 8–12.
- Henrikson, R.C.; Gedeon Matoltsy, A. The fine structure of teleost epidermis. I. Introduction and filament-containing cells. J. Ultrastruct. Res. 1968, 21, 194–212.
- Nagashima, Y.; Hamada, Y.; Ushio, H.; Nishio, S.; Shimakura, K.; Shiomi, K. Subcellular distribution of tetrodotoxin in puffer fish liver. Toxicon 1999, 37, 1833–1837.
- Matsumura, K. Tetrodotoxin as a pheromone. Nature 1995, 378, 563–564.
- Brown, M.S.; Mosher, H.S. Tarichatoxin: Isolation and purification. Science 1963, 140, 295–296.
- Mosher, H.S.; Fuhrman, F.A.; Buchwald, H.D.; Fischer, H.G. Tarichatoxin—Tetrodotoxin: A potent neurotoxin. Science 1964, 144, 1100–1110.
- Buchwald, D.H.; Durham, L.; Fischer, H.G.; Harada, R.; Mosher, H.S.; Kao, C.Y.; Fuhrman, F.A. Identity of tarichatozin and tetrodotoxin. Science 1964, 143, 474–475.
- Wakely, J.F.; Fuhrman, G.J.; Fuhrman, F.A.; Fischer, H.G.; Mosher, H.S. The occurrence of tetrodotoxin (tarichatoxin) in amphibia and the distribution of the toxin in the organs of newts (Taricha). Toxicon 1966, 3, 195–203.
- Brodie, E.D., Jr.; Hensel, J.L., Jr.; Johnson, J.A. Toxicity of the urodele amphibians Taricha, Notophthalmus, Cynops and Paramesotriton (Salamandridae). Copeia 1974, 1974, 506–511.
- Yotsu, M.; Iorizzi, M.; Yasumoto, T. Distribution of tetrodotoxin, 6-epitetrodotoxin, and 11-deoxytetrodotoxin in newts. Toxicon 1990, 28, 238–241.
- Yotsu-Yamashita, M.; Mebs, D.; Kwet, A.; Schneider, M. Tetrodotoxin and its analogue 6-epitetrodotoxin in newts (Triturus spp.; Urodela, Salamandridae) from southern Germany. Toxicon 2007, 50, 306–309.
- Yotsu-Yamashita, M.; Toennes, S.W.; Mebs, D. Tetrodotoxin in Asian newts (Salamandridae). Toxicon 2017, 134, 14–17.
- Hanifin, C.T.; Yotsu-Yamashita, M.; Yasumoto, T.; Brodie, E.D., Jr.; Brodie, E.D., III. Toxicity of dangerous prey: Variation of tetrodotoxin levels within and among populations of the newt Taricha granulosa. J. Chem. Ecol. 1999, 25, 2161–2175.
- Mebs, D.; Schneider, J.V.; Schröder, O.; Yotsu-Yamashita, M.; Harley, J.R.; Mogk, L.; Köhler, G. A study on the genetic population structure and the tetrodotoxin content of rough-skinned newts, Taricha granulosa (Salamandridae), from their northern range of distribution. Toxicon 2022, 206, 38–41.
- Yotsu-Yamashita, M.; Gilhen, J.; Russell, R.W.; Krysko, K.L.; Melaun, C.; Kurz, A.; Kauferstein, S.; Kordis, D.; Mebs, D. Variability of tetrodotoxin and of its analogues in the red-spotted newt, Notophthalmus viridescens (Amphibia: Urodela: Salamandridae). Toxicon 2012, 59, 257–264.
- Mochida, K.; Kitada, M.; Ikeda, K.; Toda, M.; Takatani, T.; Arakawa, O. Spatial and temporal instability oflocal biotic community mediate a form of aposematic defense in newts, consisting of carotenoid-based coloration and tetrodotoxin. J. Chem. Ecol. 2013, 39, 1186–1192.
- Hanifin, C.T.; Brodie, E.D., III; Brodie, E.D., Jr. Tetrodotoxin levels of the rough-skin newt, Taricha granulosa, inscreas in long-term captivity. Toxicon 2002, 40, 1149–1153.
- Mebs, D.; Arakawa, O.; Yotsu-Yamashita, M. Tissue distribution of tetrodotoxin in the red-spotted newt Notophthalmus viridescens. Toxicon 2010, 55, 1353–1357.
- Tsuruda, K.; Arakawa, O.; Noguchi, K. Toxicity and toxin profile of the newt Cynops pyrrhogaster from western Japan. J. Nat. Toxins 2001, 10, 79–89.
- Tsuruda, K.; Arakawa, O.; Kawatsu, K.; Hamano, Y.; Takatani, T.; Noguchi, T. Secretory glands of tetrodotoxin in the skin of the Japanese newt Cynops pyrrhogaster. Toxicon 2002, 40, 131–136.
- Mailho-Fontana, P.L.; Jared, C.; Antoniazzi, M.M.; Sciani, J.M.; Pimenta, D.C.; Stokes, A.N.; Grant, T.; Brodie, E.D.; Brodie, E.D. Variations in tetrodotoxin levels in populations of Taricha granulosa are expressed in the morphology of their cutaneous glands. Sci. Rep. 2019, 9, 18490.
- Spicer, M.M.; Stokes, A.N.; Chapman, T.L.; Brodie, E.D., Jr.; Brodie, E.D., III; Gall, B.G. An investigation into tetrodotoxin (TTX) levels associated with the red dorsal spots in eastern newt (Notophthalmus viridescens) efts and adults. J. Toxicol. 2018, 2018, 1–5.
- Mebs, D.; Yotsu-Yamashita, M.; Seitz, H.M.; Arakawa, O. Tetrodotoxin does not protect red-spotted newts, Notophthalmus viridescens, from intestinal parasites. Toxicon 2012, 60, 66–69.
- Kim, Y.H.; Brown, G.B.; Mosher, H.S.; Fuhrman, F.A. Tetrodotoxin: Occurrence in atelopid frogs of Costa Rica. Science 1975, 189, 151–152.
- Pavelka, L.A.; Kim, Y.H.; Mosher, H.S. Tetrodotoxin and tetrodotoxin-like compounds from the eggs of the costa rican frog, Atelopus chiriquiensis. Toxicon 1977, 15, 135–139.
- Mebs, D.; Schmidt, K. Occurrence of tetrodotoxin in the frog Atelopus oxyrhynchus. Toxicon 1989, 27, 819–822.
- Yotsu-Yamashita, M.; Mebs, D.; Yasumoto, T. Tetrodotoxin and its analogues in extracts from the toad Atelopus oxyrhynchus (family: Bufonidae). Toxicon 1992, 30, 1489–1492.
- Daly, J.W.; Gusovsky, F.; Myers, C.W.; Yotsu-Yamashita, M.; Yasumoto, T. First occurrence of tetrodotoxin in a dendrobatid frog (Colostethus inguinalis), with further reports for the bufonid genus Atelopus. Toxicon 1994, 32, 279–285.
- Mebs, D.; Yotsu-Yamashita, M.; Yasumoto, T.; Lötters, S.; Andreas, S. Further report of the occurrence of tetrodotoxin in Atelopus species (Family: Bufonidae)). Toxicon 1995, 33, 246–249.
- Yotsu-Yamashita, M.; Tateki, E. First report on toxins in the Panamanian toads Atelopus limosus, A. glyphus and A. certus. Toxicon 2010, 55, 153–156.
- Mebs, D.; Lorentz, M.; Yotsu-Yamashita, M.; Rößler, D.C.; Ernst, R.; Lötters, S. Geographic range expansion of tetrodotoxin in amphibians—First record in Atelopus hoogmoedi from the Guiana Shield. Toxicon 2018, 150, 175–179.
- Pires, O.R.; Sebben, A.; Schwartz, E.F.; Largura, S.W.R.; Bloch, C.; Morales, R.A.V.; Schwartz, C.A. Occurrence of tetrodotoxin and its analogues in the brazilian frog Brachycephalus ephippium (Anura: Brachycephalidae). Toxicon 2002, 40, 761–766.
- Pires, O.R.; Sebben, A.; Schwartz, E.F.; Bloch, C.; Morales, R.A.V.; Schwartz, C.A. The occurrence of 11-oxotetrodotoxin, a rare tetrodotoxin analogue, in the brachycephalidae frog Brachycephalus ephippium. Toxicon 2003, 42, 563–566.
- Pires, O.R.; Sebben, A.; Schwartz, E.F.; Morales, R.A.V.; Bloch, C.; Schwartz, C.A. Further report of the occurrence of tetrodotoxin and new analogues in the Anuran family Brachycephalidae. Toxicon 2005, 45, 73–79.
- Tanu, M.B.; Mahmud, Y.; Tsuruda, K.; Arakawa, O.; Noguchi, T. Occurrence of tetrodotoxin in the skin of a rhacophoridid frog Polypedates sp. from Bangladesh. Toxicon 2001, 39, 937–941.
- Sheumack, D.D.; Howden, M.E.H.; Spence, I.; Quinn, R.J. Maculotoxin: A neurotoxin from the venom glands of the octopus Hapalochlaena maculosa identified as tetrodotoxin. Science 1978, 199, 188–189.
- Hwang, D.F.; Arakawa, O.; Saito, T.; Noguchi, T.; Simidu, U.; Tsukamoto, K.; Shida, Y.; Hashimoto, K. Tetrodotoxin-producing bacteria from the blue-ringed octopus Octopus maculosus. Mar. Biol. 1989, 100, 327–332.
- Yotsu-Yamashita, M.; Mebs, D.; Flachsenberger, W. Distribution of tetrodotoxin in the body of the blue-ringed octopus (Hapalochlaena maculosa). Toxicon 2007, 49, 410–412.
- Williams, B.L.; Caldwell, R.L. Intra-organismal distribution of tetrodotoxin in two species of blue-ringed octopuses (Hapalochlaena fasciata and H. lunulata). Toxicon 2009, 54, 345–353.
- Williams, B.L.; Lovenburg, V.; Huffard, C.L.; Caldwell, R.L. Chemical defense in pelagic octopus paralarvae: Tetrodotoxin alone does not protect individual paralarvae of the greater blue-ringed octopus (Hapalochlaena lunulata) from common reef predators. Chemoecology 2011, 21, 131–141.
- Williams, B.L.; Stark, M.R.; Caldwell, R.L. Microdistribution of tetrodotoxin in two species of blue-ringed octopuses (Hapalochlaena lunulata and Hapalochlaena fasciata) detected by fluorescent immunolabeling. Toxicon 2012, 60, 1307–1313.
- Asakawa, M.; Matsumoto, T.; Umezaki, K.; Kaneko, K.; Yu, X.; Gomez-Delan, G.; Tomano, S.; Noguchi, T.; Ohtsuka, S. Toxicity and toxin composition of the greater blue-ringed octopus Hapalochlaena lunulata from ishigaki island, Okinawa prefecture, Japan. Toxins 2019, 11, 245.
- Narita, H.; Noguchi, T.; Maruyama, J.; Ueda, Y.; Hashimoto, K.; Watanabe, Y.; Hida, K. Occurrence of tetrodotoxin in a trumpet shell, “Boshubora” Charonia sauliae. Bull. Jpn. Soc. Sci. Fish. 1981, 47, 935–941.
- Nocuchi, T.; Maruyama, J.; Ueda, Y.; Hashimoto, K.; Harada, T. Occurrence of tetrodotoxin in the japanese ivory shell Babylonia japonica. Bull. Jpn. Soc. Sci. Fish. 1981, 47, 909–913.
- Noguchi, T.; Maruyama, J.; Narita, H.; Kanehisa, H. Occurrence of tetrodotoxin in the gastropod mollusk Tutufa lissostoma (frog shell). Toxicon 1984, 22, 219–226.
- Hort, V.; Arnich, N.; Guérin, T.; Lavison-Bompard, G.; Nicolas, M. First detection of tetrodotoxin in bivalves and gastropods from the French mainland coasts. Toxins 2020, 12, 599.
- Silva, M.; Rodríguez, I.; Barreiro, A.; Kaufmann, M.; Neto, A.I.; Hassouani, M.; Sabour, B.; Alfonso, A.; Botana, L.M.; Vasconcelos, V. Tetrodotoxins occurrence in non-traditional vectors of the north atlantic waters (Portuguese maritime territory, and Morocco coast). Toxins 2019, 11, 306.
- Hwang, D.F.; Tai, R.P.; Chueh, C.H.; Lin, L.C.; Jeng, S.S. Tetrodotoxin and derivatives in several species of the gastropod Naticidae. Toxicon 1991, 29, 1019–1024.
- Hwang, D.F.; Chueh, C.H.; Jeng, S.S. Tetrodotoxin secretion from the lined moon shell Natica lineata in response to external stimulation. Toxicon 1990, 28, 1133–1136.
- Lin, S.J.; Hwang, D.F. Possible source of tetrodotoxin in the starfish Astropecten scoparius. Toxicon 2001, 39, 573–579.
- Silva, M.; Azevedo, J.; Rodriguez, P.; Alfonso, A.; Botana, L.M.; Vasconcelos, V. New gastropod vectors and tetrodotoxin potential expansion in temperate waters of the Atlantic Ocean. Mar. Drugs 2012, 10, 712–726.
- Jeon, J.K.; Narita, H.; Nara, M.; Noguchi, T.; Maruyama, J.; Hashimoto, K. Occurrence of tetrodotoxin in a gastropod mollusk, “Araregai” Niotha clathrata. Bull. Jpn. Soc. Sci. Fish. 1984, 50, 2099–2102.
- Narita, H.; Noguchi, T.; Maruyama, J.; Nara, M.; Hashimoto, K. Occurrence of a tetrodotoxin-associated substance in a gastropod, “Hanamushirogai” Zeuxis siquijore. Bull. Jpn. Soc. Sci. Fish. 1984, 50, 85–88.
- Hwang, D.F.; Lin, L.C.; Jeng, S.S. Occurrence of a new toxin and tetrodotoxin in two species of the gastropod mollusk Nassariidae. Toxicon 1992, 30, 41–46.
- Hwang, D.F.; Shiu, Y.C.; Hwang, P.A.; Lu, Y.H. Tetrodotoxin in gastropods (snails) implicated in food poisoning in Northern Taiwan. J. Food Prot. 2002, 65, 1341–1344.
- Taniyama, S.; Takatani, T.; Sorimachi, T.; Sagara, T.; Kubo, H.; Oshiro, N.; Ono, K.; Xiao, N.; Tachibana, K.; Arakawa, O. Toxicity and toxin profile of scavenging and carnivorous gastropods from the coastal waters of okinawa prefecture, Japan. J. Food Hyg. Soc. Japan 2013, 54, 49–55.
- Hwang, P.A.; Tsai, Y.H.; Deng, J.F.; Cheng, C.A.; Ho, P.H.; Hwang, D.F. Identification of tetrodotoxin in a marine gastropod (Nassarius glans) responsible for human morbidity and mortality in Taiwan. J. Food Prot. 2005, 68, 1696–1701.
- Jen, H.C.; Lin, S.J.; Huang, Y.W.; Liao, I.C.; Arakawa, O.; Hwang, D.F. Occurrence of tetrodotoxin and paralytic shellfish poisons in a gastropod implicated in food poisoning in southern Taiwan. Food Addit. Contam. 2007, 24, 902–909.
- Huang, H.N.; Lin, J.; Lin, H.L. Identification and quantification of tetrodotoxin in the marine gastropod Nassarius by LC-MS. Toxicon 2008, 51, 774–779.
- Wang, X.J.; Yu, R.C.; Luo, X.; Zhou, M.J.; Lin, X.T. Toxin-screening and identification of bacteria isolated from highly toxic marine gastropod Nassarius semiplicatus. Toxicon 2008, 52, 55–61.
- Luo, X.; Yu, R.C.; Wang, X.J.; Zhou, M.J. Toxin composition and toxicity dynamics of marine gastropod Nassarius spp. collected from lianyungang, China. Food Addit. Contam.-Part A Chem. Anal. Control. Expo. Risk Assess. 2012, 29, 117–127.
- Matsumoto, T.; Nagashima, Y.; Kusuhara, H.; Sugiyama, Y.; Ishizaki, S.; Shimakura, K.; Shiomi, K. Involvement of carrier-mediated transport system in uptake of tetrodotoxin into liver tissue slices of puffer fish Takifugu rubripes. Toxicon 2007, 50, 173–179.
- Bacchiocchi, S.; Campacci, D.; Siracusa, M.; Dubbini, A.; Leoni, F.; Tavoloni, T.; Accoroni, S.; Gorbi, S.; Giuliani, M.E.; Stramenga, A.; et al. Tetrodotoxins (Ttxs) and vibrio alginolyticus in mussels from central adriatic sea (Italy): Are they closely related? Mar. Drugs 2021, 19, 304.
- Hwang, D.F.; Chueh, C.H.; Jeng, S.S. Occurrence of tetrodotoxin in the gastropod mollusk Natica lineata (lined moon shell). Toxicon 1990, 28, 21–27.
- Hwang, D.F.; Lin, L.C.; Jeng, S.S. Variation and secretion of toxins in gastropod mollusc Niotha clathrata. Toxicon 1992, 30, 1189–1194.
- McNabb, P.; Selwood, A.I.; Munday, R.; Wood, S.A.; Taylor, D.I.; MacKenzie, L.A.; van Ginkel, R.; Rhodes, L.L.; Cornelisen, C.; Heasman, K.; et al. Detection of tetrodotoxin from the grey side-gilled sea slug—Pleurobranchaea maculata, and associated dog neurotoxicosis on beaches adjacent to the Hauraki Gulf, Auckland, New Zealand. Toxicon 2010, 56, 466–473.
- Wood, S.A.; Casas, M.; Taylor, D.I.; McNabb, P.; Salvitti, L.; Ogilvie, S.; Cary, S.C. Depuration of tetrodotoxin and changes in bacterial communities in Pleurobranchea maculata adults and egg vasses vaintained in captivity. J. Chem. Ecol. 2012, 38, 1342–1350.
- Wood, S.A.; Taylor, D.I.; McNabb, P.; Walker, J.; Adamson, J.; Cary, S.C. Tetrodotoxin concentrations in Pleurobranchaea maculata: Temporal, spatial and individual variability from New Zealand Populations. Mar. Drugs 2012, 10, 163–176.
- Salvitti, L.R.; Wood, S.A.; Winsor, L.; Cary, S.C. Intracellular immunohistochemical detection of tetrodotoxin in Pleurobranchaea maculata (Gastropoda) and Stylochoplana sp. (Turbellaria). Mar. Drugs 2015, 13, 765–769.
- McNabb, P.S.; Taylor, D.I.; Ogilvie, S.C.; Wilkinson, L.; Anderson, A.; Hamon, D.; Wood, S.A.; Peake, B.M. First detection of tetrodotoxin in the bivalve Paphies australis by liquid chromatography coupled to triple quadrupole mass spectrometry with and without precolumn reaction. J. AOAC Int. 2014, 97, 325–333.
- Biessy, L.; Smith, K.F.; Harwood, D.T.; Boundy, M.J.; Hawes, I.; Wood, S.A. Spatial variability and depuration of tetrodotoxin in the bivalve Paphies australis from New Zealand. Toxicon X 2019, 2, 100008.
- Biessy, L.; Smith, K.F.; Boundy, M.J.; Webb, S.C.; Hawes, I.; Wood, S.A. Distribution of tetrodotoxin in the New Zealand Clam, Paphies Australis, established using immunohistochemistry and liquid chromatography-tandem quadrupole mass spectrometry. Toxins 2018, 10, 282.
- Turner, A.D.; Powell, A.; Schofield, A.; Lees, D.N.; Baker-Austin, C. Detection of the pufferfish toxin tetrodotoxin in European bivalves, England, 2013 to 2014. Eurosurveillance 2015, 20, 21009.
- Bordin, P.; Dall’Ara, S.; Tartaglione, L.; Antonelli, P.; Calfapietra, A.; Varriale, F.; Guiatti, D.; Milandri, A.; Dell’Aversano, C.; Arcangeli, G.; et al. First occurrence of tetrodotoxins in bivalve mollusks from Northern Adriatic Sea (Italy). Food Control 2021, 120, 107510.
- Dhanji-Rapkova, M.; Turner, A.D.; Baker-Austin, C.; Huggett, J.F.; Ritchie, J.M. Distribution of tetrodotoxin in pacific oysters (Crassostrea gigas). Mar. Drugs 2021, 19, 84.
- Turner, A.D.; Dhanji-Rapkova, M.; Coates, L.; Bickerstaff, L.; Milligan, S.; O’Neill, A.; Faulkner, D.; McEneny, H.; Baker-Austin, C.; Lees, D.N.; et al. Detection of tetrodotoxin shellfish poisoning (TSP) toxins and causative factors in bivalve molluscs from the UK. Mar. Drugs 2017, 15, 277.
- Gerssen, A.; Bovee, T.H.F.; Klijnstra, M.D.; Poelman, M.; Portier, L.; Hoogenboom, R.L.A.P. First report on the occurrence of tetrodotoxins in bivalve mollusks in the Netherlands. Toxins 2018, 10, 450.
- Dell’Aversano, C.; Tartaglione, L.; Polito, G.; Dean, K.; Giacobbe, M.; Casabianca, S.; Capellacci, S.; Penna, A.; Turner, A.D. First detection of tetrodotoxin and high levels of paralytic shellfish poisoning toxins in shellfish from Sicily (Italy) by three different analytical methods. Chemosphere 2019, 215, 881–892.
- Noguchi, T.; Maruyama, J.; Hashimoto, K.; Narita, H. Tetrodotoxin in the starfish Astropecten polyacanthus, in association with toxification of a trumpet shell, “Boshubora” Charonia sauliae. Nippon Suisan Gakkaishi 1982, 48, 1173–1177.
- Maruyama, J.; Noguchi, T.; Jeon, J.K.; Harada, T.; Hashimoto, K. Occurrence of tetrodotoxin in the starfish Astropecten latespinosus. Experientia 1984, 40, 1395–1396.
- Maruyama, J.; Noguchi, T.; Narita, H.; Nara, M.; Jeon, J.K.; Otsuka, M.; Hashimoto, K. Occurrence of tetrodotoxin in a starfish, Astropecten scoparius. Agric. Biol. Chem. 1985, 49, 3069–3070.
- Kadota, N.; Narita, H.; Murakami, R.; Noguchi, T. The toxicity of starfishes, Astropecten genus, inhabiting the coast of Toyama Bay. Food Hyg. Saf. Sci. 2008, 49, 422–427.
- Khor, S.; Wood, S.A.; Salvitti, L.; Taylor, D.I.; Adamson, J.; McNabb, P.; Cary, S.C. Investigating diet as the source of tetrodotoxin in Pleurobranchaea maculata. Mar. Drugs 2014, 12, 1–16.
- Lin, S.J.; Tsai, Y.H.; Lin, H.P.; Hwang, D.F. Paralytic toxins in Taiwanese starfish Astropecten scoparius. Toxicon 1998, 36, 799–803.
- Miyazawa, K.; Higashiyama, M.; Ito, K.; Noguchi, T.; Arakawa, O.; Shida, Y.; Hashimoto, K. Tetrodotoxin in two species of ribbon worm (Nemertini), Lineus fuscoviridis and Tubulanus punctatus. Toxicon 1988, 26, 867–874.
- Kem, W.R. Purification and characterization of a new family of polypeptide neurotoxins from the heteronemertine Cerebratulus lacteus (Leidy). J. Biol. Chem. 1976, 251, 4184–4192.
- Ali, A.E.; Arakawa, O.; Noguchi, T.; Miyazawa, K.; Shida, Y.; Hashimoto, K. Tetrodotoxin and related substances in a ribbon worm Cephalothrix linearis (Nemertean). Toxicon 1990, 28, 1083–1093.
- Noguchi, T.; Ali, A.E.; Arakawa, O.; Miyazawa, K.; Kanoh, S.; Shida, Y.; Nishio, S.; Hashimoto, K. Tetrodonic acid-like substance; a possible precursor of tetrodotoxin. Toxicon 1991, 29, 845–855.
- Asakawa, M.; Toyoshima, T.; Shida, Y.; Noguchi, T.; Miyazawa, K. Paralytic toxins in a ribbon worm Cephalothrix species (Nemertean) adherent to cultured oysters in Hiroshima Bay, Hiroshima Prefecture, Japan. Toxicon 2000, 38, 763–773.
- Asakawa, M.; Toyoshima, T.; Ito, K.; Bessho, K.; Yamaguchi, C.; Tsunetsugu, S.; Shida, Y.; Kajihara, H.; Mawatari, S.F.; Noguchi, T.; et al. Paralytic toxicity in the ribbon worm Cephalothrix species (Nemertea) in Hiroshima Bay, Hiroshima Prefecture, Japan and the isolation of tetrodotoxin as a main component of its toxins. Toxicon 2003, 41, 747–753.
- Asakawa, M.; Ito, K.; Kajihara, H. Highly toxic ribbon worm Cephalothrix simula containing tetrodotoxin in Hiroshima Bay, Hiroshima Prefecture, Japan. Toxins 2013, 5, 376–395.
- Turner, A.D.; Fenwick, D.; Powell, A.; Dhanji-Rapkova, M.; Ford, C.; Hatfield, R.G.; Santos, A.; Martinez-Urtaza, J.; Bean, T.P.; Baker-Austin, C.; et al. New invasive nemertean species (Cephalothrix simula) in england with high levels of tetrodotoxin and a microbiome linked to toxin metabolism. Mar. Drugs 2018, 16, 452.
- Carroll, S.; McEvoy, E.G.; Gibson, R. The production of tetrodotoxin-like substances by nemertean worms in conjunction with bacteria. J. Exp. Mar. Biol. Ecol. 2003, 288, 51–63.
- Vlasenko, A.E.; Velansky, P.V.; Chernyshev, A.V.; Kuznetsov, V.G.; Magarlamov, T.Y. Tetrodotoxin and its analogues profile in nemertean species from the sea of Japan. Toxicon 2018, 156, 48–51.
- Vlasenko, A.E.; Magarlamov, T.Y. Tetrodotoxin and its analogues in Cephalothrix cf. simula (Nemertea: Palaeonemertea) from the Sea of Japan (Peter the Great Gulf): Intrabody distribution and secretions. Toxins 2020, 12, 745.
- Magarlamov, T.Y.; Shokur, O.A.; Chernyshev, A.V. Distribution of tetrodotoxin in the ribbon worm Lineus alborostratus (Takakura, 1898) (nemertea): Immunoelectron and immunofluorescence studies. Toxicon 2016, 112, 29–34.
- Vlasenko, A.E.; Kuznetsov, V.G.; Malykin, G.V.; Pereverzeva, A.O.; Velansky, P.V.; Yakovlev, K.V.; Magarlamov, T.Y. Tetrodotoxins secretion and voltage-gated sodium channel adaptation in the ribbon worm Kulikovia alborostrata (Takakura, 1898) (Nemertea). Toxins 2021, 13, 606.
- Kwon, Y.S.; Min, S.K.; Yeon, S.J.; Hwang, J.H.; Hong, J.S.; Shin, H.S. Assessment of neuronal cell-based cytotoxicity of neurotoxins from an estuarine nemertean in the han river estuary. J. Microbiol. Biotechnol. 2017, 27, 725–730.
- Tanu, M.B.; Mahmud, Y.; Arakawa, O.; Takatani, T.; Kajihara, H.; Kawatsu, K.; Hamano, Y.; Asakawa, M.; Miyazawa, K.; Noguchi, T. Immunoenzymatic visualization of tetrodotoxin (TTX) in Cephalothrix species (Nemertea: Anopla: Palaeonemertea: Cephalotrichidae) and Planocera reticulata (Platyhelminthes: Turbellaria: Polycladida: Planoceridae). Toxicon 2004, 44, 515–520.
- Malykin, G.V.; Chernyshev, A.V.; Magarlamov, T.Y. Intrabody tetrodotoxin distribution and possible hypothesis for its migration in ribbon worms Cephalothrix cf. simula (palaeonemertea, nemertea). Mar. Drugs 2021, 19, 494.
- Campbell, M.E.; Schwartz, M. Immunohistological Visualization of Tetrodotoxin in Micrura Verrili and Dushia Atra (Phylum Nemertea). In Proceedings of the National Conferences for Undergraduate Research (NCUR), Salisbury, MD, USA, 10–12 April 2008.
- Arndt, W. Polycladen und maricole tricladen als giftträge. In Memórias e Estudios do Museu Zoológico da Universidade de Coimbra; Universidade de Coimbra: Coimbra, Portugal, 1943; Volume 148, pp. 1–15.
- Miyazawa, K.; Jeon, J.K.; Maruyama, J.; Noguchi, T.; Ito, K.; Hashimoto, K. Occurrence of tetrodotoxin in the flatworm Planocera multitentaculata. Toxicon 1986, 24, 645–650.
- Miyazawa, K.; Jeon, J.K.; Noguchi, T.; Ito, K.; Hashimoto, K. Distribution of tetrodotoxin in the tissues of the flatworm Planocera multitentaculata (Platyhelminthes). Toxicon 1987, 25, 975–980.
- Kashitani, M.; Okabe, T.; Oyama, H.; Noguchi, K.; Yamazaki, H.; Suo, R.; Mori, T.; Sugita, H.; Itoi, S. Taxonomic distribution of tetrodotoxin in acotylean flatworms (Polycladida: Platyhelminthes). Mar. Biotechnol. 2020, 22, 805–811.
- Jeon, J.K.; Miyazawa, K.; Narita, H.; Nara, M.; Noguchi, T.; Ito, K.; Matsubara, S.; Hashimoto, K. Occurrence of Paralytic Toxicity in Marine Flatworms. Nippon Suisan Gakkaishi 1986, 52, 1065–1069.
- Ritson-Williams, R.; Yotsu-Yamashita, M.; Paul, V.J. Ecological functions of tetrodotoxin in a deadly polyclad flatworm. Proc. Natl. Acad. Sci. USA 2006, 103, 3176–3179.
- Salvitti, L.R.; Wood, S.A.; Taylor, D.I.; McNabb, P.; Cary, S.C. First identification of tetrodotoxin (TTX) in the flatworm Stylochoplana sp.; A source of TTX for the sea slug Pleurobranchaea maculata. Toxicon 2015, 95, 23–29.
- Suo, R.; Kashitani, M.; Oyama, H.; Adachi, M.; Nakahigashi, R.; Sakakibara, R.; Nishikawa, T.; Sugita, H.; Itoi, S. First Detection of tetrodotoxins in the cotylean rlatworm Prosthiostomum trilineatum. Mar. Drugs 2021, 19, 40.
- Stokes, A.N.; Ducey, P.K.; Neuman-Lee, L.; Hanifin, C.T.; French, S.S.; Pfrender, M.E.; Brodie, E.D., III; Brodie, E.D., Jr. Confirmation and distribution of tetrodotoxin for the first time in terrestrial invertebrates: Two terrestrial flatworm species (Bipalium adventitium and Bipalium kewense). PLoS ONE 2014, 9, e100718.
- Yamada, R.; Tsunashima, T.; Takei, M.; Sato, T.; Wajima, Y.; Kawase, M.; Oshikiri, S.; Kajitani, Y.; Kosoba, K.; Ueda, H.; et al. Seasonal changes in the tetrodotoxin content of the flatworm Planocera multitentaculata. Mar. Drugs 2017, 15, 56.
- Yasumoto, T.; Nagai, H.; Yasumura, D.; Michishita, T.; Endo, A.; Yotsu, M.; Kotaki, Y. Interspecies distribution and possible origin of tetrodotoxin. Ann. N. Y. Acad. Sci. 1986, 479, 44–51.
- Thuesen, E.V.; Kogure, K.; Hashimoto, K.; Nemoto, T. Poison arrowworms: A tetrodotoxin venom in the marine phylum Chaetognatha. J. Exp. Mar. Biol. Ecol. 1988, 116, 249–256.
- Noguchi, T.; Uzu, A.; Koyama, K.; Maruyama, J.; Nagashima, Y.; Hashimoto, K. Occurrence of tetrodotoxin as the major toxin in a xanthid crab Atergatis floridus. Nippon Suisan Gakkaishi 1983, 49, 1887–1892.
- Tsai, Y.H.; Hwang, D.F.; Chai, T.J.; Jeng, S.S. Occurrence of tetrodotoxin and paralytic shellfish poison in the Taiwanese crab Lophozozymus pictor. Toxicon 1995, 33, 1669–1673.
- Hwang, D.F.; Tsai, Y.-H.; Chai, T.; Jeng, S.-S. Occurrence of tetrodotoxin and paralytic shellfish poison in Taiwan crab Zosimus aeneus. Fish. Sci. 1996, 62, 500–501.
- Tsai, Y.; Hwang, D.F.; Chai, T.J.; Jeng, S.S. Occurance of paralytic toxin in Taiwanese crab Atergatopsis germaini. Toxicon 1996, 34, 467–474.
- Tsai, Y.H.; Hwang, D.F.; Chai, T.J.; Jeng, S.S. Toxicity and toxic components of two xanthid crabs, Atergatis floridus and Demania reynaudi, in Taiwan. Toxicon 1997, 35, 1327–1335.
- Sagara, T.; Taniyama, S.; Takatani, T.; Nishibori, N.; Nishio, S.; Noguchi, T.; Arakawa, O. Toxicity and toxin profiles of xanthid crabs collected around Nakanoshima, the Tokara islands, Japan. Shokuhin Eiseigaku Zasshi 2009, 50, 237–242.
- Asakawa, M.; Gomez-Delan, G.; Tsuruda, S.; Shimomura, M.; Shida, Y.; Taniyama, S.; Barte-Quilantang, M.; Shindo, J. Toxicity assessment of the xanthid crab Demania cultripes from Cebu Island, Philippines. J. Toxicol. 2010, 2010, 1–7.
- Saito, T.; Kohama, T.; Ui, K.; Watabe, S. Distribution of tetrodotoxin in the xanthid crab (Atergatis floridus) collected in the coastal waters of Kanagawa and Wakayama Prefectures. Comp. Biochem. Physiol.-Part D Genom. Proteom. 2006, 1 Pt D1, 158–162.
- Kungsuwan, A.; Noguchi, T.; Hashimoto, K.; Nagashima, Y.; Shida, Y.; Suvapeepan, S.; Suwansakornku, P. Tetrodotoxin in the horseshoe crab Carcinoscorpius rotundicauda inhabiting Thailand. Nippon Suisan Gakkaishi 1987, 53, 261–266.
- Ikeda, K.; Venmathi Maran, B.A.; Honda, S.; Ohtsuka, S.; Arakawa, O.; Takatani, T.; Asakawa, M.; Boxshall, G.A. Accumulation of tetrodotoxin (TTX) in Pseudocaligus fugu, a parasitic copepod from panther puffer Takifugu pardalis, but without vertical transmission-Using an immunoenzymatic technique. Toxicon 2006, 48, 116–122.
- Ito, K.; Okabe, S.; Asakawa, M.; Bessho, K.; Taniyama, S.; Shida, Y.; Ohtsuka, S. Detection of tetrodotoxin (TTX) from two copepods infecting the grass puffer Takifugu niphobles: TTX attracting the parasites? Toxicon 2006, 48, 620–626.
- Kodama, T.; Ikeda, K.; Arakawa, O.; Kondo, Y.; Asakawa, M.; Kawatsu, K.; Ohtsuka, S. Evidence of accumulation of tetrodotoxin (TTX) in tissues and body parts of ectoparasitic copepods via their feeding on mucus of TTX-bearing pufferfish. Toxicon 2021, 204, 37–43.
- Koyama, K.; Noguchi, T.; Uzu, A.; Hashimoto, K. Resistibility of toxic and nontoxic crabs against paralytic shellfish poison and tetrodotoxin. Nippon Suisan Gakkaishi 1983, 49, 485–489.
- Saito, T.; Maruyama, J.; Kanoh, S.; Jeon, J.K.; Noguchi, T.; Harada, T.; Murata, O.; Hashimoto, K. Toxicity of the cultured pufferfish Fugu rubripes rubripes along with their resistibility against tetrodotoxin. Nippon Suisan Gakkaishi 1984, 50, 1573–1575.
- Daigo, K.; Arakawa, O.; Noguchi, T.; Uzu, A.; Hashimoto, K. Resistibility of two xanthid crab Zoslmus aeneus and Daira perlata against paralytic shellfish poison and tetrodotoxin. Nippon Suisan Gakkaishi 1987, 53, 881–884.
- Shiomi, K.; Yamaguchi, S.; Kikuchi, T.; Yamamori, K.; Matsui, T. Occurrence of tetrodotoxin-binding high molecular weight substances in the body fluid of shore crab (Hemigrapsus sanguineus). Toxicon 1992, 30, 1529–1537.
- Hwang, P.A.; Tsai, Y.H.; Lin, H.P.; Hwang, D.F. Tetrodotoxin-binding proteins isolated from five species of toxic gastropods. Food Chem. 2007, 103, 1153–1158.
- Matsui, T.; Yamamori, K.; Furukawa, K.; Kono, M. Purification and some properties of a tetrodotoxin binding protein from the blood plasma of kusafugu, Takifugu niphobles. Toxicon 2000, 38, 463–468.
- Yotsu-Yamashita, M.; Sugimoto, A.; Terakawa, T.; Shoji, Y.; Miyazawa, T.; Yasumoto, T. Purification, characterization, and cDNA cloning of a novel soluble saxitoxin and tetrodotoxin binding protein from plasma of the puffer fish, Fugu pardalis. Eur. J. Biochem. 2001, 268, 5937–5946.
- Matsumoto, T.; Tanuma, D.; Tsutsumi, K.; Jeon, J.K.; Ishizaki, S.; Nagashima, Y. Plasma protein binding of tetrodotoxin in the marine puffer fish Takifugu rubripes. Toxicon 2010, 55, 415–420.
This entry is offline, you can click here to edit this entry!
