MicroRNAs are promising novel biomarkers for the diagnosis and prognosis of cardiovascular diseases. These molecules are defined as a class of short-sequence non-coding RNAs that influence the expression of numerous genes. Each miRNA is a short sequence of non-coding RNA that influences countless genes’ expression and, thereby, contributes to several physiological pathways and diseases. It has been demonstrated that miRNAs participate in the development of many cardiovascular diseases (CVDs). The growing understanding of cardiac biology contributed to recognising specific abnormal microRNA expression when diseases are present, which makes them potential biomarkers and therapeutical targets.
- microRNAs
- cardiovascular diseases
- biomarker
- dog
- cat
1. Introduction
MicroRNAs are a class of single-stranded endogenous small non-coding RNAs, about 21 nucleotides in length. They are transcriptionally regulated in a manner identical to typical messenger RNA (mRNA) and, when processed, can silence or downregulate the expression of their targets [1]. Most mature miRNAs are processed from long primary transcripts in a stepwise process involving a series of endonucleolytic cleavages. The mature miRNAs are loaded in a large protein complex known as the RNA-induced silencing complex. MicroRNAs guide the RNA-induced silencing complex to complementary target mRNAs, which are translationally repressed or cleaved [2].
In the clinical setting, biomarkers can help with the early diagnosis of diseases, evaluate and manage the response to therapy, and assess patient prognosis. MicroRNAs have numerous characteristics that potentially make them suitable new and non-invasive biomarkers [3].
Circulating miRNAs have been detected in peripheral blood and body fluids such as saliva, urine, and breast milk [4]. They can be secreted or produced due to various events like passive leakage from cells and active secretion via cell-derived membrane vesicles or a protein–miRNA complex [5]. There are two circulating miRNA populations; one can be found in proteins like argonaute-2 and high-density lipoproteins. The other is associated with vesicles such as exosomes, microvesicles, and apoptotic bodies [6]. Exosomes are phospholipid bilayer nanovesicles that carry substances such as lipids, proteins, DNA, and non-coding RNA [7]. They can be secreted by almost all eukaryotic cells, and they are a form of cell-to-cell communication in physiological and pathological conditions [8]. Several clinical studies characterised exosomal miRNAs (ex-miRNAs) and validated their potential clinical applications. MicroRNAs can be quantified in cells, tissues, and biological fluids. Many commercial RNA extraction kits, such as chloroform-phenol-based extraction, magnetic bead extraction, and column-based extraction, are available. Afterward, RNA expression can be analysed using different assays like northern blot, microarray, next-generation sequencing (RNAseq), droplet digital polymerase chain reaction, and real-time polymerase chain reaction (RT-qPCR). Among these methods, RT-qPCR is the gold standard for measuring circulating miRNAs [9].
MicroRNAs regulate various biological processes, including immune response, hematopoietic development and function, tumour suppression, and tumorigenesis [10]. The growing understanding of cardiac biology contributed to recognising specific miRNAs as novel biomarkers for several cardiovascular diseases (CVDs) as they participate in processes such as cardiomyocyte differentiation, growth and contractility, and cardiac rhythm [11]. Abnormal miRNA expression is associated with pathological processes such as congenital defects, arrhythmias, cardiac hypertrophy, and heart failure (HF) [12]. In fact, these molecules play a role in several molecular pathways related to CVDs, such as cardiac fibrosis, through the transforming growth factor-beta and mitogen-activated protein kinase pathways, among others; cardiac hypertrophy, by calcium signalling and cell cycle-related pathways; and angiogenesis, via the vascular endothelial growth factor and other angiogenic pathways [13][14][15].
2. MicroRNAs in the Diagnosis of Cardiovascular Diseases
2.1. Heart Development
2.2. Arrhythmias
The miRNA expression level is altered in patients with AF [22]. In atrial fibroblasts, the expression of the extracellular matrix (ECM) genes collagen-1A1 (COL1A1), collagen-3A1 (COL3A1), and fibrillin is modulated by miR-29b. Plasma from human patients with AF and atrial tissue from dogs with AF showed decreased expression of miR-29b. Therefore, miR-29b likely plays a role in atrial fibrotic remodelling and might have value as a biomarker or therapeutic target [23]. Moreover, miR-21 seems to be involved in profibrotic collagen production by regulating COL1A1 indirectly by targeting Sprouty homolog-1 [24]. On the other hand, miR-133 and miR-30 are anti-fibrotic and play a relevant role in the structural alterations observed in chronic AF [25]. Qiao et al. stated that miR-132 is downregulated in canine and human patients with AF. The downregulation of miR-132 and the upregulation of the connective tissue growth factor (CTGF), which is an essential player in the process of fibrosis, suggests a molecular mechanism associated with the development of AF-dependent fibrosis. This fact may provide a potential therapeutic target for AF treatment in the future [26].
2.3. Myxomatous Mitral Valve Disease
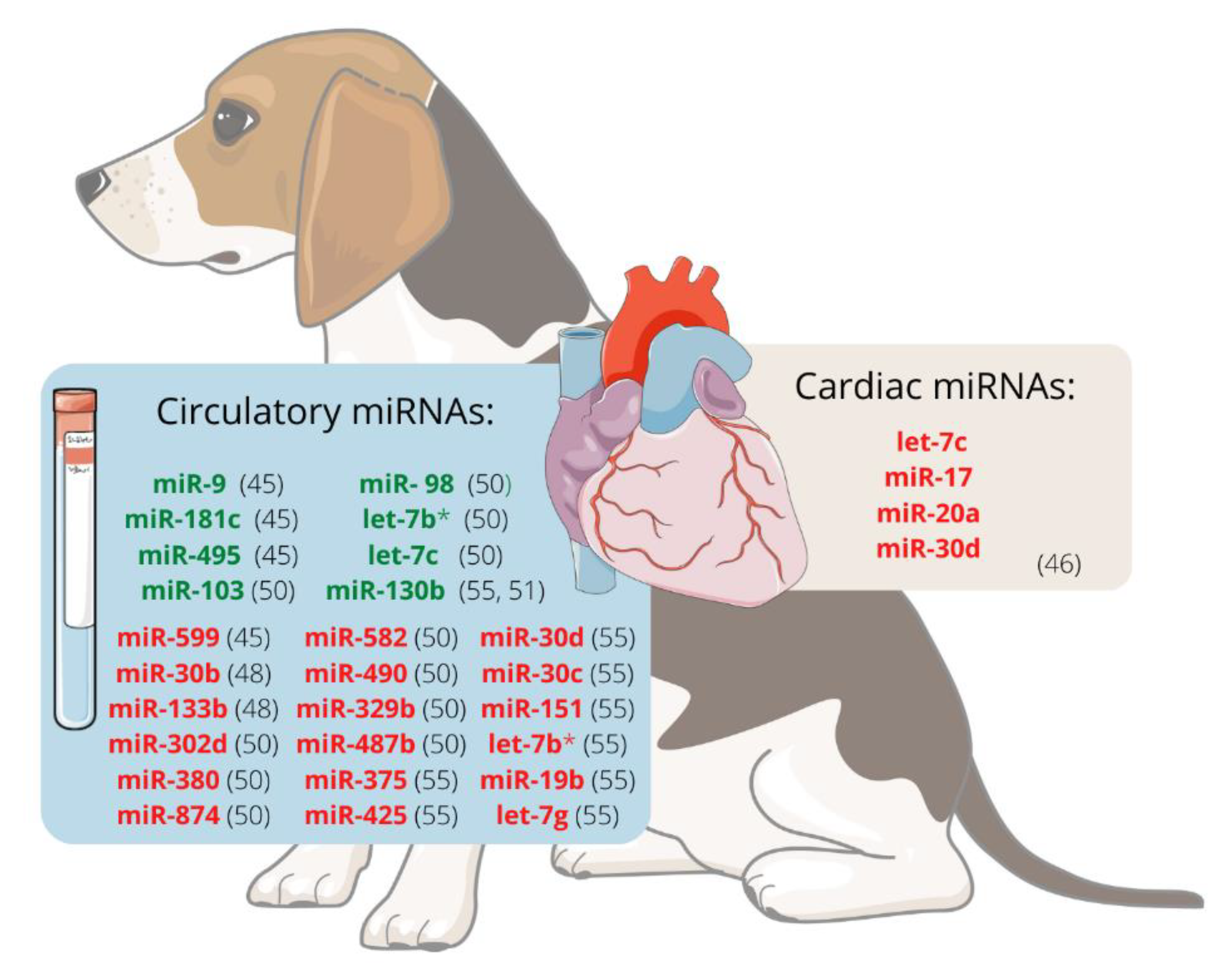
When evaluating the expression of circulating ex-miRNAs in dogs with MMVD, changes in these miRNAs are detected in dogs as they become older (miR-9, miR-495, and miR-599) and develop MMVD (miR-9 and miR-599) or CHF (miR-181c and miR-495) [27].
It was hypothesised that miRNAs are involved in the development of MMVD and that valvular interstitial cells (VIC) probably endure disease-relevant changes. The expression of miRNAs in VIC from canine mitral valve tissues was analysed using RT-qPCR and RNAseq. Using both methods, let-7c, miR-17, miR-20a, and miR-30d were significantly downregulated in VICs from diseased valves compared to healthy ones, suggesting that they may participate in the development of canine MMVD. This decrease in let-7c, mir-17, and miR-20a is thought to contribute to myofibroblastic differentiation and cell senescence. In contrast, reducing miR-30d may disinhibit cell apoptosis, so these four miRNAs should be further investigated as potential therapeutic targets [28].
There is evidence that the miRNA expression profile in dogs with MMVD differs between ACVIM stages. Dachshund is one breed prone to develop MMVD, which ultimately causes heart failure (HF). Compared with unaffected dogs, miR-30b is significantly downregulated in ACVIM stage B [29]. This miRNA downregulates CTGF, a profibrotic protein [30], and could potentially be used as a biomarker of ACVIM stage B [29]. Moreover, it appears that expression changes are greater as the disease progresses. Seven miRNAs, cfa-miR-302d, cfa-miR-380, cfa-miR-874, cfa-miR-582, cfa-miR-490, cfa-miR-329b, and cfa-miR-487b appeared to be downregulated whereas four, cfa-miR-103, cfa-miR-98, cfa-let-7b, and cfa-let-7c, were upregulated in stage B1/B2 or C/D, when compared with stage A dogs. When comparing stages B1/B2 and C/D, the expression of cfa-miR-582 and cfa-miR-487b was higher in the latest groups, whereas cfa-miR-103, cfa-miR-98, cfa-let-7b, and cfa-let-7c were downregulated in the stage C/D dogs. This fact suggests that miRNAs might be useful biomarkers for diagnosis, prognosis, or monitoring response to treatment in dogs with MMVD [31].
Figure 1 represents the miRNAs differentially expressed, either in plasma/serum or cardiac tissue, in dogs with MMVD.
2.4. Cardiomyopathies
A study carried out in 2013 compared miRNA expression patterns between Doberman Pinschers with DCM and healthy ones. They screened serum miRNA expression profiles using miRNA microarray but did not find statistical significance in the 22 miRNAs that appeared to be differently expressed in the two groups. From these 22 miRNAs, five (miR-142-3p, miR-144*, miR-21, let-7c, and miR92a) were selected for further analysis using RT-qPCR, as these were previously mentioned as being involved in cardiovascular pathology. Note that miR-144* and miR-144 are the two strands of the double-stranded precursor miRNA (mir-144). The absence of no statistically significant differences observed in this RT-qPCR analysis may be explained by the small sample size (only four animals per group) [34]. Hypertrophic cardiomyopathy (HCM) is a spontaneously occurring cardiac disease of the cat. In diseased cats, miR-381-3p, miR-486-3p, miR-4751, miR-476c-3p, miR-5700, miR-513a-3p, miR-320e and miR-1246 appeared to be upregulated [35]. In humans, HCM is a common inherited cardiomyopathy [36]. Several studies analysed miRNAs’ role in HCM progression in humans, though only a few miRNAs overlap between studies (miR-29, miR-21, miR-133, and miR-1) [37]. The study of HCM may help to disclose pathophysiologic pathways that may be useful for diagnosis, prognosis, and therapeutics of human HCM.
2.5. Heartworm Disease
In 2014, the presence of circulating filarial-derived miRNAs in the host bloodstream was studied by Tritten et al. They detected over 200 mature miRNA sequences that possibly originated from nematodes in the plasma of dogs infected with D. immitis. Both miR-34 and miR-71 were found in all samples from D. immitis-infected dogs. However, these miRNAs were also detected in samples from dogs infected with Brugia pahangi (but never appeared in the plasma of uninfected dogs). Since these two miRNA mature sequences can also be found completely conserved in other nematodes, they may not distinguish the presence of different species. Besides, a low correlation was found between miRNA copy numbers and microfilaria counts, implying that adults also significantly release miRNAs into the bloodstream. This also means that filarial-derived miRNAs can be found in plasma/serum even when the parasite does not exist in the bloodstream [38]. By measuring plasma levels of miR-34 and miR-71, there was no significant difference in expression between low- and high-intensity infections in dogs infected with D. immitis adult worms. When comparing the infected and non-infected groups, there was a substantial difference as the copy number of both these miRNAs was elevated in dogs with D. immitis. This reiterates that miR-34 and miR-71 could be used as biomarkers for identifying D. immitis infection in dogs, even though their copy number does not reflect the intensity of adults in the host.This determined if the intensity of adult worm infection could be distinguished using miRNAs as biomarkers and if they could be used to identify new infections.
2.6. Heart Failure
MicroRNAs could influence gene expression changes which are consistent with the pathophysiology of HF [24]. This makes miRNAs potential biomarkers as they may offer valuable information on the severity of the disease and risk stratification and guide the therapy plan [39]. Jung et al. found significant differences between the miRNA expression profiles in dogs with CHF secondary to MMVD and healthy controls. Of all the 326 miRNAs identified, four (miR-133, miR-1, cfa-let-7e, and miR-125a) were significantly upregulated, and four (miR-30c, miR-128, miR-142, and miR-423) were downregulated in dogs with CHF. These downregulated miRNAs were associated, for example, with cardiac hypertrophy and endothelial-to-mesenchymal transition, which makes them potential biomarkers for this disease in dogs [40].
2.7. Coronary Artery Disease and Myocardial Infarction
In humans, CAD is the most frequent cause of unexpected cardiac death in adults and the most significant cause of global morbidity and mortality [41]. MicroRNAs are potential biomarkers of CAD since this disease and its risk factors (e.g., abnormal lipid metabolism, inflammation) cause alterations in miRNAs expression profiles [42]. Of the dysregulated miRNAs, miR-1, miR133a, miR-133b, miR-208, and miR-499 appeared to be the most promising for diagnosing acute myocardial infarction. On the other hand, miR-126, miR-199a, miR-132, miR-140-3p, and miR-210 seemed more useful as prognostic biomarkers [41]. In people with ST-segment elevation myocardial infarction, miR-208a is the most promising biomarker. It allows an earlier diagnosis compared with the gold standard, cardiac troponin (cTn), since it can be detected within 2 hours of the onset of acute myocardial infarction. Besides, within 24 hours, miR-208a values decline to baseline, allowing for the detection of other minor cardiac events post-infarction [43].
2.8. Cardiac Toxicity
Three miRNAs were differentially expressed in canine patients after administration of DOX: miR-107 and miR-146a were significantly downregulated, while miR-502 was significantly upregulated [88].Similarly, in human paediatric patients, it was also demonstrated downregulation of mir-107 and mir-146a [44]. MicroRNAs are promising biomarkers for cardiotoxicity detection as they may help clinicians modify treatment or implement early cardioprotective strategies.Some miRNAs play a crucial role in cardiac remodelling after cardiomyocyte injury and are regulators of cardiac fibrosis [45].
2.9. Hypertensive Vascular Conditions
The renin–angiotensin–aldosterone system is a crucial point in maintaining an adequate extravascular volume and blood pressure [46]. Several miRNAs regulate renin–angiotensin–aldosterone system genes. The miRNA expression is altered in hypertensive human patients [47]. MiR-136 was significantly downregulated in peripheral blood serum of hypertensive patients [48]. Some miRNAs, like miR-202, were overexpressed and might exert a protective role against hypertension [49]. Other miRNAs with high expression in hypertensive patients were miR-21, miR-126, miR-196a, and miR-451, whereas miR-181a, miR-638, and miR-663 were under-expressed [50]. Moreover, it was noted that miR-181a, miR-663, and miR-25 regulate the renin gene [50][51].
MiR-150 levels were significantly reduced in human patients with PH and samples harvested from the lungs of a rat model of PH [52]. Moreover, it was observed that miR-26a, miR-29c, miR-34b, and miR-451 were downregulated, and miR-21, miR-130a, miR-133b, miR-191, miR-204, and miR-208b were upregulated. These plasma miRNAs were thought to be candidates for diagnostic biomarkers of PH in humans [53].
3. Conclusions
The discovery of noncoding RNA has revolutionised gene expression knowledge. MicroRNA’s contribution to diverse physiological pathways in the cardiovascular field is evident and highly promising but is yet to be fully understood. Furthermore, miRNA’s high stability in biological samples and their presence in circulation mirroring the changes within cells imply a potential role as biomarkers.
This entry is adapted from the peer-reviewed paper 10.3390/vetsci9100533
References
- Krol, J.; Loedige, I.; Filipowicz, W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010, 11, 597–610.
- Wahid, F.; Shehzad, A.; Khan, T.; Kim, Y.Y. MicroRNAs: Synthesis, mechanism, function, and recent clinical trials. Biochim. Biophys. Acta 2010, 1803, 1231–1243.
- Fiedler, J.; Batkai, S.; Thum, T. MicroRNA-based therapy in cardiology. Herz 2014, 39, 194–200.
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The MicroRNA Spectrum in 12 Body Fluids. Clin. Chem. 2010, 56, 1733–1741.
- Redis, R.S.; Calin, S.; Yang, Y.; You, M.J.; Calin, G.A. Cell-to-cell miRNA transfer: From body homeostasis to therapy. Pharmacol. Ther. 2012, 136, 169–174.
- Kamal, N.N.S.B.N.M.; Shahidan, W.N.S. Non-exosomal and exosomal circulatory MicroRNAs: Which are more valid as biomarkers? Front. Pharmacol. 2020, 10, 1500.
- Meng, Y.; Sun, J.; Wang, X.; Hu, T.; Ma, Y.; Kong, C.; Piao, H.; Yu, T.; Zhang, G. Exosomes: A Promising Avenue for the Diagnosis of Breast Cancer. Technol. Cancer Res. Treat 2019, 18, 1533033818821421.
- Asgarpour, K.; Shojaei, Z.; Amiri, F.; Ai, J.; Mahjoubin-Tehran, M.; Ghasemi, F.; ArefNezhad, R.; Hamblin, M.R.; Mirzaei, H. Exosomal microRNAs derived from mesenchymal stem cells: Cell-to-cell messages. Cell Commun. Signal 2020, 18, 149.
- Dave, V.P.; Ngo, T.A.; Pernestig, A.K.; Tilevik, D.; Kant, K.; Nguyen, T.; Wolff, A.; Bang, D.D. MicroRNA amplification and detection technologies: Opportunities and challenges for point of care diagnostics. Lab. Investig. 2019, 99, 452–469.
- Baltimore, D.; Boldin, M.P.; O’Connell, R.M.; Rao, D.S.; Taganov, K.D. MicroRNAs: New regulators of immune cell development and function. Nat. Immunol. 2008, 9, 839–845.
- Islas, J.F.; Moreno-Cuevas, J.E. A MicroRNA perspective on cardiovascular development and diseases: An update. Int. J. Mol. Sci. 2018, 19, 2075.
- Çakmak, H.A.; Demir, M. MicroRNA and Cardiovascular Diseases. Balk. Med. J. 2020, 37, 60–71.
- Yang, C.; Zheng, S.D.; Wu, H.J.; Chen, S.-J. Regulatory Mechanisms of the Molecular Pathways in Fibrosis Induced by MicroRNAs. Chin. Med. J. 2016, 129, 2365–2372.
- Wehbe, N.; Nasser, S.A.; Pintus, G.; Badran, A.; Eid, A.H.; Baydoun, E. MicroRNAs in Cardiac Hypertrophy. Int. J. Mol. Sci. 2019, 20, 4714.
- Wang, H.; Cai, J. The role of microRNAs in heart failure. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 2019–2030.
- Park, C.Y.; Choi, Y.S.; McManus, M.T. Analysis of microRNA knockouts in mice. Hum. Mol. Genet. 2010, 19, R169–R175.
- Rao, P.K.; Toyama, Y.; Chiang, H.R.; Gupta, S.; Bauer, M.; Medvid, R.; Reinhardt, F.; Liao, R.; Krieger, M.; Jaenisch, R.; et al. Loss of cardiac microRNA-mediated regulation leads to dilated cardiomyopathy and heart failure. Circ. Res. 2009, 105, 585–594.
- Ivey, K.N.; Muth, A.; Arnold, J.; King, F.W.; Yeh, R.F.; Fish, J.E.; Hsiao, E.C.; Schwartz, R.J.; Conklin, B.R.; Bernstein, H.S.; et al. MicroRNA Regulation of Cell Lineages in Mouse and Human Embryonic Stem Cells. Cell Stem Cell 2008, 2, 219–229.
- van Rooij, E.; Quiat, D.; Johnson, B.A.; Sutherland, L.B.; Qi, X.; Richardson, J.A.; Kelm, R.J.; Olson, E.N. A Family of microRNAs Encoded by Myosin Genes Governs Myosin Expression and Muscle Performance. Dev. Cell 2009, 17, 662–673.
- Porrello, E.R.; Johnson, B.A.; Aurora, A.B.; Simpson, E.; Nam, Y.J.; Matkovich, S.J.; Dorn, G.W.; Van Rooij, E.; Olson, E.N. MiR-15 family regulates postnatal mitotic arrest of cardiomyocytes. Circ. Res. 2011, 109, 670–679.
- Ventura, A.; Young, A.G.; Winslow, M.M.; Lintault, L.; Meissner, A.; Erkeland, S.J.; Newman, J.; Bronson, R.T.; Crowley, D.; Stone, J.R.; et al. Targeted Deletion Reveals Essential and Overlapping Functions of the miR-17∼92 Family of miRNA Clusters. Cell 2008, 132, 875–886.
- Zhang, L.; Wang, X.; Huang, C. A narrative review of non-coding RNAs in atrial fibrillation: Potential therapeutic targets and molecular mechanisms. Ann. Transl. Med. 2021, 9, 1486.
- Dawson, K.; Wakili, R.; Ördög, B.; Clauss, S.; Chen, Y.; Iwasaki, Y.; Voigt, N.; Qi, X.Y.; Sinner, M.F.; Dobrev, D.; et al. MicroRNA29: A mechanistic contributor and potential biomarker in atrial fibrillation. Circulation 2013, 127, 1466–1475.
- Chen, Y.; Wakili, R.; Xiao, J.; Wu, C.T.; Luo, X.; Clauss, S.; Dawson, K.; Qi, X.; Naud, P.; Shi, Y.F.; et al. Detailed characterization of microRNA changes in a canine heart failure model: Relationship to arrhythmogenic structural remodeling. J. Mol. Cell. Cardiol. 2014, 77, 113–124.
- Li, H.; Li, S.; Yu, B.; Liu, S. Expression of miR-133 and miR-30 in chronic atrial fibrillation in canines. Mol. Med. Rep. 2012, 5, 1457–1460.
- Qiao, G.; Xia, D.; Cheng, Z.; Zhang, G. miR-132 in atrial fibrillation directly targets connective tissue growth factor. Mol. Med. Rep. 2017, 16, 4143–4150.
- Yang, V.K.; Loughran, K.A.; Meola, D.M.; Juhr, C.M.; Thane, K.E.; Davis, A.M.; Hoffman, A.M. Circulating exosome microRNA associated with heart failure secondary to myxomatous mitral valve disease in a naturally occurring canine model. J. Extracell. Vesicles 2017, 6, 1350088.
- Yang, V.K.; Tai, A.K.; Huh, T.P.; Meola, D.M.; Juhr, C.M.; Robinson, N.A.; Hoffman, A.M. Dysregulation of valvular interstitial cell let-7c, miR-17, miR-20a, and miR-30d in naturally occurring canine myxomatous mitral valve disease. PLoS ONE 2018, 13, e0188617.
- Hulanicka, M.; Garncarz, M.; Parzeniecka-Jaworska, M.; Jank, M. Plasma miRNAs as potential biomarkers of chronic degenerative valvular disease in Dachshunds. BMC Vet. Res. 2014, 10, 205.
- Duisters, R.F.; Tijsen, A.J.; Schroen, B.; Leenders, J.J.; Lentink, V.; van der Made, I.; Herias, V.; van Leeuwen, R.E.; Schellings, M.W.; Barenbrug, P.; et al. miR-133 and miR-30 regulate connective tissue growth factor: Implications for a role of microRNAs in myocardial matrix remodeling. Circ. Res. 2009, 104, 170–178.
- Li, Q.; Freeman, L.M.; Rush, J.E.; Laflamme, D.P. Expression Profiling of Circulating MicroRNAs in Canine Myxomatous Mitral Valve Disease. Int. J. Mol. Sci. 2015, 16, 14098–14108.
- Ro, W.-B.; Kang, M.-H.; Song, D.-W.; Lee, S.-H.; Park, H.-M. Expression Profile of Circulating MicroRNAs in Dogs With Cardiac Hypertrophy: A Pilot Study. Front. Vet. Sci. 2021, 8, 652224.
- Balistreri, C.R.; Allegra, A.; Crapanzano, F.; Pisano, C.; Ruvolo, G. Matrix Metalloproteinases (MMPs), Their Genetic Variants and miRNA in Mitral Valve Diseases: Potential Biomarker Tools and Targets for Personalized Treatments. J. Heart Valve Dis. 2016, 25, 463–474.
- Steudemann, C.; Bauersachs, S.; Weber, K.; Wess, G. Detection and comparison of microRNA expression in the serum of Doberman Pinschers with dilated cardiomyopathy and healthy controls. BMC Vet. Res. 2013, 9, 12.
- Weber, K.; Rostert, N.; Bauersachs, S.; Wess, G. Serum microRNA profiles in cats with hypertrophic cardiomyopathy. Mol. Cell. Biochem. 2015, 402, 171–180.
- Maron, B.J.; Desai, M.Y.; Nishimura, R.A.; Spirito, P.; Rakowski, H.; Towbin, J.A.; Rowin, E.J.; Maron, M.S.; Sherrid, M.V. Diagnosis and Evaluation of Hypertrophic Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 372–389.
- Chiti, E.; Paolo, M.D.; Turillazzi, E.; Rocchi, A. MicroRNAs in Hypertrophic, Arrhythmogenic and Dilated Cardiomyopathy. Diagnostics 2021, 11, 1720.
- Tritten, L.; Burkman, E.; Moorhead, A.; Satti, M.; Geary, J.; Mackenzie, C.; Geary, T. Detection of Circulating Parasite-Derived MicroRNAs in Filarial Infections. PLoS Negl. Trop. Dis. 2014, 8, e2971.
- Magnussen, C.; Blankenberg, S. Biomarkers for heart failure: Small molecules with high clinical relevance. J. Intern. Med. 2018, 283, 530–543.
- Jung, S.W.; Bohan, A. Genome-wide sequencing and quantification of circulating microRNAs for dogs with congestive heart failure secondary to myxomatous mitral valve degeneration. Am. J. Vet. Res. 2018, 79, 163–169.
- Borghini, A.; Andreassi, M.G. Genetic polymorphisms offer insight into the causal role of microRNA in coronary artery disease. Atherosclerosis 2018, 269, 63–70.
- Fazmin, I.T.; Achercouk, Z.; Edling, C.E.; Said, A.; Jeevaratnam, K. Circulating microrna as a biomarker for coronary artery disease. Biomolecules 2020, 10, 1354.
- Wang, C.; Jing, Q. Non-coding RNAs as biomarkers for acute myocardial infarction review-article. Acta Pharmacol. Sin. 2018, 39, 1110–1119.
- Oatmen, K.E.; Toro-Salazar, O.H.; Hauser, K.; Zellars, K.N.; Mason, K.C.; Hor, K.; Gillan, E.; Zeiss, C.J.; Gatti, D.M.; Spinale, F.G. Identification of a novel microRNA profile in pediatric patients with cancer treated with anthracycline chemotherapy. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1443–H1452.
- van Rooij, E.; Sutherland, L.B.; Thatcher, J.E.; DiMaio, J.M.; Naseem, R.H.; Marshall, W.S.; Hill, J.A.; Olson, E.N. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl. Acad. Sci. USA 2008, 105, 13027–13032.
- Patel, S.; Rauf, A.; Khan, H.; Abu-Izneid, T. Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomed. Pharm. 2017, 94, 317–325.
- Improta-Caria, A.C.; Aras, M.G.; Nascimento, L.; De Sousa, R.A.L.; Aras-Júnior, R.; Souza, B.S.F. MicroRNAs Regulating Renin-Angiotensin-Aldosterone System, Sympathetic Nervous System and Left Ventricular Hypertrophy in Systemic Arterial Hypertension. Biomolecules 2021, 11, 1771.
- Chu, H.T.; Li, L.; Jia, M.; Diao, L.L.; Li, Z.B. Correlation between serum microRNA-136 levels and RAAS biochemical markers in patients with essential hypertension. Eur. Rev. Med. Pharm. Sci. 2020, 24, 11761–11767.
- Li, L.; Zhong, D.; Xie, Y.; Yang, X.; Yu, Z.; Zhang, D.; Jiang, X.; Wu, Y.; Wu, F. Blood microRNA 202-3p associates with the risk of essential hypertension by targeting soluble ST2. Biosci. Rep. 2020, 40, BSR20200378.
- Marques, F.Z.; Campain, A.E.; Tomaszewski, M.; Zukowska-Szczechowska, E.; Yang, Y.H.; Charchar, F.J.; Morris, B.J. Gene expression profiling reveals renin mRNA overexpression in human hypertensive kidneys and a role for microRNAs. Hypertension 2011, 58, 1093–1098.
- Li, H.; Xie, Y.; Liu, Y.; Qi, Y.; Tang, C.; Li, X.; Zuo, K.; Sun, D.; Shen, Y.; Pang, D.; et al. Alteration in microRNA-25 expression regulate cardiac function via renin secretion. Exp. Cell Res. 2018, 365, 119–128.
- Rhodes, C.J.; Wharton, J.; Boon, R.A.; Roexe, T.; Tsang, H.; Wojciak-Stothard, B.; Chakrabarti, A.; Howard, L.S.; Gibbs, J.S.; Lawrie, A.; et al. Reduced microRNA-150 is associated with poor survival in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2013, 187, 294–302.
- Wei, C.; Henderson, H.; Spradley, C.; Li, L.; Kim, I.K.; Kumar, S.; Hong, N.; Arroliga, A.C.; Gupta, S. Circulating miRNAs as potential marker for pulmonary hypertension. PLoS ONE 2013, 8, e64396.
