Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Obesity is defined as the accumulation of an excessive amount of body fat. The correlation of obesity with increased cancer incidence and death has been well established.
- adipocytes
- adiponcosis
- cancer
- cytokines
- inflammation
- metabolic dysregulation
- survival
- tumorigenesis
1. Obesity: A Cause of Cancer
Obesity and overweight are two prime risk factors for disorders related to hormones, blood circulation, and pulmonary functions, as they significantly contribute to chronic diseases such as atherosclerotic cardiovascular disease (ASCVD), type 2 diabetes, and asthma. Obesity causes inflammation, oxidative stress, and dysfunctions related to the hypothalamic–pituitary–adrenal (HPA) axis, as well as mitochondrial and synaptic activities, leading to depression, anxiety, cognitive deficits, and post-traumatic stress disorder (PTSD). Interestingly, people with obesity also have a decreased brain volume and are at higher risk of dementia [25]. Importantly, several types of cancer are directly related to obesity [26,27]. Figure 1 shows some of the biological mechanisms through which obesity can lead to cognitive decline, various psychological disorders, or neuropsychiatric manifestations.
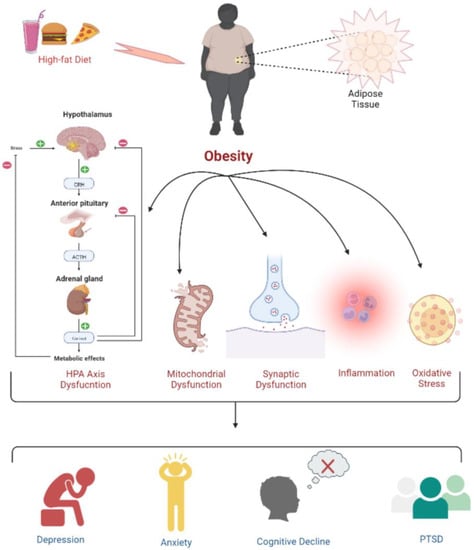
Figure 1. Psychophysiological changes due to high-fat-diet induced obesity. Obesity leads to a dysregulated HPA axis, mitochondrial and synaptic dysfunction, inflammation, and oxidative stress, which is further associated with the development of depression, anxiety, cognitive deficits, and PTSD.
Over the last two decades, the correlation of obesity with increased cancer incidence and death has been well established. The estimated percentage of cancer cases caused by obesity alone is 20% [28]. Obesity has also accounted for a significant percentage of deaths in both males and females [28]. The risk of malignancies is influenced by weight change, diet, body fat distribution, and overall physical activity [29]. To demonstrate the characteristic features of cancers (e.g., uncontrolled proliferations), the normal cells must undergo a neoplastic transformation. Obesity causes the chronic activation of cellular growth factor signaling pathways due to excess nutrients, leading to an increased risk for neoplastic transformation [30]. Moreover, the proliferation and growth of cells also depend on the nutrients that support the macromolecule synthesis. Excess energy due to excess food intake gets stored in the form of lipids inside the body, skeletal muscles, and organs (e.g., the liver). The increased level of lipids changes the usual metabolic milieu, which generates an environment that transmits signals for excess nutrients to the cells. As a result, the signaling pathways for angiogenesis, proliferation, glucose uptake, and cell growth are activated, dropping the barrier for oncogenic transformation [31,32].
A recent research study by Calle and Thun established a link between being overweight, obesity, and cancer [27], as they found a risk of colorectal cancer in obese adults. Of note, obese postmenopausal women had a 30–50% greater possibility of breast cancer according to breast cancer studies [27]. The evidence of the association between obesity and numerous types of cancer is shown in Table 2.
Table 2. This shows the link between obesity and the development of various types of cancer.
| S. No. | Cancer Type | Association with Obesity | Reference |
|---|---|---|---|
| 1. | Prostate Cancer | Obesity promotes low-grade inflammation linked to prostate cancer progression by compromising treatment and diagnosis. | [33] |
| 2. | Breast Cancer | In obese women, aromatization activity due to elevated levels of estrogen, the overexpression of insulin resistance, proinflammatory cytokines, IGFs, oxidative stress, and hypercholesterolemia contributes to the development of breast cancer. | [34] |
| 3. | Lung Cancer | Abdominal obesity has a significant role in the development of lung cancer. Smoking is one of the leading causes of the development of lung cancer; people who smoke regularly have an increased BMI. The reduced levels of sex-hormone-binding globulin and elevated estrogens and androgens are associated with obesity and an increased risk of lung cancer. However, the exact mechanism is not understood. | [35] |
| 4. | Bladder cancer | Obesity is a potential risk factor for recurrence, progression, or death with bladder cancer. | [36] |
| 5. | Colorectal Cancer | Visceral and abdominal fat increase the risks of colorectal cancer by up to 30–70% in men and are linked with worse outcomes and recurrence. | [37] |
| 6. | Kidney Cancer | Renal cell cancer (RCC) is the main form of kidney cancer. Studies have reported that people with a high waist-to-hip ratio (WHR), waist circumference (WC), and increased BMI are risk factors associated with RCC. | [38] |
| 7. | Hodgkin Lymphoma (HL) | It has been elucidated that inflammation is common both in HL and obesity. The interaction of molecules released by adipocytes and the tumor microenvironment associates obesity with an increased risk of developing HL. | [39] |
| 8. | Melanoma | Adipocytes provide nutrients to melanoma cells. Adipokines released by adipocytes stimulate the progression of myeloma cells. Moreover, it has been reported that insulin resistance and hyperinsulinemia may promote the growth of myeloma. | [40] |
| 9. | Pancreatic Cancer | Obesity increases the risk of pancreatic cancer through mechanisms that are not fully understood. Inflammation and hormone imbalance could be plausible causes. Excess abdominal adiposity is one of the few controllable risk factors for developing pancreatic cancer. | [41] |
| 10. | Thyroid Cancer | Adiponectin (APN) is one of the vitally essential adipocytokines. In obese individuals, there are reduced levels of APN. Similarly, reduced levels of APN have been found in patients with thyroid cancer and metabolic syndrome. | [42] |
| 11. | Endometrial Cancer | Visfatin, leptin, and resistin are associated with endometrial cancer proliferation, growth metastasis, and invasion. | [43] |
| 12. | Bone Cancer | Bone marrow adipocytes (BMAds) increase in size and number during obesity and can initiate bone cancer or cancer within the bone marrow. The BMAds provide nutrients to tumor cells and help in tumor cell proliferation. | [44] |
| 13. | Gastric Cancer | Obesity is associated with the occurrence of gastric cancer. The mechanism involved includes insulin resistance; higher levels of IGFs; and altered leptin, ghrelin, and adiponectin levels. | [45] |
| 14. | Neuroblastoma | Lim et al. reported on a 29-month-old Korean female who developed neuroblastoma and showed clinical features of ROHHAD. The laboratory examinations revealed high levels of IGF-1, prolactin, sex hormone, cortisol, and lactate dehydrogenase. | [46] |
2. Understanding the General Causes of Obesity
The research studies indicate several factors contributing to obesity; however, the primary cause has been related to an imbalance of energy research studies. Energy imbalances mainly occur when the energy consumed via food (or calorie intake) is not equivalent to the energy utilized (or calories spent) via regular bodily functions such as proper food digestion or staying active. This section will discuss some of the important factors that contribute to obesity [47].
2.1. A Sedentary Routine
Many people do not maintain an active lifestyle and spend countless hours in front of computers, televisions, and other digital gadgets. Technology and more advanced facilities have made it easier for people to do less physical work at home and in the workplace. People also prefer driving rather than walking and eating junk foods over cooking homemade meals. Hence, people with a sedentary lifestyle are prone to obesity and health issues such as coronary artery disease, cancer, and diabetes [48].
2.2. Family History
The probability of being overweight or obese increases if one or both parents are overweight or obese. Moreover, the food patterns and lifestyle habits of the parents are picked up by their children. As a result, a child born to obese parents who eat high-calorie foods and are physically inactive will most probably grow up obese [49]. On the other hand, if the family members are not obese and follow healthy eating and exercise habits, the chances of the child being overweight or obese are reduced [49].
2.3. Medicines
It is well established that atypical antipsychotic medications (clozapine, olanzapine, risperidone, and quetiapine) induce significant weight gain. Antidepressants, including amitriptyline, mirtazapine, and selective serotonin reuptake inhibitors (SSRIs), may also cause weight gain that cannot be attributed primarily to improved depressive symptoms [50]. Likewise, low mood is associated with increased food consumption, leading to lowered metabolic activity [50]. These changes in stabilizers such as lithium, valproic acid, and carbamazepine have demonstrated a similar effect. Importantly, antiepileptic medications (AEDs) such as sodium valproate, pregabalin, vigabatrin, carbamazepine, and gabapentin can cause weight gain [51,52]. Weight gain associated with antiepileptic medicines (AEDs) is a significant issue in the care of epilepsy patients. Moreover, weight gain is a reported side effect of corticosteroid medications [53]. Furthermore, histamine, a neurotransmitter secreted by the posterior hypothalamus, is also involved in body weight regulation [54], as antagonistic histamine stimulation increases appetite and slows body fat breakdown [55].
2.4. Smoking Cessation
Quitting smoking extends the life expectancy by decreasing the risk of major diseases, but is often associated with weight gain issues [56]. The most likely cause of this is an increase in appetite and a decrease in energy expenditure [57]. Nicotine or smoking aids in regulating or managing compulsive eating and overeating. One possibility for weight gain is that nicotine’s appetite-suppressing effect gets reversed [58]. Substitution reinforcement may occur when food is substituted for cigarettes [59]. A meta-analysis examination of weight gain in former smokers (quit for around 12 months) reported that weight gain following smoking cessation was more than previously believed. On average, without using nicotine replacement therapy or other medications, some showed 1.1 kg of weight gain in the 1st month, 2.3 kg in the 2nd month, and a continued increase of up to 4.7 kg following 12 months of smoking cessation. Furthermore, 13% of individuals gained over 10 kg upon cessation; interestingly, 16% lost weight after giving up smoking too [60]. Smokers with a particular history of binge eating during smoking cessation gain significantly more weight than non-smokers [61]. Another way of describing this is that sugary and fatty food consumption stimulates reward circuits in the brain, similar to smoking. [62]. Nicotine withdrawal results in an increased reward threshold, which may induce individuals to consume more carbohydrates and sugar-containing snacks [63].
3. Development of Tumor Due to Obesity: Underlying the Biological Mechanism
Obesity is defined as the accumulation of an excessive amount of body fat [64]. Several published statistical studies show that obesity-related cancer alone causes around 100,000 deaths per year. [65]. Of note, BMI, i.e., the ratio of weight to height squared, is used to determine the body fat content (square meters). Whereas a BMI of 25–29 kg/m2 is regarded as overweight, a BMI of 30 kg/m2 or above is considered obese [64]. Since increased adiposity is highly associated with an increased risk of developing or suffering from various cancers, researchers recently proposed the term “adiponcosis,” which is derived from the fusion of the Latin word ‘adiposis’ (excessive body fat accumulation) with the Greek word ‘oncosis’ (the development of tumors) [66].
The association of obesity with a very high risk of metabolic diseases such as insulin resistance, dyslipidemia, and non-alcoholic fatty liver disease (NAFLD) can lead to carcinogenesis. Chronic inflammation has been described as a significant contributing factor in the evolution and progression of such chronic diseases [67]. In obesity, the inflammatory trigger is metabolic and triggered by extra nutrients. Moreover, specific metabolic cells initiate the inflammatory response via specific metabolic signals to disrupt the normal cellular metabolic homeostasis [68].
The visceral adipose tissue is the largest endocrine organ, which produces proinflammatory cytokines (TNF-alpha, IL-6, and IL-17) and growth factors referred to as adipokines (adiponectin, omentin, chemerin), leading to dyslipidemia, insulin resistance, diabetes, cardiovascular disorders, and cancer [69]. Adipokines are aimed explicitly at storing energy as triglycerides within the lipid droplets of the cytoplasm [69]. Excess nutrients stimulate metabolic signaling pathways such as c-Jun N-terminal kinase (JNK), nuclear factor B (NFB), and protein kinase R. When these pathways are activated, lower levels of inflammatory cytokines are produced, leading to low inflammatory reactions [67]. Excess nutrition and obesity also cause hyperplasia and the enlargement of white adipose tissue adipocytes, significant tissue remodeling, and a rise in free fatty acids, all of which result in adipokine synthesis alterations and low-grade inflammatory responses [70].
Moreover, obesity results in increased endoplasmic reticulum stress, which activates the unfolded protein response, activating NF-kB, JNK, and oxidative stress, leading to the overexpression of proinflammatory cytokines [71]. Interestingly, macrophages in visceral adipose tissue have a unique function in significantly increased obesity. They are involved with adipose tissue inflammation and the production of inflammatory cytokines (TNF alpha, IL-6, IL-8, IL-17, IL-18, MCP-1), as well as additional adipokines (resistin, visfatin, and retinol-binding protein 4) [71]. All of these pathways direct to the onset of obesity-related inflammation. While inflammation linked with obesity is predominantly focused on the white adipose tissue, several other tissue types, such as the liver, pancreas, and brain, have also exhibited elevated inflammation under obesity [72]. Figure 2 outlines the various underlying primary mechanisms involved in tumor formation due to obesity.
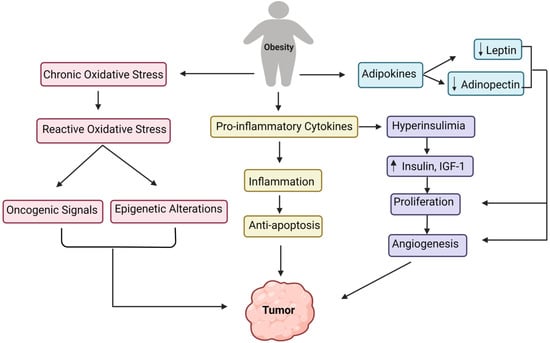
Figure 2. Converging pathways of the underlying mechanisms involved in the tumor development due to obesity.
This entry is adapted from the peer-reviewed paper 10.3390/life12101673
This entry is offline, you can click here to edit this entry!
