Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Diabetic retinopathy (DR), with increasing incidence, is the major cause of vision loss and blindness worldwide in working-age adults. Diabetic macular edema (DME) remains the main cause of vision impairment in diabetic patients, with its pathogenesis still not completely elucidated. Vascular endothelial growth factor (VEGF) plays a pivotal role in the pathogenesis of DR and DME.
- diabetic retinopathy
- diabetic macular edema
- blood-retinal barrier breakdown
1. Overview of Diabetic Retinopathy (DR) and Diabetic Macular Edema (DME)
Diabetes mellitus is a chronic, metabolic disease, which is characterized by a prolonged period of hyperglycemia. According to the reports of International Diabetes Federation (IDF) Diabetes Atlas 10th edition (https://diabetesatlas.org, accessed on 21 October 2022), the prevalence of diabetes has continued to increase globally, and diabetes still remains a significant challenge to the health and well-being of people.
Given the global pandemic of diabetes, diabetes causes serious damage to many organs, including the heart, blood vessels, eyes, and kidneys, which remains a major cause of heart attacks, stroke, blindness, and kidney failure (https://www.who.int, accessed on 21 October 2022). As one of the common complications of diabetes, diabetic retinopathy (DR) remains a major cause of visual impairment and blindness in working-age adults. Despite the advances in optimal control of systemic risk factors, i.e., hyperglycemia, hypertension and hyperglycemia, and the application of anti-vascular endothelial growth factor (VEGF) agents (Anti-VEGF), the prevalence of DR remains high in diabetic patients. Among diabetic patients, about one third of patients suffered from DR, which increases markedly after the age of 60 years due to the longer duration of diabetes [1].
Diabetic macular edema (DME) represents the major cause of vision impairment in diabetic patients with an increasing prevalence worldwide [2]. DME is considered as the retinal thickening, which involves or approaches the fovea due to abnormal accumulation of fluid in the macula under diabetic condition. In diabetic retina, fluid accumulation in the macular area leads to increased central retinal/macular thickness, resulting in DME.
2. The Pathogenesis of DME
DME is due to an imbalance between fluid entry, fluid exit and retinal hydraulic conductivity, leading to the accumulation of intraretinal fluid (IRF) or SRF [2]. IRF is the fluid accumulates in retinal parenchyma, mainly in the extracellular spaces of INL, OPL and ONL, while SRF is the fluid accumulation in subretinal space right underneath the neurosensory retina and above retinal pigment epithelium (RPE). Based on the Starling equation, in normal retina, the balance of influx and efflux of the fluid in retina is maintained by the blood-retinal barrier (BRB) integrity and the active drainage function of Müller glia and RPE [2][3]. The intact BRB and the active drainage function of both Müller glia and RPE maintain the retina under a relative dehydrated condition and normal function [2]. Under physiologic conditions, Müller glia removes the fluid from the retinal interstitial tissue to the blood vessels or vitreous, while RPE removes the SRF to the choroid by active transport [4][5]. However, the pathogenesis of DME is really complex, and it has still not been fully elucidated yet. Among the multiple, intricate mechanisms (Figure 1), DME develops mainly due to the two major underlying mechanisms, i.e., BRB breakdown, increasing fluid influx into retina parenchyma, and the decrease in drainage functions by Müller glia and RPE, resulting in the reduced fluid efflux out of retina [6][7]. Moreover, inflammation also plays a contributory role in BRB breakdown (Figure 1), resulting in DME [2][8][9][10].
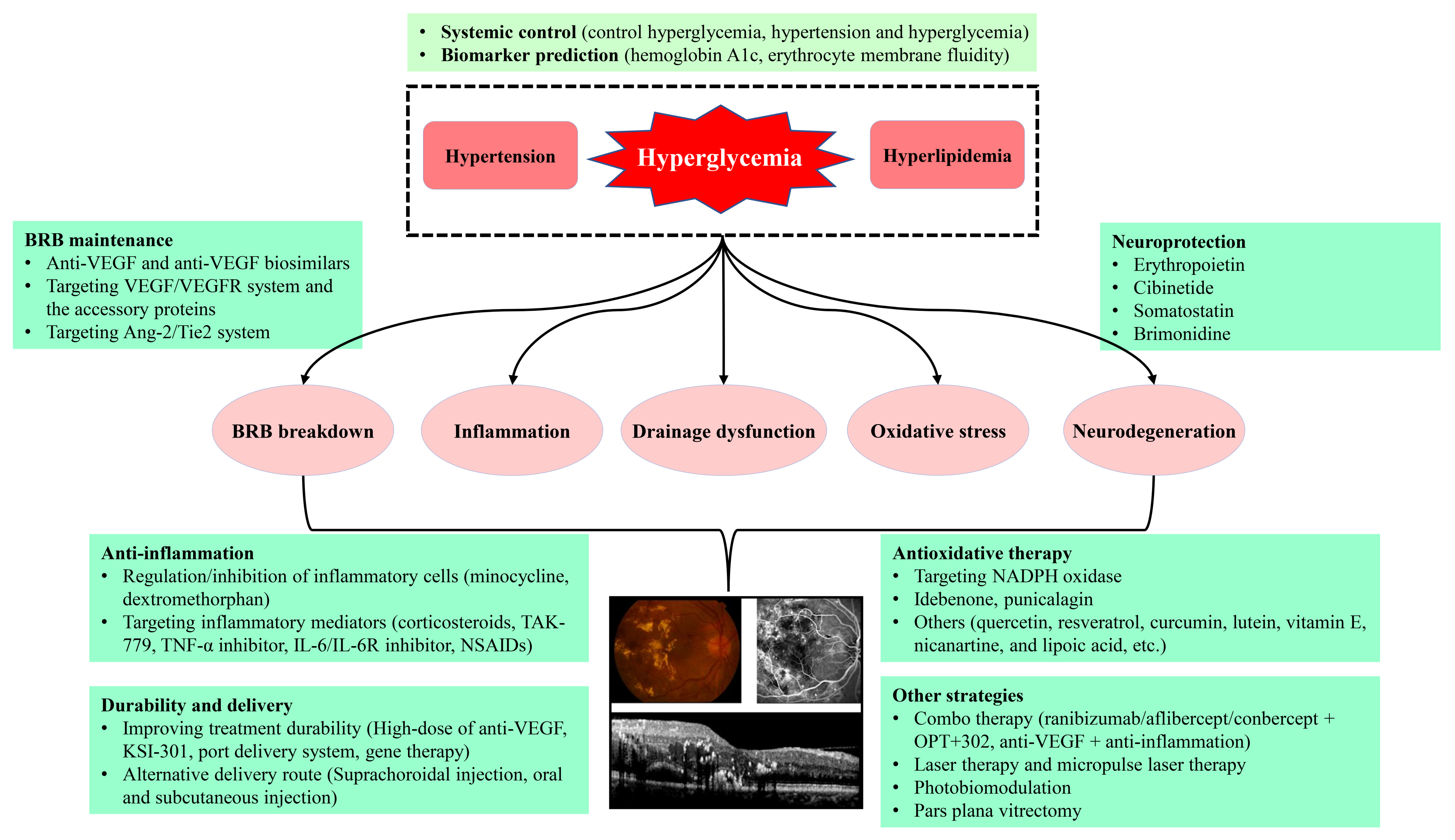
Figure 1. The proposed diagram for the pathogenesis and treatments of DR and DME. Anti-VEGF, anti-vascular endothelial growth factor; Ang-2, angiopoietin 2; DME, diabetic macular edema; DR, diabetic retinopathy; IL-6, interleukin 6; IL-6R, interleukin 6 receptor; NADPH, the reduced form of nicotinamide adenine dinucleotide phosphate; NSAIDs, nonsteroidal anti-inflammatory drugs; Tie2, tyrosine kinase with immunoglobulin-like and epidermal growth factor-like domains 2; TNF-α, tumor necrosis factor α; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor.
The pathogenesis of DME starts with retinal hypoxia, resulting in the hyperpermeability of retinal capillary and increased intravascular pressure due to dysfunction of vascular autoregulation. The capillary hyperpermeability is mainly attributed to hypoxia-induced upregulation of VEGF, which plays a major role in the pathogenesis of DME.
Moreover, it remains largely unknown whether or not these anti-VEGF agents can restore the drainage functions of Müller glia and RPE to facilitate the absorbance and transport of excess fluid out of retina. Alongside the VEGF pathway, DME formation is also attributed to other factors including leakage of microaneurysms, tractional effects by epiretinal membranes or posterior vitreous cortex, and inflammation from both retina and vitreous pool [2][9][10].
3. Therapeutic Strategies for DME
Since the pathogenesis of DME involves different mechanisms with multiple factors and pathways participation, the treatment of DME should be the multimodality therapies, comprising the systemic control of the risk factors, regulating the potential targets, anti-inflammation, anti-oxidative stress, neuroprotection, laser and subthreshold micropulse laser therapy, photobiomodulation, as well as the vitrectomy, etc. (Figure 1).
3.1. Control of Systemic Risk Factors
Since DME remains a common complication of DR caused by diabetes, control of systemic risk factors including tight control of hyperglycemia, hyperlipidemia and hypertension should be considered as the fundamental strategy for the prevention and treatment of DR and DME. The Diabetes Control and Complications Trial (DCCT) showed that intensive glycemic control in T1DM reduced the risk of developing retinopathy by 76% and also reduced the risk of proliferative disease and the need for laser treatment [11]. For patients with T2DM, the UK Prospective Diabetes Study (UKPDS) showed that intensive glycemic control can reduce the need of laser photocoagulation treatment and decrease the risk of progression to blindness [12]. The UKPDS analyzed the effect of intensive control of blood pressure with captopril or atenolol on microvascular complications in 1148 hypertensive patients with T2DM [13].
3.2. Laser Therapy
The efficacy and safety of focal laser for treating DME was validated by the Early Treatment of Diabetic Retinopathy Study (ETDRS) in the 1980s [14]. Today, the focal/grid laser is an alternative in eyes with DME, mostly for non-center involved DME (Non-CI-DME). The subthreshold micropulse laser has been accepted as a potential and promising treatment in some cases for DME [15], due to its safe, non-scarring alternative procedure [16][17][18][19]. Subthreshold micropulse laser therapy is known to improve RPE function, modulate the activation of heat-shock proteins and normalize cytokine expression [20], and it seems to result in the normalization of retinal neuroinflammatory metabolic pathways [21].
3.3. Intravitreal Injection of Anti-VEGF Agents
Since many cytokines and various pathways are implicated in the pathogenic process of DME, treating targets become the fundamental strategy for DME treatment [22]. In the pathogenesis of DR and DME, VEGF is upregulated and plays a pivotal role leading to BRB breakdown, macular edema, and neovascularization [23], and the severity of leakage in DME correlates with the level of VEGF [24]. Targeting VEGF (anti-VEGF) treatment has demonstrated significant benefits for patients with DME, which has become the first-line treatment for DME [22], supplanting focal photocoagulation. Currently, there are several anti-VEGF agents which are commercially available for DME, including Lucentis (ranibizumab), Eylea (aflibercept), Lumitin (conbercept), Beovu (brolucizumab), and off-label Avastin (bevacizumab) [25][26][27][28][29][30], differing in molecular weight and structure, binding affinity, targeted VEGF isoforms, and concentration, etc.
3.4. Emerging Therapeutic Strategies Targeting VEGF/VEGFR System and the Accessory Proteins
The current trend for anti-VEGF development is toward either smaller molecular weight targeting VEGF-A (e.g., Beovu and abicipar), fusion proteins targeting VEGF-A in combination with other factors (e.g., faricimab), targeting other VEGF family members (OPT-302), reducing the cost of burden (developing biosimilars), or improving treatment durability (KSI-301, port delivery system, gene therapy), and etc.
3.4.1. Abicipar Pegol
Abicipar pegol (AGN-150998, Allergan plc/Molecular Partners) belongs to a family of the designed ankyrin repeat proteins (DARPin). Abicipar pegol binds VEGF-A with high affinity [31]. Compared with ranibizumab, abicipar pegol improved its pharmacokinetic properties, i.e., lower molecular weight (34 vs. 48 kDa), higher target binding affinity (2 vs. 46 pM) and longer ocular half-life (≥13 vs. 7 days in the aqueous humor) [32][33][34]. In phase I/II, open-label, multicenter dose-escalation trial for DME, prolonged edema reduction and visual improvement was achieved in several patients, however, ocular inflammation was a major concern [33].
3.4.2. OPT-302
OPT-302 (Opthea; Victoria, Australia) is a soluble form of VEGF receptor 3 (VEGFR-3) consisting of the extracellular domains 1–3 of human VEGFR-3 and the Fc fragment of human IgG1. OPT-302 blocks the activity of the proteins VEGF-C and VEGF-D [35], which may serve a complementary therapeutic role in VEGF-mediated DR pathogenesis, and overcome the limitation of the current anti-VEGF drugs that only target VEGF-A. Intravitreal OPT-302 was safe and well tolerated, and the combined treatment with OPT-302 may enhance the efficacy in neovascular suppression in nAMD [35]. A multicenter phase 1b/2a trial has evaluated OPT-302 in combination with aflibercept for refractory DME [36]. Combo-therapy using OPT-302 and aflibercept or conbercept may target all the VEGF family members, which might be effective in patients with retinal vascular diseases and is worth trying.
3.4.3. Anti-VEGF Biosimilars
The anti-VEGF medications have been available for more than a decade and their patent expiration dates are coming. For example, ranibizumab’s patent expired in June 2020 in the United States (2022 in the European Union) and aflibercept’s patent will expire in 2023 in the United States (2025 in the European Union) [37]. With the expiry of these patents, the transition to biosimilars can have a significant impact worldwide due to the favorable cost-effectiveness [38].
Many bevacizumab biosimilars were approved for cancer treatment. However, due to a cheaper alternative to ranibizumab, the off-label use of bevacizumab is still increasing in ophthalmology [38]. Currently, there are several anti-VEGF biosimilars to ranibizumab and aflibercept in the development stage or acquiring approval [38]. For example, Razumab® (Intas Pharmaceutical Ltd., Ahmedabad, GJ, India) is the first biosimilar to ranibizumab approved for ophthalmic use in India by the drug controller general of India for nAMD, myopic choroidal neovascularization, DME, and retinal vein occlusion-macular edema (RVO-ME) [38][39][40][41].
3.4.4. KSI-301
KSI-301 (KODIAK sciences, Palo Alto, CA, USA) comprises a specific anti-VEGF IgG1 antibody and an inert immune effector, covalently linked to a high molecular weight phosphorycholine biopolymer (950 kDa). Intravitreal injection of KSI-301 showed prolonged intravitreal half-life (about 6 months) due to slow diffusion and decreased elimination in the eye [42][43]. Clinical trials (GLEAM Study and GLIMMER study) are underway. The patients are randomized into two groups receiving either intravitreal KSI-301 or aflibercept [44]. Phase 2b/3 clinical trial failed to meet the primary endpoint of visual acuity gains in nAMD patients treated with KSI-301 compared to aflibercept [45].
3.4.5. Port Delivery System (PDS) with Ranibizumab
Currently, the delivery of anti-VEGF drugs is largely dependent on repeated intravitreal injections. PDS allows continuous release of ranibizumab, and minimizes the need for frequent injections [46]. Sustained and controlled release is achieved by the porous metal element allowing passive diffusion of drugs from PDS to the vitreous [47]. ARCHWAY (NCT03677934) randomized Phase 3 trial of PDS with ranibizumab showed that PDS with ranibizumab met its primary objective, demonstrating equivalent efficacy of monthly ranibizumab injection [48]. Phase 3 clinical trials for DR (PAVILION; NCT04503551), and DME (PAGODA; NCT04108156) are currently in progress.
3.4.6. High-Dose of Anti-VEGF Agents
An intravitreal injection of high-dose anti-VEGF agents might prolong the intravitreal injection intervals and improve drug efficacy. Using rabbits, Kim et al. showed that a two-fold increase in retinal half-life and prolonged effective concentration of ranibizumab in retina when administered a 10-fold dose of ranibizumab with good safety in rabbit eyes [49]. Currently, phase 3 clinical trials are underway in DME (PHOTON; NCT04429503) and nAMD (PULSAR; NCT04423718).
3.4.7. Gene Therapy to Deliver Anti-VEGF Agents
Given the burden of repeated anti-VEGF treatments, gene therapy can achieve long-term expression of anti-VEGF proteins to suppress of VEGF in retinal vascular diseases. Several gene therapy drugs, including RGX-314, ADVM-022 and rAAV-sFlt1, are currently under clinical evaluation.
RGX-314 is an adeno-associated virus 8 (AAV8) vector encoding ranibizumab. The Phase II ALTITUDE trial is studying the patients with DR but without DME, who are treated with a single dose of RGX-314, delivered in suprachoroidal space [50]. Positive 3-month interim data from cohort 1, treated with a single injection at a dose of 2.5 × 1011 genomic copies per eye, showed that treatment was well-tolerated and 33% of patients had a ≥ 2-step improvement from baseline [50].
ADVM-022, an AAV2-7m8 vector encoding aflibercept, is optimized for intravitreal delivery. Prolonged expression and efficacy of ADVM-022 was evaluated in a laser-induced CNV model in non-human primates with promising outcomes [51]. Clinical trials for nAMD (NCT04645212; NCT03748784) and DME (NCT04418427) are currently underway, evaluating safety and efficacy following a single intravitreal injection of ADVM-022.
rAAV-sFlt1, a recombinant AAV2 vector expressing soluble VEGF receptor 1, works as a decoy receptor for VEGF. A pre-clinical study showed safety and well-toleration in non-human primates after a single subretinal injection of rAAV-sFlt1 [52]. Although phase I study (NCT01494805) demonstrated the safety in nAMD patients [53], phase IIa clinical trial (NCT01494805) showed no obvious benefit in visual acuity or anatomy [54]. The potential effect of rAAV-sFlt1 on DME deserves further study [55].
3.4.8. Targeting VEGFRs
The inhibition of VEGFRs is one of the promising strategies for treatment of VEGF-driven neovascular diseases [56][57][58][59][60]. Targeting VEGFRs has been extensively studied in clinical oncology. There are several approaches to inhibiting VEGFR signaling, i.e., VEGFR antibodies, VEGFR allosteric inhibitors, and inhibition of the intracellular tyrosine kinase of VEGFR by tyrosine kinase inhibitors (TKIs).
Ramucirumab (Cyramza®), a fully humanized anti-VEGFR-2 monoclonal antibody, was approved for the treatment of cancer patients who experience disease progression during chemotherapy [58]. Its ophthalmic use is to treat retinal vascular diseases, including DME, which deserves further exploration.
GB-102 (GrayBug Vision; Redwood City, CA, USA), sunitinib maleate and a TKI with activity against both VEGF-A and PDGF, is encapsulated within bioerodible polymer nanoparticles degrading slowly over time [61]. Single GB-102 treatment can last up to 6 months with comparable visual acuity and CSFT outcomes [62][63].
X-82 (Tyrogenex) is an oral anti-PDGF and VEGF-A inhibitor. In a Phase 1 study (NCT02348359) for nAMD, 29% patients (10 of 35) did not complete the 24-week endpoint, with 6 (17%) withdrawing due to adverse events, including diarrhea, nausea, fatigue, and transaminase elevation [64]. Phase 2 APEX study (NCT02348359) is underway, which compares X-82 (Tyrogenex) with as-needed aflibercept injections to aflibercept monotherapy.
PAN-90806 (PanOptica; Mount Arlington, NJ, USA), a TKI eyedrop, was shown to inhibit VEGF signaling with topical once daily dosing. According to a phase 1/2 study (NCT03479372), PAN-90806 showed favorable safety and effectiveness as monotherapy. However, it may be applicable for certain patients and further studies are needed [62].
3.4.9. Targeting Neuropilin-1
Vesencumab is a human IgG1 monoclonal antibody against neuropilin-1 (NRP-1), with potential anti-angiogenic and anti-neoplastic activities. Vesencumab specifically targets and binds to NRP-1, preventing the subsequent coupling of NRP-1 to VEGFR-2, thereby decreasing VEGF-mediated signaling. When combined with other anti-VEGF therapies, vesencumab may enhance their anti-angiogenic effect [65]. Vesencumab is currently undergoing clinical study for cancer patients [66].
3.5. Anti-Inflammatory Therapy
Since inflammation plays a critical role in DR and DME, suppression of inflammation seems to be a reasonable approach for treating DR and DME [67]. Corticosteroids have been proven to be beneficial in treating DR and DME due to their anti-inflammatory and anti-angiogenic properties [68]. At present, intravitreal preservative-free triamcinolone, the extended-release dexamethasone implant (Ozurdex) and the fluocinolone acetonide implant (Iluvein) are FDA-approved for treating DME. Intravitreal injection of sustainable dexamethasone (Ozurdex, Allergen) was safe and effective in DME treatment, achieving visual improvement, reducing edema, and decreasing the inflammatory cytokines, such as VEGF, MCP-1, and IL-6. A MEAD study (NCT00168337 and NCT00168389) evaluated the safety and efficacy of Ozurdex (0.7 mg and 0.35 mg) and demonstrated both doses of the Ozurdex implant met the primary objective for visual improvement with acceptable safety profile [69].
Based on the inflammatory theory of DME formation, the ongoing translational research targeting inflammatory cells and factors is shedding new light on the management of DME beyond anti-VEGF therapy. Anti-inflammation treatment can be roughly classified into several categories, i.e., regulation/inhibition of inflammatory cells (such as minocycline, dextromethorphan), targeting various inflammatory mediators (such as corticosteroids, TAK-779, TNF-α inhibitor, IL-6/IL-6R inhibitor, non-steroid anti-inflammatory drugs (NSAIDs)), and alternative delivery route (Suprachoroidal injection, oral and subcutaneous injection), and etc.
3.5.1. Minocycline and Dextromethorphan
Minocycline, besides its antimicrobial activity, has anti-inflammatory, anti-oxidant, anti-apoptotic, neuroprotective, and immunomodulatory effects [70]. In phase I/II clinical trial (ClinicalTrials.gov number, NCT01120899), oral minocycline treatment improved visual function and reduced central macular edema [71]. Dextromethorphan was effective in decreasing vascular leakage in 5 DME patients in phase I/II clinical trial, in which oral dextromethorphan was administered 60 mg twice daily for 6 months as monotherapy [72].
3.5.2. Difluprednate and Dexamethasone-Cyclodextrin
Difluprednate (difluprednisolone butyrate acetate, DFBA) is an anti-inflammatory steroid, effective in the treatment of anterior uveitis, postoperative ocular inflammation, and pain [73][74]. Difluprednate ophthalmic emulsion 0.05% (Durezol (TM), Sirion Therapeutics Inc., Tampa, FL, USA) effectively reduces refractory DME post-vitrectomy [75], and diffuse DME without surgical intervention [76]. Topical dexamethasone-cyclodextrin eye drops were safe, improved visual acuity and decreased central macular thickness in DME patients [77]. In a randomized, controlled trial, topical dexamethasone-cyclodextrin nanoparticle eye drops (1.5%) significantly improved the vision and decreased macular thickness in DME patients [78].
3.5.3. TAK-779
TAK-779, a dual CCR2/CCR5 inhibitor, significantly reduced retinal vascular permeability in diabetic mice [79]. TAK-779 also decreased infiltration of macrophage/microglia, reduced the expressions of ICAM-1 and stromal cell-derived factor 1 (SDF-1), and restored zonula occludens-1 (ZO-1) in diabetic mouse retina [79]. Targeting CCR2/CCR5 might provide a novel strategy for DME management.
3.5.4. Targeting Integrin
Integrins are involved in many biological processes and play a critical role in the pathogenesis of many diseases. Some integrins are associated with vitreolysis, angiogenesis, and ocular surface diseases [80]. Anti-β2-integrin or anti-ICAM-1 decreased leukocyte adhesion, the death of endothelial cells, and BRB breakdown [81][82][83]. Therefore, targeting integrins, independent of anti-VEGF therapies, has the potential to prevent vision loss.
Risuteganib (Luminate, ALG-1001, Allegro Ophthalmics, LLC, San Juan Capistrano, CA, USA) is an engineered arginyl-glycyl-aspartic acid (RGD) class synthetic peptide targeting integrin. RGD peptide treatment suppressed retinal neovascularization and released cellular adhesion to induce posterior vitreous detachment [84][85]. Risuteganib has potential as a therapy for DR and DME [80][86]. SB-267268 (GlaxoSmithKline) is a small molecule inhibitor of αvβ3 and αvβ5 integrins [87]. In an animal model of retinopathy of prematurity (ROP), SB-267268 decreased the mRNA expressions of VEGF and VEGFR2, and reduced the pathological angiogenesis by 50% [87].
3.5.5. Targeting TNF-α
TNF-α is an inflammatory cytokine that promotes the upregulation of adhesion molecule expression, leukocyte recruitment and monocyte attraction. TNF-α was increased in the aqueous and vitreous of diabetic patients compared to control subjects [88][89][90]. The targeting TNF-α might provide an option for treating DR and DME. Currently, there are monoclonal anti-TNF-α full IgG1 antibodies (infliximab, adalimumab, and golimumab), PEGylated Fab’ fragment of anti-TNF-α antibody (certolizumab pegol) and extracellular domain of TNF receptor 2/IgG1-Fc fusion protein (etanercept), effective for the treatment of rheumatoid arthritis [91]. In fact, a clinical study with infliximab achieved functional and anatomical improvement in DME patients, highlighting the pathogenic role of TNF-α in DR [92].
3.5.6. Targeting IL-6/IL-6R
IL-6/IL-6R exerts an important role in initiating the breakdown of BRB in DR [93][94], due to the disrupting of the barrier function and increasing vascular leakage via the downregulation of tight junction proteins [95]. IL-6 signaling occurs through its membrane-bound receptor IL-6R (classical signaling) or through the soluble IL-6R (sIL-6R, trans-signaling) [96][97]. Anti-IL-6 and anti-IL-6R strategies target both classical and trans-signaling pathways to block IL-6 signaling. Several therapeutic strategies targeting IL-6 signaling pathways are in progress [98], including anti-IL-6 antibodies (e.g., siltuximab and sirukumab), anti-IL-6R antibodies (e.g., tocilizumab and vobarilizumab), and IL-6 trans-signaling selective inhibitor (olamkicept). Tocilizumab is effective in the treatment of various autoimmune and inflammatory diseases, including rheumatoid arthritis, with a favorable outcome [99]. Thus, blocking IL-6 and IL-6R may be potential approaches for treating DR.
3.5.7. Vascular Adhesion Protein-1 (VAP-1) Inhibitor
VAP-1, known as amine oxidase copper-containing 3 (AOC3) and semicarbazide-sensitive amine oxidase, is a membrane-bound adhesion protein facilitating leukocyte adhesion and transmigration to the inflammatory site [100]. Previous studies showed that the level of soluble VAP-1 was higher in the vitreous of PDR patients than in that of nondiabetic patients [101]. In diabetic rats, the leukocyte transmigration rate was reduced by UV-002 (a specific inhibitor of VAP-1) [100]. In diabetic animals, VAP-1 inhibition improved retinal function and structure as evidenced by electroretinogram and histopathological studies [102]. Thus, VAP-1 could be an underlying target for DR treatment [103]. A phase 2 study (VIDI study, NCT02302079) tested the effect of ASP8232, a specific VAP-1 inhibitor, on CI-DME [104]. The primary data showed that ASP8232 nearly inhibited the activity of plasma VAP-1, while had no effect on CSFT in patients with CI-DME. The clinical application of VAP-1 inhibition still requires further study.
3.5.8. Non-Steroid Anti-Inflammatory Drugs (NSAIDs)
NSAIDs inhibit the cyclooxygenase (COX) enzyme that is an essential mediator through the regulation of prostaglandin dependent pathways [105]. Bromfenac mainly inhibits the activity of COX-2 [106], and nepafenac, a prodrug, acts on COX-1 and COX-2 through its active metabolite amfenac [107]. NSAIDs were reported effective in DME with various and heterogeneous results. In a pilot study, topical bromfenac significantly reduced central macular thickness in patients with DME, however, without obvious effect on visual acuity [108]. The safety and efficacy of topical nepafenac 0.1% were tested in 6 eyes of 5 patients with DME, which showed that topical nepafenac treatment improved vision and decreased retinal thickness [109]. Postoperative topical nepafenac was shown to be effective for prophylaxis of macular edema in diabetic patients underwent phacoemulsification and intraocular lens implantation [110]. Further investigations on whether topical NSAIDs could serve as an alternative or adjunctive treatment to intravitreal anti-VEGF therapy are required.
3.5.9. Suprachoroidal Injection of Steroid
The suprachoroidal space (SCS) has become an applicable route to deliver drugs to the back of the eye via suprachoroidal injection [111]. When delivered to the suprachoroidal space, the drug can target both the retina and the choroid, overcoming multiple ocular tissue barriers and achieving the efficacy at low dose [42][112]. The phase 2 TYBEE clinical trial enrolled 71 eyes with treatment-naïve DME [113], with 36 eyes received SCS injection of triamcinolone acetonide (TA) (CLS-TA, 4 mg/100 µL) and aflibercept (2 mg/0.05 mL) at baseline and week 12 (active group) and 35 eyes which were treated with aflibercept (control group). At 24 weeks from baseline, the visual acuity gain was similar between two groups, with mild anatomic improvement and less treatment burden in the active group [113].
3.6. Targeting Ang-2/Tyrosine Kinase with Immunoglobulin-like and Epidermal Growth Factor-like Domains 2 (Tie2) System
The angiopoietin (Ang)/Tie2 pathway is involved in many retinal vascular diseases. Angiopoietin-1 (Ang-1) and Ang-2 ligands compete for the Tie2 receptor. Tie2 is a tyrosine kinase receptor in vascular endothelial cells and maintains vascular stability. Tie2 activation by Ang-1 increases the survival, adhesion, and cell junction integrity of endothelial cells, while Ang-2 interferes with the Ang-1/Tie2 axis, resulting in vascular instability. Vascular endothelial-protein tyrosine phosphatase (VE-PTP) is an endothelial cell-specific phosphatase, which forms a complex with Tie2 and dephosphorylates Tie2, against the actions of Ang-1 [114]. In DR, there is an increased production of Ang-2, competitively binding Tie2 to reduce Ties phosphorylation, whereas VE-PTP directly decreases Tie2 phosphorylation. Tie2 inactivation destabilizes the vasculature, resulting in pericyte dropout, reduction in endothelial cell viability, decreased endothelial cell anchor and cell junction integrity. Thus, activating the Tie2 signaling pathway, by the inhibition of Ang-2 or VE-PTP, should be a therapeutic strategy for retinal vascular diseases.
3.6.1. Targeting Ang-2
Nesvacumab (Regeneron, Tarrytown, NY, USA) is a fully human IgG1 monoclonal antibody selectively binding Ang-2. In phase 2 studies of nAMD and DME, nesvacumab co-formulated with aflibercept failed to show beneficial effects over aflibercept in visual gains improvement [115].
AXT107 (Asclepix Therapeutics, Baltimore, MD, USA) is a peptide derived from the non-collagenous domain of collagen IV [115]. AXT107 modifies Ang-2 and promotes its conversion into the Tie2 agonist, and AXT107 also inhibits the signaling of VEGFR-2 and other receptor tyrosine kinases [115]. In the presence of AXT107 and Ang-2, α5β1 integrin is disrupted, promoting Tie2 clustering and converting Ang-2 into a Tie2 agonist [116]. Currently, AXT107 is in the preclinical phase of study [115].
3.6.2. Bispecific Drug
Faricimab (faricimab-svoa; Vabysmo™), known as RG7716, (Roche, Basel, Switzerland and Genentech, South San Francisco, CA, USA), is a bispecific antibody binding both VEGF-A and Ang-2. Phase 3 trials for DME (YOSEMITE NCT03622580 and RHINE NCT03622593) showed robust vision gains and anatomical improvements in patients treated with faricimab and the personalized treatment interval was extended to 16 weeks [117]. In 2022, faricimab received its first approvals in the USA for the treatment of nAMD or DME [118].
3.6.3. Targeting VE-PTP
ARP-1536 (Aerpio Therapeutics, Cincinnati, OH, USA) is a monoclonal antibody targeting VE-PTP. ARP-1536 is intravitreally administered, currently undergoing preclinical studies [61]. AKB-9778 (Aerpio Therapeutics, Cincinnati, OH, USA) is a small molecule antagonist of VE-PTP, increasing Tie2 phosphorylation. AKB-9778 is administered by subcutaneous injection. In preclinical studies, AKB-9778 reduced vascular leakage and ocular neovascularization, with synergistic effect when combined with VEGF inhibition [119]. AKB-9778 reduced macular edema more effectively when combined with monthly ranibizumab in a phase 2 study of DME [120].
3.7. Neuroprotection in DME Management
Since DR is also a neurovegetative disease, neuroprotection should be considered in the management of DR and DME. The neuroprotective agents include but not limited to erythropoietin (EPO), Cibinetide (known as ARA 290 and helix B surface peptide (HBSP)), somatostatin and brimonidine.
EPO’s protective mechanisms comprise anti-apoptosis and neuroprotection via activating the ERK and AKT pathways [121][122], neurotrophic effect and anti-reactive gliosis [123], anti-VEGF via inhibition of HIF-1α [124], anti-inflammatory effect by decreasing inflammatory factors from Müller glia [125], increase in the expression of zinc transporter 8 (ZnT8) [126], downregulation of glutamate [127], and maintenance of VE-cadherin expression via inhibiting VEGF/VEGFR-2/Src pathway. In addition, EPO is able to improve the integrity of the inner BRB [128], and maintain outer BRB integrity through downregulation of HIF-1α and c-Jun N-terminal kinase (JNK) signaling, and upregulation of ZO-1 and occludin expressions in RPE cells [129]. Recently, the researchers found that EPO protects the inner BRB by inhibiting microglia phagocytosis via Src/Akt/cofilin signaling in experimental DR [130].
Cibinetide is a synthetic 11 amino acid peptide, derived from EPO, having anti-apoptotic, anti-permeability and anti-inflammatory functions, with no erythropoietic function [131][132][133]. Both somatostatin and brimonidine were tested in diabetic patients, however, no neuroprotective effect was found for both drugs to achieve the primary endpoint [134]. However, the topical administration of somatostatin and brimonidine appears to be useful in preventing the worsening of preexisting retinal dysfunction [134]. Topical treatment with either somatostatin or brimonidine was observed to cause retinal arteriolar and venous dilation in patients with T2DM and early DR [135].
3.8. Antioxidative Therapy
Oxidative stress, resulting from the metabolic abnormalities, is regarded as a pivotal contributor to the pathogenesis of DR [136]. The reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Nox) system is as a key enzymatic source of oxidative stress [136][137]. Nox-derived ROS contributes to retinal damage through inducing the expressions of pro-angiogenic and pro-inflammatory cytokines, including VEGF-A, EPO, Ang-2 and ICAM-1 [138][139]. The Nox2 gene knockout reduced oxidative stress, attenuated vascular permeability, and reduced leucocyte-endothelial interaction and leukostasis in diabetic mice [137]. Nox4 knockdown with small interfering RNA significantly decreased retinal vascular permeability, indicating the causal role of Nox4 in BRB breakdown [140]. Thus, the inhibition of Nox would provide a potential strategy for the treatment of DR and DME.
Idebenone, a ubiquinone short-chain synthetic analog, is believed to restore mitochondrial ATP synthesis with antioxidant properties [141]. Punicalagin (2,3-hexahydroxydiphenoyl-gallagyl-D-glucose), a polyphenol extracted from pomegranate (Punica granatum), is a potent antioxidant in several cell types [142]. Previous studies showed that both idebenone and punicalagin could protected RPE from oxidative damage, suggesting their possible roles in DR and DME treatment. Idebenone protected RPE through modulation of the intrinsic mitochondrial pathway of apoptosis [141]. Punicalagin exerted its effect to reduce oxidative stress and decrease the apoptosis via enhancing mitochondrial functions [142].
Other potential antioxidants, such as quercetin, resveratrol, curcumin, lutein, vitamin E, nicanartine, and lipoic acid, etc., are promising against oxidative stress in treatment of DR and DME [136], deserving further exploration.
3.9. Combo Therapy and Other Strategies
Based on the severity of the DME, combo therapy can be proposed, such as ranibizumab + OPT + 302, aflibercept/conbercept + OPT + 302 or anti-VEGF + anti-inflammatory treatment. Moreover, other approaches are also attempted to treat DME, including targeted laser photocoagulation for non-perfusion area, micropulse laser for macular microaneurysms, photobiomodulation to enhance RPE function, vitrectomy to relieve the abnormalities of vitreoretinal interface and clear vitreous body, and etc. The growing achievements of translational research will lead to future treatments for DME with better efficacy, longer duration, and greater cost-effectiveness.
This entry is adapted from the peer-reviewed paper 10.3390/cells11213362
References
- Hashemi, H.; Rezvan, F.; Pakzad, R.; Ansaripour, A.; Heydarian, S.; Yekta, A.; Ostadimoghaddam, H.; Pakbin, M.; Khabazkhoob, M. Global and Regional Prevalence of Diabetic Retinopathy; A Comprehensive Systematic Review and Meta-Analysis. Semin. Ophthalmol. 2022, 37, 291–306.
- Daruich, A.; Matet, A.; Moulin, A.; Kowalczuk, L.; Nicolas, M.; Sellam, A.; Rothschild, P.-R.; Omri, S.; Gélizé, E.; Jonet, L.; et al. Mechanisms of Macular Edema: Beyond the Surface. Prog. Retin. Eye Res. 2018, 63, 20–68.
- Bringmann, A.; Reichenbach, A.; Wiedemann, P. Pathomechanisms of Cystoid Macular Edema. Ophthalmic Res. 2004, 36, 241–249.
- Reichenbach, A.; Wurm, A.; Pannicke, T.; Iandiev, I.; Wiedemann, P.; Bringmann, A. Müller Cells as Players in Retinal Degeneration and Edema. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 245, 627–636.
- Simó, R.; Villarroel, M.; Corraliza, L.; Hernández, C.; Garcia-Ramírez, M. The Retinal Pigment Epithelium: Something More than a Constituent of the Blood-Retinal Barrier—Implications for the Pathogenesis of Diabetic Retinopathy. J. Biomed. Biotechnol. 2010, 2010, 190724.
- Reichenbach, A.; Bringmann, A. New Functions of Müller Cells. Glia 2013, 61, 651–678.
- Caplan, M.J. Membrane Polarity in Epithelial Cells: Protein Sorting and Establishment of Polarized Domains. Am. J. Physiol. 1997, 272, F425–F429.
- Rangasamy, S.; McGuire, P.G.; Franco Nitta, C.; Monickaraj, F.; Oruganti, S.R.; Das, A. Chemokine Mediated Monocyte Trafficking into the Retina: Role of Inflammation in Alteration of the Blood-Retinal Barrier in Diabetic Retinopathy. PLoS ONE 2014, 9, e108508.
- Romero-Aroca, P.; Baget-Bernaldiz, M.; Pareja-Rios, A.; Lopez-Galvez, M.; Navarro-Gil, R.; Verges, R. Diabetic Macular Edema Pathophysiology: Vasogenic versus Inflammatory. J. Diabetes Res. 2016, 2016, 2156273.
- Rübsam, A.; Parikh, S.; Fort, P.E. Role of Inflammation in Diabetic Retinopathy. Int. J. Mol. Sci. 2018, 19, 942.
- The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N. Engl. J. Med. 1993, 329, 977–986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive Blood-Glucose Control with Sulphonylureas or Insulin Compared with Conventional Treatment and Risk of Complications in Patients with Type 2 Diabetes (UKPDS 33). Lancet Lond. Engl. 1998, 352, 837–853.
- UK Prospective Diabetes Study Group. Tight Blood Pressure Control and Risk of Macrovascular and Microvascular Complications in Type 2 Diabetes: UKPDS 38. BMJ 1998, 317, 703–713.
- Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for Diabetic Macular Edema. Early Treatment Diabetic Retinopathy Study Report Number 1. Arch. Ophthalmol. Chic. Ill 1960 1985, 103, 1796–1806.
- Passos, R.M.; Malerbi, F.K.; Rocha, M.; Maia, M.; Farah, M.E. Real-Life Outcomes of Subthreshold Laser Therapy for Diabetic Macular Edema. Int. J. Retina Vitr. 2021, 7, 4.
- Scholz, P.; Altay, L.; Fauser, S. A Review of Subthreshold Micropulse Laser for Treatment of Macular Disorders. Adv. Ther. 2017, 34, 1528–1555.
- Lavinsky, D.; Wang, J.; Huie, P.; Dalal, R.; Lee, S.J.; Lee, D.Y.; Palanker, D. Nondamaging Retinal Laser Therapy: Rationale and Applications to the Macula. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2488–2500.
- Luttrull, J.K.; Sramek, C.; Palanker, D.; Spink, C.J.; Musch, D.C. Long-Term Safety, High-Resolution Imaging, and Tissue Temperature Modeling of Subvisible Diode Micropulse Photocoagulation for Retinovascular Macular Edema. Retina 2012, 32, 375–386.
- Lavinsky, D.; Sramek, C.; Wang, J.; Huie, P.; Dalal, R.; Mandel, Y.; Palanker, D. Subvisible Retinal Laser Therapy: Titration Algorithm and Tissue Response. Retina 2014, 34, 87–97.
- Mainster, M.A. Wavelength Selection in Macular Photocoagulation. Tissue Optics, Thermal Effects, and Laser Systems. Ophthalmology 1986, 93, 952–958.
- Frizziero, L.; Calciati, A.; Midena, G.; Torresin, T.; Parrozzani, R.; Pilotto, E.; Midena, E. Subthreshold Micropulse Laser Modulates Retinal Neuroinflammatory Biomarkers in Diabetic Macular Edema. J. Clin. Med. 2021, 10, 3134.
- Kim, E.J.; Lin, W.V.; Rodriguez, S.M.; Chen, A.; Loya, A.; Weng, C.Y. Treatment of Diabetic Macular Edema. Curr. Diab. Rep. 2019, 19, 68.
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264.
- Funatsu, H.; Yamashita, H.; Sakata, K.; Noma, H.; Mimura, T.; Suzuki, M.; Eguchi, S.; Hori, S. Vitreous Levels of Vascular Endothelial Growth Factor and Intercellular Adhesion Molecule 1 Are Related to Diabetic Macular Edema. Ophthalmology 2005, 112, 806–816.
- Glassman, A.R.; Wells, J.A.; Josic, K.; Maguire, M.G.; Antoszyk, A.N.; Baker, C.; Beaulieu, W.T.; Elman, M.J.; Jampol, L.M.; Sun, J.K. Five-Year Outcomes after Initial Aflibercept, Bevacizumab, or Ranibizumab Treatment for Diabetic Macular Edema (Protocol T Extension Study). Ophthalmology 2020, 127, 1201–1210.
- Ciulla, T.A.; Harris, A.; McIntyre, N.; Jonescu-Cuypers, C. Treatment of Diabetic Macular Edema with Sustained-Release Glucocorticoids: Intravitreal Triamcinolone Acetonide, Dexamethasone Implant, and Fluocinolone Acetonide Implant. Expert Opin. Pharmacother. 2014, 15, 953–959.
- Rajendram, R.; Fraser-Bell, S.; Kaines, A.; Michaelides, M.; Hamilton, R.D.; Esposti, S.D.; Peto, T.; Egan, C.; Bunce, C.; Leslie, R.D.; et al. A 2-Year Prospective Randomized Controlled Trial of Intravitreal Bevacizumab or Laser Therapy (BOLT) in the Management of Diabetic Macular Edema: 24-Month Data: Report 3. Arch. Ophthalmol. Chic. Ill 1960 2012, 130, 972–979.
- Nguyen, Q.D.; Brown, D.M.; Marcus, D.M.; Boyer, D.S.; Patel, S.; Feiner, L.; Gibson, A.; Sy, J.; Rundle, A.C.; Hopkins, J.J.; et al. Ranibizumab for Diabetic Macular Edema: Results from 2 Phase III Randomized Trials: RISE and RIDE. Ophthalmology 2012, 119, 789–801.
- Brown, D.M.; Schmidt-Erfurth, U.; Do, D.V.; Holz, F.G.; Boyer, D.S.; Midena, E.; Heier, J.S.; Terasaki, H.; Kaiser, P.K.; Marcus, D.M.; et al. Intravitreal Aflibercept for Diabetic Macular Edema: 100-Week Results From the VISTA and VIVID Studies. Ophthalmology 2015, 122, 2044–2052.
- Schmidt-Erfurth, U.; Lang, G.E.; Holz, F.G.; Schlingemann, R.O.; Lanzetta, P.; Massin, P.; Gerstner, O.; Bouazza, A.S.; Shen, H.; Osborne, A.; et al. Three-Year Outcomes of Individualized Ranibizumab Treatment in Patients with Diabetic Macular Edema: The Restore Extension Study. Ophthalmology 2014, 121, 1045–1053.
- Thomas, C.N.; Sim, D.A.; Lee, W.H.; Alfahad, N.; Dick, A.D.; Denniston, A.K.; Hill, L.J. Emerging Therapies and Their Delivery for Treating Age-Related Macular Degeneration. Br. J. Pharmacol. 2022, 179, 1908–1937.
- Souied, E.H.; Devin, F.; Mauget-Faÿsse, M.; Kolář, P.; Wolf-Schnurrbusch, U.; Framme, C.; Gaucher, D.; Querques, G.; Stumpp, M.T.; Wolf, S.; et al. Treatment of Exudative Age-Related Macular Degeneration with a Designed Ankyrin Repeat Protein That Binds Vascular Endothelial Growth Factor: A Phase I/II Study. Am. J. Ophthalmol. 2014, 158, 724–732.e2.
- Campochiaro, P.A.; Channa, R.; Berger, B.B.; Heier, J.S.; Brown, D.M.; Fiedler, U.; Hepp, J.; Stumpp, M.T. Treatment of Diabetic Macular Edema with a Designed Ankyrin Repeat Protein That Binds Vascular Endothelial Growth Factor: A Phase I/II Study. Am. J. Ophthalmol. 2013, 155, 697–704.e2.
- Krohne, T.U.; Liu, Z.; Holz, F.G.; Meyer, C.H. Intraocular Pharmacokinetics of Ranibizumab Following a Single Intravitreal Injection in Humans. Am. J. Ophthalmol. 2012, 154, 682–686.e2.
- Dugel, P.U.; Boyer, D.S.; Antoszyk, A.N.; Steinle, N.C.; Varenhorst, M.P.; Pearlman, J.A.; Gillies, M.C.; Finger, R.P.; Baldwin, M.E.; Leitch, I.M. Phase 1 Study of OPT-302 Inhibition of Vascular Endothelial Growth Factors C and D for Neovascular Age-Related Macular Degeneration. Ophthalmol. Retina 2020, 4, 250–263.
- Boyer, D.S. Phase 1b/2a DME Study Results of OPT-302 to Block VEGF-C/-D in Combination with Aflibercept. In Proceedings of the AAO 2020, Virtual, 13 November 2020.
- Biosimilars for the Treatment of Wet AMD. Available online: Https://Www.Ophthalmologymanagement.Com/Newsletters/Amd-Update/July-2020 (accessed on 21 October 2022).
- Kapur, M.; Nirula, S.; Naik, M.P. Future of Anti-VEGF: Biosimilars and Biobetters. Int. J. Retina Vitr. 2022, 8, 2.
- Sharma, A.; Reddy, P.; Kuppermann, B.D.; Bandello, F.; Lowenstein, A. Biosimilars in Ophthalmology: “Is There a Big Change on the Horizon?”. Clin. Ophthalmol. 2018, 12, 2137–2143.
- Sharma, A.; Kumar, N.; Kuppermann, B.D.; Francesco, B.; Lowenstein, A. Ophthalmic Biosimilars: Lessons from India. Indian J. Ophthalmol. 2019, 67, 1384–1385.
- Kumar, A.; Agarwal, D.; Kumar, A. Commentary: Use of Biosimilars for Retinal Diseases in India: Challenges and Concerns. Indian J. Ophthalmol. 2021, 69, 357.
- Kim, H.M.; Woo, S.J. Ocular Drug Delivery to the Retina: Current Innovations and Future Perspectives. Pharmaceutics 2021, 13, 108.
- Del Amo, E.M.; Rimpelä, A.-K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic Aspects of Retinal Drug Delivery. Prog. Retin. Eye Res. 2017, 57, 134–185.
- Chandrasekaran, P.R.; Madanagopalan, V.G. KSI-301: Antibody Biopolymer Conjugate in Retinal Disorders. Ther. Adv. Ophthalmol. 2021, 13, 25158414211027708.
- Kodiak Sciences Announces Top-Line Results from Its Initial Phase 2b/3 Study of KSI-301 in Patients with Neovascular (Wet) Age-Related Macular Degeneration. Available online: Https://Ir.Kodiak.Com/News-Releases/News-Release-Details/Kodiak-Sciences-Announces-Top-Line-Results-Its-Initial-Phase-2b3 (accessed on 21 October 2022).
- Khanani, A.M.; Aziz, A.A.; Weng, C.Y.; Lin, W.V.; Vannavong, J.; Chhablani, J.; Danzig, C.J.; Kaiser, P.K. Port Delivery System: A Novel Drug Delivery Platform to Treat Retinal Diseases. Expert Opin. Drug Deliv. 2021, 18, 1571–1576.
- Campochiaro, P.A.; Marcus, D.M.; Awh, C.C.; Regillo, C.; Adamis, A.P.; Bantseev, V.; Chiang, Y.; Ehrlich, J.S.; Erickson, S.; Hanley, W.D.; et al. The Port Delivery System with Ranibizumab for Neovascular Age-Related Macular Degeneration: Results from the Randomized Phase 2 Ladder Clinical Trial. Ophthalmology 2019, 126, 1141–1154.
- Holekamp, N.M.; Campochiaro, P.A.; Chang, M.A.; Miller, D.; Pieramici, D.; Adamis, A.P.; Brittain, C.; Evans, E.; Kaufman, D.; Maass, K.F.; et al. Archway Randomized Phase 3 Trial of the Port Delivery System with Ranibizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2022, 129, 295–307.
- Kim, H.M.; Park, Y.J.; Lee, S.; Son, J.Y.; Hong, H.K.; Ham, M.H.; Jin, X.; Chung, J.Y.; Park, K.H.; Park, K.D.; et al. Intraocular Pharmacokinetics of 10-Fold Intravitreal Ranibizumab Injection Dose in Rabbits. Transl. Vis. Sci. Technol. 2020, 9, 7.
- REGENXBIO Presents Positive Initial Data from Phase II ALTITUDE™ Trial of RGX-314 for the Treatment of Diabetic Retinopathy Using Suprachoroidal Delivery at American Society of Retina Specialists Annual Meeting. Available online: https://www.prnewswire.com/news-releases/regenxbio-presents-positive-initial-data-from-phase-ii-altitude-trial-of-rgx-314-for-the-treatment-of-diabetic-retinopathy-using-suprachoroidal-delivery-at-american-society-of-retina-specialists-annual-meeting-301396478.html (accessed on 21 October 2022).
- Grishanin, R.; Vuillemenot, B.; Sharma, P.; Keravala, A.; Greengard, J.; Gelfman, C.; Blumenkrantz, M.; Lawrence, M.; Hu, W.; Kiss, S.; et al. Preclinical Evaluation of ADVM-022, a Novel Gene Therapy Approach to Treating Wet Age-Related Macular Degeneration. Mol. Ther. 2019, 27, 118–129.
- Lai, C.-M.; Estcourt, M.J.; Himbeck, R.P.; Lee, S.-Y.; Yew-San Yeo, I.; Luu, C.; Loh, B.K.; Lee, M.W.; Barathi, A.; Villano, J.; et al. Preclinical Safety Evaluation of Subretinal AAV2.SFlt-1 in Non-Human Primates. Gene Ther. 2012, 19, 999–1009.
- Rakoczy, E.P.; Lai, C.-M.; Magno, A.L.; Wikstrom, M.E.; French, M.A.; Pierce, C.M.; Schwartz, S.D.; Blumenkranz, M.S.; Chalberg, T.W.; Degli-Esposti, M.A.; et al. Gene Therapy with Recombinant Adeno-Associated Vectors for Neovascular Age-Related Macular Degeneration: 1 Year Follow-up of a Phase 1 Randomised Clinical Trial. Lancet Lond. Engl. 2015, 386, 2395–2403.
- Constable, I.J.; Pierce, C.M.; Lai, C.-M.; Magno, A.L.; Degli-Esposti, M.A.; French, M.A.; McAllister, I.L.; Butler, S.; Barone, S.B.; Schwartz, S.D.; et al. Phase 2a Randomized Clinical Trial: Safety and Post Hoc Analysis of Subretinal RAAV.SFLT-1 for Wet Age-Related Macular Degeneration. EBioMedicine 2016, 14, 168–175.
- Heier, J.S.; Kherani, S.; Desai, S.; Dugel, P.; Kaushal, S.; Cheng, S.H.; Delacono, C.; Purvis, A.; Richards, S.; Le-Halpere, A.; et al. Intravitreous Injection of AAV2-SFLT01 in Patients with Advanced Neovascular Age-Related Macular Degeneration: A Phase 1, Open-Label Trial. Lancet Lond. Engl. 2017, 390, 50–61.
- Atzori, M.G.; Tentori, L.; Ruffini, F.; Ceci, C.; Bonanno, E.; Scimeca, M.; Lacal, P.M.; Graziani, G. The Anti-Vascular Endothelial Growth Factor Receptor-1 Monoclonal Antibody D16F7 Inhibits Glioma Growth and Angiogenesis In Vivo. J. Pharmacol. Exp. Ther. 2018, 364, 77–86.
- Lee, S.H. Tanibirumab (TTAC-0001): A Fully Human Monoclonal Antibody Targets Vascular Endothelial Growth Factor Receptor 2 (VEGFR-2). Arch. Pharm. Res. 2011, 34, 1223–1226.
- Poole, R.M.; Vaidya, A. Ramucirumab: First Global Approval. Drugs 2014, 74, 1047–1058.
- Di Stasi, R.; De Rosa, L.; Diana, D.; Fattorusso, R.; D’Andrea, L.D. Human Recombinant VEGFR2D4 Biochemical Characterization to Investigate Novel Anti-VEGFR2D4 Antibodies for Allosteric Targeting of VEGFR2. Mol. Biotechnol. 2019, 61, 513–520.
- Bhargava, P.; Robinson, M.O. Development of Second-Generation VEGFR Tyrosine Kinase Inhibitors: Current Status. Curr. Oncol. Rep. 2011, 13, 103–111.
- Al-Khersan, H.; Hussain, R.M.; Ciulla, T.A.; Dugel, P.U. Innovative Therapies for Neovascular Age-Related Macular Degeneration. Expert Opin. Pharmacother. 2019, 20, 1879–1891.
- Samanta, A.; Aziz, A.A.; Jhingan, M.; Singh, S.R.; Khanani, A.M.; Chhablani, J. Emerging Therapies in Neovascular Age-Related Macular Degeneration in 2020. Asia-Pac. J. Ophthalmol. 2020, 9, 250–259.
- Hussain, R.M.; Shaukat, B.A.; Ciulla, L.M.; Berrocal, A.M.; Sridhar, J. Vascular Endothelial Growth Factor Antagonists: Promising Players in the Treatment of Neovascular Age-Related Macular Degeneration. Drug Des. Devel. Ther. 2021, 15, 2653–2665.
- Jackson, T.L.; Boyer, D.; Brown, D.M.; Chaudhry, N.; Elman, M.; Liang, C.; O’Shaughnessy, D.; Parsons, E.C.; Patel, S.; Slakter, J.S.; et al. Oral Tyrosine Kinase Inhibitor for Neovascular Age-Related Macular Degeneration: A Phase 1 Dose-Escalation Study. JAMA Ophthalmol. 2017, 135, 761–767.
- Patnaik, A.; LoRusso, P.M.; Messersmith, W.A.; Papadopoulos, K.P.; Gore, L.; Beeram, M.; Ramakrishnan, V.; Kim, A.H.; Beyer, J.C.; Mason Shih, L.; et al. A Phase Ib Study Evaluating MNRP1685A, a Fully Human Anti-NRP1 Monoclonal Antibody, in Combination with Bevacizumab and Paclitaxel in Patients with Advanced Solid Tumors. Cancer Chemother. Pharmacol. 2014, 73, 951–960.
- Weekes, C.D.; Beeram, M.; Tolcher, A.W.; Papadopoulos, K.P.; Gore, L.; Hegde, P.; Xin, Y.; Yu, R.; Shih, L.M.; Xiang, H.; et al. A Phase I Study of the Human Monoclonal Anti-NRP1 Antibody MNRP1685A in Patients with Advanced Solid Tumors. Investig. New Drugs 2014, 32, 653–660.
- Tang, J.; Kern, T.S. Inflammation in Diabetic Retinopathy. Prog. Retin. Eye Res. 2011, 30, 343–358.
- Silva, P.S.; Sun, J.K.; Aiello, L.P. Role of Steroids in the Management of Diabetic Macular Edema and Proliferative Diabetic Retinopathy. Semin. Ophthalmol. 2009, 24, 93–99.
- Boyer, D.S.; Yoon, Y.H.; Belfort, R.; Bandello, F.; Maturi, R.K.; Augustin, A.J.; Li, X.-Y.; Cui, H.; Hashad, Y.; Whitcup, S.M.; et al. Three-Year, Randomized, Sham-Controlled Trial of Dexamethasone Intravitreal Implant in Patients with Diabetic Macular Edema. Ophthalmology 2014, 121, 1904–1914.
- Singh, S.; Khanna, D.; Kalra, S. Minocycline and Doxycycline: More Than Antibiotics. Curr. Mol. Pharmacol. 2021, 14, 1046–1065.
- Cukras, C.A.; Petrou, P.; Chew, E.Y.; Meyerle, C.B.; Wong, W.T. Oral Minocycline for the Treatment of Diabetic Macular Edema (DME): Results of a Phase I/II Clinical Study. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3865–3874.
- Valent, D.J.; Wong, W.T.; Chew, E.Y.; Cukras, C.A. Oral Dextromethorphan for the Treatment of Diabetic Macular Edema: Results From a Phase I/II Clinical Study. Transl. Vis. Sci. Technol. 2018, 7, 24.
- Korenfeld, M.S.; Silverstein, S.M.; Cooke, D.L.; Vogel, R.; Crockett, R.S. Difluprednate Ophthalmic Emulsion 0.05% (Durezol) Study Group Difluprednate Ophthalmic Emulsion 0.05% for Postoperative Inflammation and Pain. J. Cataract. Refract. Surg. 2009, 35, 26–34.
- Foster, C.S.; Davanzo, R.; Flynn, T.E.; McLeod, K.; Vogel, R.; Crockett, R.S. Durezol (Difluprednate Ophthalmic Emulsion 0.05%) Compared with Pred Forte 1% Ophthalmic Suspension in the Treatment of Endogenous Anterior Uveitis. J. Ocul. Pharmacol. Ther. 2010, 26, 475–483.
- Nakano, S.; Yamamoto, T.; Kirii, E.; Abe, S.; Yamashita, H. Steroid Eye Drop Treatment (Difluprednate Ophthalmic Emulsion) Is Effective in Reducing Refractory Diabetic Macular Edema. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 248, 805–810.
- Nakano Goto, S.; Yamamoto, T.; Kirii, E.; Abe, S.; Yamashita, H. Treatment of Diffuse Diabetic Macular Oedema Using Steroid Eye Drops. Acta Ophthalmol. 2012, 90, 628–632.
- Tanito, M.; Hara, K.; Takai, Y.; Matsuoka, Y.; Nishimura, N.; Jansook, P.; Loftsson, T.; Stefánsson, E.; Ohira, A. Topical Dexamethasone-Cyclodextrin Microparticle Eye Drops for Diabetic Macular Edema. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7944–7948.
- Ohira, A.; Hara, K.; Jóhannesson, G.; Tanito, M.; Ásgrímsdóttir, G.M.; Lund, S.H.; Loftsson, T.; Stefánsson, E. Topical Dexamethasone γ-Cyclodextrin Nanoparticle Eye Drops Increase Visual Acuity and Decrease Macular Thickness in Diabetic Macular Oedema. Acta Ophthalmol. 2015, 93, 610–615.
- Monickaraj, F.; Oruganti, S.R.; McGuire, P.; Das, A. A Potential Novel Therapeutic Target in Diabetic Retinopathy: A Chemokine Receptor (CCR2/CCR5) Inhibitor Reduces Retinal Vascular Leakage in an Animal Model. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 93–100.
- Bhatwadekar, A.D.; Kansara, V.; Luo, Q.; Ciulla, T. Anti-Integrin Therapy for Retinovascular Diseases. Expert Opin. Investig. Drugs 2020, 29, 935–945.
- Miyamoto, K.; Khosrof, S.; Bursell, S.E.; Rohan, R.; Murata, T.; Clermont, A.C.; Aiello, L.P.; Ogura, Y.; Adamis, A.P. Prevention of Leukostasis and Vascular Leakage in Streptozotocin-Induced Diabetic Retinopathy via Intercellular Adhesion Molecule-1 Inhibition. Proc. Natl. Acad. Sci. USA 1999, 96, 10836–10841.
- Joussen, A.M.; Murata, T.; Tsujikawa, A.; Kirchhof, B.; Bursell, S.E.; Adamis, A.P. Leukocyte-Mediated Endothelial Cell Injury and Death in the Diabetic Retina. Am. J. Pathol. 2001, 158, 147–152.
- Barouch, F.C.; Miyamoto, K.; Allport, J.R.; Fujita, K.; Bursell, S.E.; Aiello, L.P.; Luscinskas, F.W.; Adamis, A.P. Integrin-Mediated Neutrophil Adhesion and Retinal Leukostasis in Diabetes. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1153–1158.
- Oliveira, L.B.; Meyer, C.H.; Kumar, J.; Tatebayashi, M.; Toth, C.A.; Wong, F.; Epstein, D.L.; McCuen, B.W. RGD Peptide-Assisted Vitrectomy to Facilitate Induction of a Posterior Vitreous Detachment: A New Principle in Pharmacological Vitreolysis. Curr. Eye Res. 2002, 25, 333–340.
- Yasukawa, T.; Hoffmann, S.; Eichler, W.; Friedrichs, U.; Wang, Y.-S.; Wiedemann, P. Inhibition of Experimental Choroidal Neovascularization in Rats by an Alpha(v)-Integrin Antagonist. Curr. Eye Res. 2004, 28, 359–366.
- Shaw, L.T.; Mackin, A.; Shah, R.; Jain, S.; Jain, P.; Nayak, R.; Hariprasad, S.M. Risuteganib-a Novel Integrin Inhibitor for the Treatment of Non-Exudative (Dry) Age-Related Macular Degeneration and Diabetic Macular Edema. Expert Opin. Investig. Drugs 2020, 29, 547–554.
- Wilkinson-Berka, J.L.; Jones, D.; Taylor, G.; Jaworski, K.; Kelly, D.J.; Ludbrook, S.B.; Willette, R.N.; Kumar, S.; Gilbert, R.E. SB-267268, a Nonpeptidic Antagonist of Alpha(v)Beta3 and Alpha(v)Beta5 Integrins, Reduces Angiogenesis and VEGF Expression in a Mouse Model of Retinopathy of Prematurity. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1600–1605.
- Feng, S.; Yu, H.; Yu, Y.; Geng, Y.; Li, D.; Yang, C.; Lv, Q.; Lu, L.; Liu, T.; Li, G.; et al. Levels of Inflammatory Cytokines IL-1β, IL-6, IL-8, IL-17A, and TNF-α in Aqueous Humour of Patients with Diabetic Retinopathy. J. Diabetes Res. 2018, 2018, 8546423.
- Wu, F.; Phone, A.; Lamy, R.; Ma, D.; Laotaweerungsawat, S.; Chen, Y.; Zhao, T.; Ma, W.; Zhang, F.; Psaras, C.; et al. Correlation of Aqueous, Vitreous, and Plasma Cytokine Levels in Patients With Proliferative Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2020, 61, 26.
- Gustavsson, C.; Agardh, C.-D.; Agardh, E. Profile of Intraocular Tumour Necrosis Factor-α and Interleukin-6 in Diabetic Subjects with Different Degrees of Diabetic Retinopathy. Acta Ophthalmol. 2013, 91, 445–452.
- Mitoma, H.; Horiuchi, T.; Tsukamoto, H.; Ueda, N. Molecular Mechanisms of Action of Anti-TNF-α Agents—Comparison among Therapeutic TNF-α Antagonists. Cytokine 2018, 101, 56–63.
- Sfikakis, P.P.; Markomichelakis, N.; Theodossiadis, G.P.; Grigoropoulos, V.; Katsilambros, N.; Theodossiadis, P.G. Regression of Sight-Threatening Macular Edema in Type 2 Diabetes Following Treatment with the Anti-Tumor Necrosis Factor Monoclonal Antibody Infliximab. Diabetes Care 2005, 28, 445–447.
- Mesquida, M.; Drawnel, F.; Lait, P.J.; Copland, D.A.; Stimpson, M.L.; Llorenç, V.; Sainz de la Maza, M.; Adan, A.; Widmer, G.; Strassburger, P.; et al. Modelling Macular Edema: The Effect of IL-6 and IL-6R Blockade on Human Blood–Retinal Barrier Integrity In Vitro. Transl. Vis. Sci. Technol. 2019, 8, 32.
- Valle, M.L.; Dworshak, J.; Sharma, A.; Ibrahim, A.S.; Al-Shabrawey, M.; Sharma, S. Inhibition of Interleukin-6 Trans-Signaling Prevents Inflammation and Endothelial Barrier Disruption in Retinal Endothelial Cells. Exp. Eye Res. 2019, 178, 27–36.
- Jo, D.H.; Yun, J.-H.; Cho, C.S.; Kim, J.H.; Kim, J.H.; Cho, C.-H. Interaction between Microglia and Retinal Pigment Epithelial Cells Determines the Integrity of Outer Blood-Retinal Barrier in Diabetic Retinopathy. Glia 2019, 67, 321–331.
- Barnes, T.C.; Anderson, M.E.; Moots, R.J. The Many Faces of Interleukin-6: The Role of IL-6 in Inflammation, Vasculopathy, and Fibrosis in Systemic Sclerosis. Int. J. Rheumatol. 2011, 2011, 1–6.
- Rose-John, S. IL-6 Trans-Signaling via the Soluble IL-6 Receptor: Importance for the Pro-Inflammatory Activities of IL-6. Int. J. Biol. Sci. 2012, 8, 1237–1247.
- Sharma, S. Interleukin-6 Trans-Signaling: A Pathway With Therapeutic Potential for Diabetic Retinopathy. Front. Physiol. 2021, 12, 689429.
- Ohsugi, Y.; Kishimoto, T. The Recombinant Humanized Anti-IL-6 Receptor Antibody Tocilizumab, an Innovative Drug for the Treatment of Rheumatoid Arthritis. Expert Opin. Biol. Ther. 2008, 8, 669–681.
- Noda, K.; Nakao, S.; Zandi, S.; Engelstädter, V.; Mashima, Y.; Hafezi-Moghadam, A. Vascular Adhesion Protein-1 Regulates Leukocyte Transmigration Rate in the Retina during Diabetes. Exp. Eye Res. 2009, 89, 774–781.
- Murata, M.; Noda, K.; Fukuhara, J.; Kanda, A.; Kase, S.; Saito, W.; Ozawa, Y.; Mochizuki, S.; Kimura, S.; Mashima, Y.; et al. Soluble Vascular Adhesion Protein-1 Accumulates in Proliferative Diabetic Retinopathy. Investig. Opthalmol. Vis. Sci. 2012, 53, 4055.
- Tékus, V.; Horváth, Á.I.; Csekő, K.; Szabadfi, K.; Kovács-Valasek, A.; Dányádi, B.; Deres, L.; Halmosi, R.; Sághy, É.; Varga, Z.V.; et al. Protective Effects of the Novel Amine-Oxidase Inhibitor Multi-Target Drug SZV 1287 on Streptozotocin-Induced Beta Cell Damage and Diabetic Complications in Rats. Biomed. Pharmacother. 2021, 134, 111105.
- Singh, A.D.; Kulkarni, Y.A. Vascular Adhesion Protein-1 and Microvascular Diabetic Complications. Pharmacol. Rep. 2022, 74, 40–46.
- Nguyen, Q.D.; Sepah, Y.J.; Berger, B.; Brown, D.; Do, D.V.; Garcia-Hernandez, A.; Patel, S.; Rahhal, F.M.; Shildkrot, Y.; Renfurm, R.W.; et al. Primary Outcomes of the VIDI Study: Phase 2, Double-Masked, Randomized, Active-Controlled Study of ASP8232 for Diabetic Macular Edema. Int. J. Retina Vitr. 2019, 5, 28.
- Rao, P.; Knaus, E.E. Evolution of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs): Cyclooxygenase (COX) Inhibition and Beyond. J. Pharm. Pharm. Sci. 2008, 11, 81s–110s.
- Jones, J.; Francis, P. Ophthalmic Utility of Topical Bromfenac, a Twice-Daily Nonsteroidal Anti-Inflammatory Agent. Expert Opin. Pharmacother. 2009, 10, 2379–2385.
- Gaynes, B.I.; Onyekwuluje, A. Topical Ophthalmic NSAIDs: A Discussion with Focus on Nepafenac Ophthalmic Suspension. Clin. Ophthalmol. 2008, 2, 355–368.
- Pinna, A.; Blasetti, F.; Ricci, G.D.; Boscia, F. Bromfenac Eyedrops in the Treatment of Diabetic Macular Edema: A Pilot Study. Eur. J. Ophthalmol. 2017, 27, 326–330.
- Callanan, D.; Williams, P. Topical Nepafenac in the Treatment of Diabetic Macular Edema. Clin. Ophthalmol. 2008, 2, 689–692.
- Howaidy, A.; Eldaly, Z.H.; Anis, M.; Othman, T.M. Prophylaxis of Macular Edema after Cataract Surgery in Diabetic Patients, Topical Nepafenac versus Intravitreal Ranibizumab. Eur. J. Ophthalmol. 2022, 32, 205–212.
- Naftali Ben Haim, L.; Moisseiev, E. Drug Delivery via the Suprachoroidal Space for the Treatment of Retinal Diseases. Pharmaceutics 2021, 13, 967.
- Ranta, V.-P.; Mannermaa, E.; Lummepuro, K.; Subrizi, A.; Laukkanen, A.; Antopolsky, M.; Murtomäki, L.; Hornof, M.; Urtti, A. Barrier Analysis of Periocular Drug Delivery to the Posterior Segment. J. Control. Release 2010, 148, 42–48.
- Barakat, M.R.; Wykoff, C.C.; Gonzalez, V.; Hu, A.; Marcus, D.; Zavaleta, E.; Ciulla, T.A. Suprachoroidal CLS-TA plus Intravitreal Aflibercept for Diabetic Macular Edema: A Randomized, Double-Masked, Parallel-Design, Controlled Study. Ophthalmol. Retina 2021, 5, 60–70.
- Fachinger, G.; Deutsch, U.; Risau, W. Functional Interaction of Vascular Endothelial-Protein-Tyrosine Phosphatase with the Angiopoietin Receptor Tie-2. Oncogene 1999, 18, 5948–5953.
- Hussain, R.M.; Neiweem, A.E.; Kansara, V.; Harris, A.; Ciulla, T.A. Tie-2/Angiopoietin Pathway Modulation as a Therapeutic Strategy for Retinal Disease. Expert Opin. Investig. Drugs 2019, 28, 861–869.
- Mirando, A.C.; Shen, J.; Silva, R.L.E.; Chu, Z.; Sass, N.C.; Lorenc, V.E.; Green, J.J.; Campochiaro, P.A.; Popel, A.S.; Pandey, N.B. A Collagen IV-Derived Peptide Disrupts A5β1 Integrin and Potentiates Ang2/Tie2 Signaling. JCI Insight 2019, 4, 122043.
- Wykoff, C.C.; Abreu, F.; Adamis, A.P.; Basu, K.; Eichenbaum, D.A.; Haskova, Z.; Lin, H.; Loewenstein, A.; Mohan, S.; Pearce, I.A.; et al. Efficacy, Durability, and Safety of Intravitreal Faricimab with Extended Dosing up to Every 16 Weeks in Patients with Diabetic Macular Oedema (YOSEMITE and RHINE): Two Randomised, Double-Masked, Phase 3 Trials. Lancet 2022, 399, 10326.
- Shirley, M. Faricimab: First Approval. Drugs 2022, 82, 825–830.
- Campochiaro, P.A.; Peters, K.G. Targeting Tie2 for Treatment of Diabetic Retinopathy and Diabetic Macular Edema. Curr. Diab. Rep. 2016, 16, 126.
- Campochiaro, P.A.; Khanani, A.; Singer, M.; Patel, S.; Boyer, D.; Dugel, P.; Kherani, S.; Withers, B.; Gambino, L.; Peters, K.; et al. Enhanced Benefit in Diabetic Macular Edema from AKB-9778 Tie2 Activation Combined with Vascular Endothelial Growth Factor Suppression. Ophthalmology 2016, 123, 1722–1730.
- Zhang, J.; Wu, Y.; Jin, Y.; Ji, F.; Sinclair, S.H.; Luo, Y.; Xu, G.; Lu, L.; Dai, W.; Yanoff, M.; et al. Intravitreal Injection of Erythropoietin Protects Both Retinal Vascular and Neuronal Cells in Early Diabetes. Investig. Ophthalmol. Vis. Sci. 2008, 49, 732–742.
- Shen, J.; Wu, Y.; Xu, J.-Y.; Zhang, J.; Sinclair, S.H.; Yanoff, M.; Xu, G.; Li, W.; Xu, G.-T. ERK- and Akt-Dependent Neuroprotection by Erythropoietin (EPO) against Glyoxal-AGEs via Modulation of Bcl-XL, Bax, and BAD. Investig. Ophthalmol. Vis. Sci. 2010, 51, 35–46.
- Hu, L.-M.; Luo, Y.; Zhang, J.; Lei, X.; Shen, J.; Wu, Y.; Qin, M.; Unver, Y.B.; Zhong, Y.; Xu, G.-T.; et al. EPO Reduces Reactive Gliosis and Stimulates Neurotrophin Expression in Muller Cells. Front. Biosci.-Elite 2011, 3, 1541–1555.
- Zhang, J.; Hu, L.-M.; Xu, G.; Wu, Y.; Shen, J.; Luo, Y.; Zhong, Y.; Sinclair, S.H.; Yanoff, M.; Li, W.; et al. Anti-VEGF Effects of Intravitreal Erythropoietin in Early Diabetic Retinopathy. Front. Biosci.-Elite 2010, 2, 912–927.
- Lei, X.; Zhang, J.; Shen, J.; Hu, L.-M.; Wu, Y.; Mou, L.; Xu, G.; Li, W.; Xu, G.-T. EPO Attenuates Inflammatory Cytokines by Muller Cells in Diabetic Retinopathy. Front. Biosci. Elite Ed. 2011, 3, 201–211.
- Xu, G.; Kang, D.; Zhang, C.; Lou, H.; Sun, C.; Yang, Q.; Lu, L.; Xu, G.-T.; Zhang, J.; Wang, F. Erythropoietin Protects Retinal Cells in Diabetic Rats Through Upregulating ZnT8 via Activating ERK Pathway and Inhibiting HIF-1α Expression. Investig. Ophthalmol. Vis. Sci. 2015, 56, 8166–8178.
- Gu, L.; Xu, H.; Wang, F.; Xu, G.; Sinha, D.; Wang, J.; Xu, J.-Y.; Tian, H.; Gao, F.; Li, W.; et al. Erythropoietin Exerts a Neuroprotective Function against Glutamate Neurotoxicity in Experimental Diabetic Retina. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8208–8222.
- Liu, D.; Xu, H.; Zhang, C.; Xie, H.; Yang, Q.; Li, W.; Tian, H.; Lu, L.; Xu, J.-Y.; Xu, G.; et al. Erythropoietin Maintains VE-Cadherin Expression and Barrier Function in Experimental Diabetic Retinopathy via Inhibiting VEGF/VEGFR2/Src Signaling Pathway. Life Sci. 2020, 259, 118273.
- Zhang, C.; Xie, H.; Yang, Q.; Yang, Y.; Li, W.; Tian, H.; Lu, L.; Wang, F.; Xu, J.-Y.; Gao, F.; et al. Erythropoietin Protects Outer Blood-Retinal Barrier in Experimental Diabetic Retinopathy by up-Regulating ZO-1 and Occludin. Clin. Experiment. Ophthalmol. 2019, 47, 1182–1197.
- Xie, H.; Zhang, C.; Liu, D.; Yang, Q.; Tang, L.; Wang, T.; Tian, H.; Lu, L.; Xu, J.-Y.; Gao, F.; et al. Erythropoietin Protects the Inner Blood-Retinal Barrier by Inhibiting Microglia Phagocytosis via Src/Akt/Cofilin Signalling in Experimental Diabetic Retinopathy. Diabetologia 2021, 64, 211–225.
- Ahmet, I.; Tae, H.-J.; Juhaszova, M.; Riordon, D.R.; Boheler, K.R.; Sollott, S.J.; Brines, M.; Cerami, A.; Lakatta, E.G.; Talan, M.I. A Small Nonerythropoietic Helix B Surface Peptide Based upon Erythropoietin Structure Is Cardioprotective against Ischemic Myocardial Damage. Mol. Med. 2011, 17, 194–200.
- Brines, M.; Patel, N.S.A.; Villa, P.; Brines, C.; Mennini, T.; De Paola, M.; Erbayraktar, Z.; Erbayraktar, S.; Sepodes, B.; Thiemermann, C.; et al. Nonerythropoietic, Tissue-Protective Peptides Derived from the Tertiary Structure of Erythropoietin. Proc. Natl. Acad. Sci. USA 2008, 105, 10925–10930.
- McVicar, C.M.; Hamilton, R.; Colhoun, L.M.; Gardiner, T.A.; Brines, M.; Cerami, A.; Stitt, A.W. Intervention With an Erythropoietin-Derived Peptide Protects Against Neuroglial and Vascular Degeneration During Diabetic Retinopathy. Diabetes 2011, 60, 2995–3005.
- Simó, R.; Hernández, C.; Porta, M.; Bandello, F.; Grauslund, J.; Harding, S.P.; Aldington, S.J.; Egan, C.; Frydkjaer-Olsen, U.; García-Arumí, J.; et al. Effects of Topically Administered Neuroprotective Drugs in Early Stages of Diabetic Retinopathy: Results of the EUROCONDOR Clinical Trial. Diabetes 2019, 68, 457–463.
- Grauslund, J.; Frydkjaer-Olsen, U.; Peto, T.; Fernández-Carneado, J.; Ponsati, B.; Hernández, C.; Cunha-Vaz, J.; Simó, R.; EUROCONDOR. Topical Treatment With Brimonidine and Somatostatin Causes Retinal Vascular Dilation in Patients With Early Diabetic Retinopathy From the EUROCONDOR. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2257–2262.
- Kang, Q.; Yang, C. Oxidative Stress and Diabetic Retinopathy: Molecular Mechanisms, Pathogenetic Role and Therapeutic Implications. Redox Biol. 2020, 37, 101799.
- Urner, S.; Ho, F.; Jha, J.C.; Ziegler, D.; Jandeleit-Dahm, K. NADPH Oxidase Inhibition: Preclinical and Clinical Studies in Diabetic Complications. Antioxid. Redox Signal. 2020, 33, 415–434.
- Wilkinson-Berka, J.L.; Deliyanti, D.; Rana, I.; Miller, A.G.; Agrotis, A.; Armani, R.; Szyndralewiez, C.; Wingler, K.; Touyz, R.M.; Cooper, M.E.; et al. NADPH Oxidase, NOX1, Mediates Vascular Injury in Ischemic Retinopathy. Antioxid. Redox Signal. 2014, 20, 2726–2740.
- Al-Shabrawey, M.; Rojas, M.; Sanders, T.; Behzadian, A.; El-Remessy, A.; Bartoli, M.; Parpia, A.K.; Liou, G.; Caldwell, R.B. Role of NADPH Oxidase in Retinal Vascular Inflammation. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3239–3244.
- Li, J.; Wang, J.J.; Yu, Q.; Chen, K.; Mahadev, K.; Zhang, S.X. Inhibition of Reactive Oxygen Species by Lovastatin Downregulates Vascular Endothelial Growth Factor Expression and Ameliorates Blood-Retinal Barrier Breakdown in Db/Db Mice: Role of NADPH Oxidase 4. Diabetes 2010, 59, 1528–1538.
- Clementi, M.E.; Pizzoferrato, M.; Bianchetti, G.; Brancato, A.; Sampaolese, B.; Maulucci, G.; Tringali, G. Cytoprotective Effect of Idebenone through Modulation of the Intrinsic Mitochondrial Pathway of Apoptosis in Human Retinal Pigment Epithelial Cells Exposed to Oxidative Stress Induced by Hydrogen Peroxide. Biomedicines 2022, 10, 503.
- Clementi, M.E.; Maulucci, G.; Bianchetti, G.; Pizzoferrato, M.; Sampaolese, B.; Tringali, G. Cytoprotective Effects of Punicalagin on Hydrogen-Peroxide-Mediated Oxidative Stress and Mitochondrial Dysfunction in Retinal Pigment Epithelium Cells. Antioxidants 2021, 10, 192.
This entry is offline, you can click here to edit this entry!
