Titanium oxide (TiO2) nanoparticles are successfully employed in human food, drugs, cosmetics, advanced medicine, and dentistry because of their non-cytotoxic, non-allergic, and bio-compatible nature when used in direct close contact with the human body. These NPs are the most versatile oxides as a result of their acceptable chemical stability, lower cost, strong oxidation properties, high refractive index, and enhanced aesthetics. These NPs are fabricated by conventional (physical and chemical) methods and the latest biological methods (biological, green, and biological derivatives), with their advantages and disadvantages in this epoch. The significance of TiO2 NPs as a medical material includes drug delivery release, cancer therapy, orthopedic implants, biosensors, instruments, and devices, whereas their significance as a dental biomaterial involves dentifrices, oral antibacterial disinfectants, whitening agents, and adhesives. In addition, TiO2 NPs play an important role in orthodontics (wires and brackets), endodontics (sealers and obturating materials), maxillofacial surgeries (implants and bone plates), prosthodontics (veneers, crowns, bridges, and acrylic resin dentures), and restorative dentistry (GIC and composites).
- nanomaterials
- nanoparticles (NPs)
- nanodentistry
- medicine
- titanium oxide (TiO2)
1. Introduction
2. Background of Nanotechnology
2.1. History of Nanotechnology
2.2. Significant Features of Nanotechnology
-
This technology can bring changes in the original matter’s structure by converting it into a nanoscale product.
-
The newly formed nanoscale structure should contain periodic repetition (i.e., the nanoparticle must periodically repeat itself in one or more than one direction) just like the parent matter.
-
The novel properties, characteristics, and functions of a newly formed nanoscale product should be like those of the parent matter or be superior to the parent matter despite being nanometric.
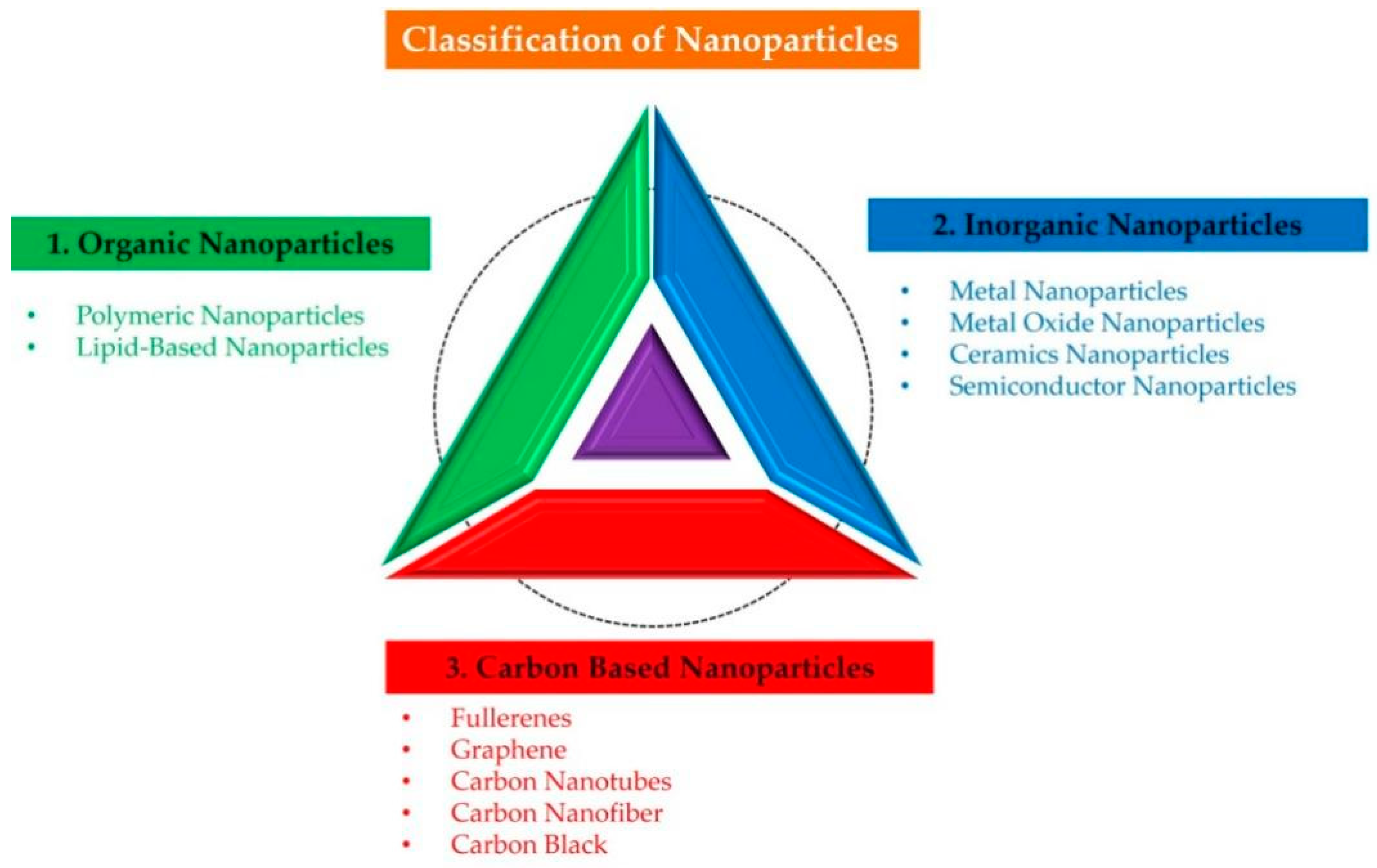
2.3. Classification of NPs

3. The Titanium Oxide NPs
This entry is adapted from the peer-reviewed paper 10.3390/nano12203670
References
- Ahmadi, E.D.; Hafeji, S.; Khurshid, Z.; Imran, E.; Zafar, M.S.; Saeinasab, M.; Sefat, F. Biophotonics in Dentistry. Appl. Sci. 2022, 12, 4254.
- Hayat, F.; Sabbir, J.; Khurshid, Z.; Zafar, M.S.; Ghabbani, H.M.; Shahbazi, M.-A.; Sefat, F. Nanoparticles in endodontics. In Biomaterials in Endodontics; Woodhead Publishers: Shaston, UK, 2022; pp. 195–209.
- Khurshid, Z.; Zafar, M.; Qasim, S.; Shahab, S.; Naseem, M.; AbuReqaiba, A. Advances in Nanotechnology for Restorative Dentistry. Materials 2015, 8, 717–731.
- Zafar, M.S.; Khurshid, Z.; Najeeb, S.; Zohaib, S.; Rehman, I.U. Therapeutic applications of nanotechnology in dentistry. Nanostruct. Oral Med. 2017, 17, 833–862.
- Najeeb, S.; Khurshid, Z.; Ghabbani, H.; Zafar, M.S.; Sefat, F. Nano glass ionomer cement: Modification for biodental applications. In Advanced Dental Biomaterials; Woodhead Publishers: Shaston, UK, 2019; pp. 217–227. ISBN 9780081024768.
- Sagadevan, S.; Imteyaz, S.; Murugan, B.; Anita, L.J.; Sridewi, N.; Weldegebrieal, G.; Fatimah, I.; Oh, W.A. Comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications. Green Process. Synth. 2022, 11, 44–63.
- American Conference of Governmental Industrial Hygienists (ACGIH). Threshold Limit Values and Biological Exposure Indices for 1992; American Conference of Governmental Industrial Hygienists: Cincinnati, OH, USA, 1992.
- ILSI Risk Science Institute. The relevance of the rat lung response to particle overload for human risk assessment: A workshop consensus report. Inhal. Toxicol. 2000, 12, 1–17.
- Maynard, A.D.; Kuempel, E.D. Airborne Nanostructured Particles and Occupational Health. J. Nanopart. Res. 2005, 7, 587–614.
- Tsuji, J.S.; Maynard, A.D.; Howard, P.C.; James, J.T.; Lam, C.W.; Warheit, D.B.; Santamaria, A.B. Research strategies for safety evaluation of nanomaterials, part IV: Risk assessment of nanoparticles. Toxicol. Sci. Off. J. Soc. Toxicol. 2006, 89, 42–50.
- Aslam, M.; Abdullah, A.Z.; Rafatullah, M. Recent development in the green synthesis of titanium dioxide nanoparticles using plant-based biomolecules for environmental and antimicrobial applications. J. Ind. Eng. Chem. 2021, 98, 1–16.
- Ahmed, A.A.; Afzal, N.; Devarajan, M.; Subramani, S. Structural, morphological, optical and electrical properties of NiO films prepared on Si (100) and glass substrates at different thicknesses. Mater. Res. Express 2016, 3, 116405.
- Medhi, R.; Marquez, M.D.; Lee, T.R. Visible-light-active doped metal oxide nanoparticles: Review of their synthesis, properties, and applications. ACS Appl. Nano Mater. 2020, 3, 6156–6185.
- Garcia, M.F.; Rodriguez, J.A. Metal Oxide Nanoparticles. In Nanomaterials: Inorganic and Bioinorganic Perspectives; BNL-79479-2007-BC; Brookhaven National Laboratory: Long Island, NY, USA, 2007.
- Nabi, G.; Qurat-ul-Aain, K.N.R.; Tahir, M.B.; Rafique, M.; Rizwan, M.; Hussain, S.; Iqbal, T.; Majid, A. A Review on Novel Eco-Friendly Green Approach to Synthesis Titanium dioxide (TiO2) nanoparticles Using Different Extracts. J. Inorg. Organomet. Polym. Mater. 2018, 28, 1552–1564.
- Lazar, M.A.; Varghese, S.; Nair, S.S. Photocatalytic water treatment by Titanium dioxide: Recent updates. Catalysts 2012, 2, 572–601.
- Zhang, W.; Li, Y.; Su, Y.; Mao, K.; Wang, Q. Effect of water composition on TiO2 photocatalytic removal of endocrine disrupting compounds (EDCs) and estrogenic activity from secondary effluent. J. Hazard Mater. 2012, 215, 252–258.
- Dastjerdi, R.; Montazer, M. A review on the application of inorganic nano-structured materials in the modification of textiles: Focus on anti-microbial properties. Colloids Surf. B Biointerfaces. 2010, 79, 5–18.
- Kubacka, A.; Diez, M.; Rojo, D. Understanding the antimicrobial mechanism of TiO2-based nanocomposite films in a pathogenic bacterium. Sci. Rep. 2014, 4, 4134.
- Venkatasubbu, D.; Ramasamy, G.; Ramakrishnan, S.; Janakiraman, K. Folate targeted PEGylated titanium dioxide nanoparticles as a nanocarrier for targeted paclitaxel drug delivery. Adv. Powder Technol. 2013, 24, 947–954.
- Yin, Z.F.; Wu, L.; Yang, H.G.; Su, Y.H. Recent progress in biomedical applications of titanium dioxide. Phys. Chem. Chem. Phys. 2013, 15, 4844–4858.
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nature 1997, 388, 431–432.
- Xu, P.; Wang, R.; Ouyang, J.; Chen, B. A new strategy for TiO2 whiskers mediated multi-mode cancer treatment. Nanoscale Res. Lett. 2015, 10, 94–104.
- Comune, M.; Rai, A.; Palma, P.; Tondaturo, C.; Ferreira, L. Antimicrobial and pro-angiogenic properties of soluble and nanoparticle-immobilized LL37 peptides. Biomater. Sci. 2021, 9, 8153–8159.
- Liu, E.; Zhou, Y.; Liu, Z.; Li, J.; Zhang, D.; Chen, J.; Cai, Z. Cisplatin loaded hyaluronic acid modified Titanium dioxide (TiO2) nanoparticles for neoadjuvant chemotherapy of ovarian cancer. J. Nanomater. 2015, 2015, 1–8.
- Behnam, M.A.; Emami, F.; Sobhani, Z.; Dehghanian, A.R. The application of titanium dioxide (TiO2) nanoparticles in the photo-thermal therapy of melanoma cancer model. Iran. J. Basic Med. Sci. 2018, 21, 1133–1139.
- Abushowmi, T.H.; AlZaher, Z.A.; Almaskin, D.F.; Qaw, M.S.; Abualsaud, R.; Akhtar, S.; Al-Thobity, A.M.; Al-Harbi, F.A.; Gad, M.M.; Baba, N.Z. Comparative Effect of Glass Fiber and Nano-Filler Addition on Denture Repair Strength. J. Prosthodont. 2020, 29, 261–268.
- Xia, Y.; Zhang, F.; Xie, H.; Gu, N. Nanoparticle-reinforced resin-based dental composites. J. Dent. 2008, 36, 450–455.
- Ohkubo, C.; Hanatani, S.; Hosoi, T. Present status of titanium removable dentures—A review of the literature. J. Oral Rehabil. 2008, 35, 706–714.
- Sodagar, A.; Akhoundi, M.; Bahador, A.; Jalali, Y.F.; Behzadi, Z.; Elhaminejad, F.; Mirhashemi, A.H. Effect of Titanium dioxide (TiO2) nanoparticles incorporation on antibacterial properties and shear bond strength of dental composite used in Orthodontics. Dent. Press J. Orthod. 2017, 22, 67–74.
- Samiei, M.; Janani, M.; Asl-Aminabadi, N.; Ghasemi, N.; Divband, B.; Shirazi, S.; Kafili, K. Effect of the Titanium dioxide (TiO2) nanoparticles on the selected physical properties of mineral trioxide aggregate. J. Clin. Exp. Dent. 2017, 9, e191–e195.
- Bertani, R.; Bartolozzi, A.; Pontefisso, A.; Quaresimin, M.; Zappalorto, M. Improving the Antimicrobial and Mechanical Properties of Epoxy Resins via Nanomodification: An Overview. Mol. Ecules 2021, 26, 5426.
- Lhotan, A.; Yates, J.; Zidan, S.; Haider, J.; Silikas, N. Assessing Fracture Toughness and Impact Strength of PMMA Reinforced with Nanoparticles and Fibre as Advanced Denture Base Materials. Materials 2021, 24, 4127.
- Chambers, C.; Stewart, S.B.; Su, B.; Jankinson, H.E.; Sandy, J.; Ireland, A. Silver doped titanium dioxide nanoparticles as antimicrobial additives to dental polymers. Dent. Mater. 2017, 33, e115–e123.
- Colvin, V.L.; Schlamp, M.C.; Alivisatos, A.P. Light emitting diodes made from cadmium selenide nanocrystals and a semiconducting polymer. Nature 1994, 370, 354–357.
- Wang, Y.H.N. Nanometer-sized semiconductor clusters: Materials synthesis, quantum size effects, and photophysical properties. J. Phys. Chem. 1991, 95, 525–532.
- Atul, R.I.; Thakare, S.R.; Khati, N.T.; Wankhade, A.V.; Burghate, D.K. Green synthesis of selenium nanoparticles under ambient condition. Chalcogenide Lett. 2010, 7, 485–489.
- Ozak, S.T.; Ozkan, P. Nanotechnology and dentistry. Eur. J. Dent. 2013, 7, 145–151.
- Saravana, K.R.; Vijayalakshmi, R. Nanotechnology in Dentistry. Ind. J. Den. Res. 2006, 17, 62–65.
- Ochekpe, A.N.; Olorunfemi, P.O.; Ngwuluka, N.C. Nanotechnology and Drug Delivery Part 1: Background and Applications. Trop. J. Pharm. Res. 2009, 8, 265–274.
- Swihart, M.T. Vapor-phase synthesis of nanoparticles. Curr. Opin. Colloid. Interface Sci. 2003, 8, 127–133.
- Narducci, D. An introduction to nanotechnologies: What’s in it for us? Vet. Res. Commun. 2007, 31 (Suppl. 1), 131–137.
- Jayapalan, A.R.; Lee, B.Y.; Kurtis, K.E. Effect of Nano-sized Titanium Dioxide on Early Age Hydration of Portland Cement. In Nanotechnology in Construction 3; Springer: Berlin/Heidelberg, Germany, 2009; pp. 267–273.
- Srimaneepong, V.; Skallevold, H.E.; Khurshid, Z.; Zafar, M.S.; Rokaya, D.; Sapkota, J. Graphene for Antimicrobial and Coating Application. Int. J. Mol. Sci. 2022, 23, 499.
- Foong, L.K.; Foroughi, M.M.; Mirhosseini, A.F.; Safaei, M.; Jahani, S.; Mostafavi, M.; Ebrahimpoor, N.; Sharifi, M.; Varma, R.S.; Khatami, M. Applications of nano-materials in diverse dentistry regimes. RSC Adv. 2020, 10, 15430–15460.
- Yan, L.; Zhao, F.; Wang, J.; Zu, Y.; Gu, Z.; Zhao, Y. A Safe-by-Design Strategy towards Safer Nanomaterials in Nanomedicines. Adv. Mater. 2019, 31, 1805391.
- Niinomi, M. Mechanical biocompatibilities of titanium alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 2008, 1, 30–42.
- Burello, E.; Worth, A. Predicting toxicity of nanoparticles. Nat. Nanotechnol. 2011, 6, 138–139.
- Tschernitschek, H.; Borchers, L.; Geurtsen, W. Nonalloyed titanium as a bioinert metal—A review. Quintessence Int. 2005, 36, 351.
- Li, Y.; Fan, Y.; Chen, Y. A novel method for preparation of nanocrystalline rutile TiO2 powders by liquid hydrolysis of TiCl. J. Mater. Chem. 2002, 12, 1387–1390.
- Wang, W.N.; Lenggoro, I.W.; Terashi, Y.; Kim, T.O.; Okuyama, K. One-step synthesis of titanium oxide nanoparticles by spray pyrolysis of organic precursors. Mater. Sci. Eng. 2005, 123, 194–202.
- Warheit, D.B.; Webb, K.L.; Reed, S.; Frerichs and Sayes, C.M. Pulmonary toxicity study in rats with three forms of ultrafi ne-TiO2 particles: Diff erential responses related to surface properties. Toxicology 2007, 230, 90–104.
- Sayes, C.M.; Wahi, R.; Kurian, P.A.; Liu, Y.; West, J.L.; Ausman, K.D.; Warheit, D.B.; Colvin, V.L. Correlating nanoscale titania structure with toxicity: A cytotoxicity and inflammatory response study with human dermal fibroblasts and human lung epithelial cells. Toxicol. Sci. 2006, 92, 174–185.
- Balayeva, N.O.; Mamiyev, Z.; Dillert, R.; Zheng, N.; Bahnemann, D.W. Rh/TiO2-Photocatalyzed Acceptorless Dehydrogenation of N-Heterocycles upon Visible-Light Illumination. ACS Catal. 2020, 10, 5542–5553.
- Balayeva, N.O.; Fleisch, M.; Bahnemann, D.W. Surface-grafted WO3/TiO2 photocatalysts: Enhanced visible-light activity towards indoor air purification. Catal. Today 2018, 313, 63–71.
- Balayeva, N.O.; Zheng, N.; Dillert, R.; Bahnemann, D.W. Visible-Light-Mediated Photocatalytic Aerobic Dehydrogenation of N-heterocycles by Surface-Grafted TiO2 and 4-amino-TEMPO. ACS Catal. 2019, 9, 10694–10704.
