Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Inkjet printing technologies have advanced significantly and found several applications in the pharmaceutical and biomedical sector. Thermal inkjet printing is one of the most widely used techniques due to its versatility in the development of bioinks for cell printing or biosensors, and the potential to fabricate personalized medications of various forms such as films and tablets.
- thermal inkjet printing
- TIJ
- bubble jet printing
1. Introduction
Over the last 20 years, we have encountered a transformation in manufacturing technologies in the area of medicinal products [1][2][3]. Traditionally marketed medicines are manufactured at fixed doses (one size fits all) targeting a large number of patients in order to reduce the production costs and time to the market. However, the widely varied responses to a particular therapeutic dose in patient populations especially for medicines with narrow therapeutic windows points out the limitations of generalized mass manufacturing [1][4]. Moreover, there is a growing number of patients worldwide with chronic diseases who have to take multiple doses of medicines per day, called polypharmacy, which increases the risk for side effects and drug–disease interactions [5]. Currently, swift advances in gene sequencing technology along with increased knowledge of genomics and better understanding of diseases on molecular level coupled with the use of toxicogenomic markers have opened a door for personalized medicine that will possibly bring a revolution in the conventional treatment approaches as well as in pharmaceutical industry [6][7][8][9]. For the materialization of these advances in personalized medicines, a wide range of 2D and 3D printing technologies have been introduced as appropriate for manufacturing print-on-demand medicinal products. Inkjet printing (IJP) technology is considered an ideal approach as it is cost effective [1][10][11][12][13][14] with high precision, repeatability, robustness, and high-throughput (Figure 1). Due to its wide applicability, inkjet printing has been extensively used for pharmaceutical applications and tissue engineering [15][16][17][18][19][20][21][22][23][24].
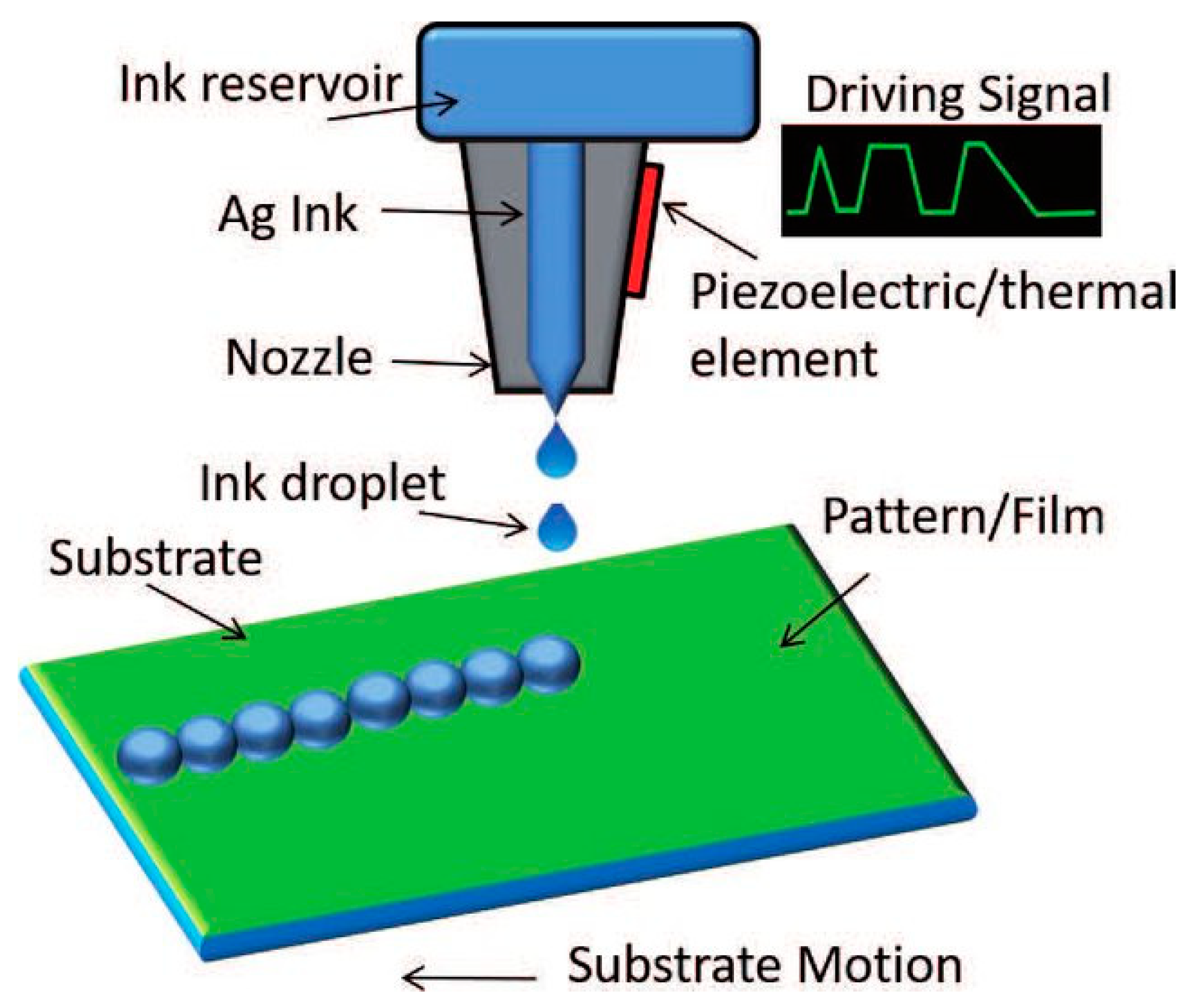
Figure 1. Schematic illustration of inkjet printing technology [25].
1.1. Inkjet Printing Technology
Inkjet printing is a reprographic method that provides for the controlled deposition of a small drop of ink (e.g., biological, synthetic and any form of therapeutic or nontherapeutic substances) on a substrate [26][27].
Today, this widely known digital printing technology that was originally developed to transfer electronic data on paper is present in almost every office and household as a common technique for printing text or graphics [26][27][28][29]. Being a noncontact deposition and direct-patterning technique, it provides minimal contamination and waste of therapeutic sample, respectively [11][30][31][32]. It has also caught the attention of researchers worldwide because of its drop placement accuracy in a precisely fixed amount (typically in volumes of picolitres, pL) of material that can be dispensed without any prior pattern [11][30][33][34].
This material-conserving patterning technique is usually used for the deposition of liquid phase materials that are technically termed ink and that contain the solute as dissolved or dispersed in a solvent. A piezoelectric inkjet printer uses a piezo-ceramic plate to apply ink droplets in order to regulate the ejection. To avoid unwanted interactions between the inks and the plate, a tiny diaphragm is connected to the piezo-ceramic plate. An electric impulse causes a piezo-ceramic plate to distort, and subsequently, the droplet is ejected from the nozzle as a result of the pressure wave this creates. The piezo-ceramic plate returns to its original shape after the electric pulse is removed, and the ink is replaced. These ejected droplets gravitate towards and settled on the surface of the substrate using the momentum obtained during the motion. Subsequently, droplets dry (see detailed schematic representation in Figure 2) via the evaporation of the solvent [35].
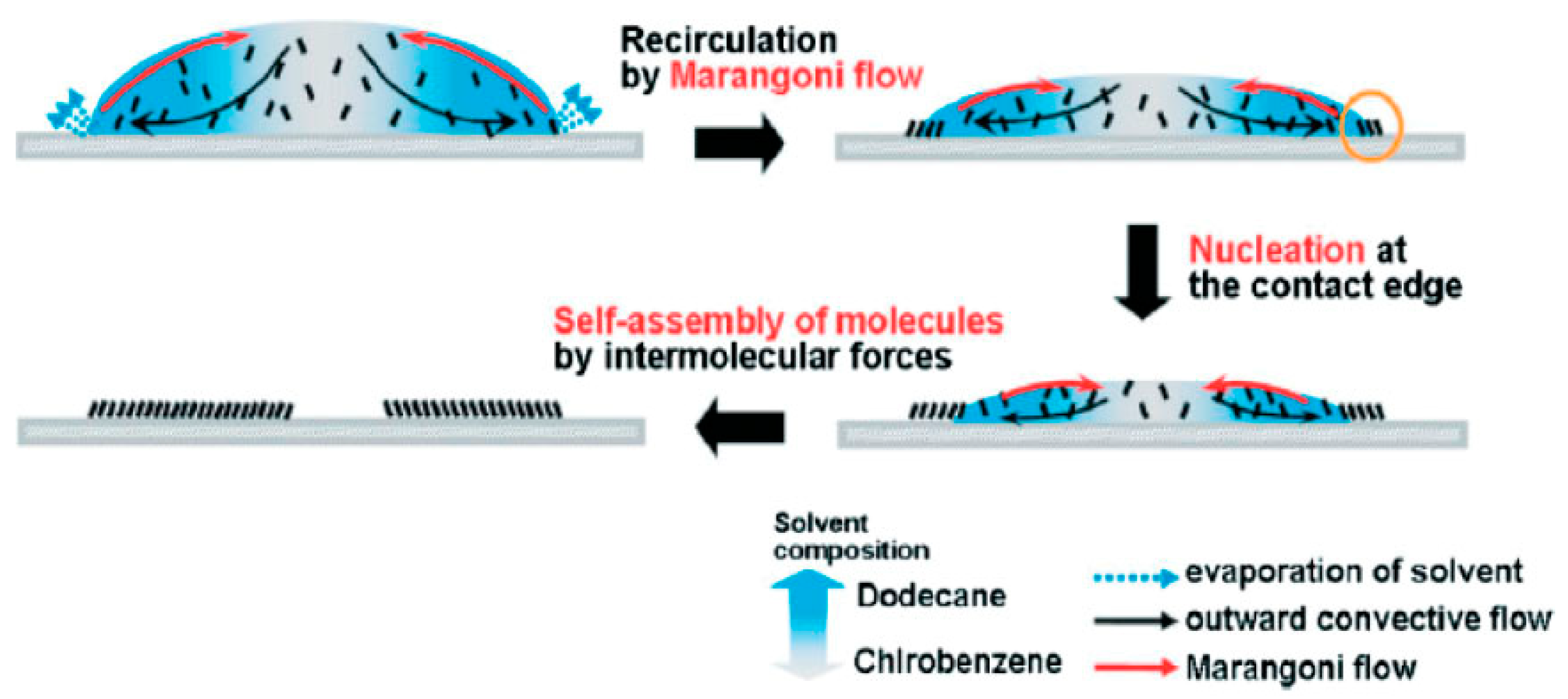
Figure 2. Schematic representation of the potential drying process and crystal formation of an organic semiconductor, 6,13-bis((triisopropylsilylethynyl) pentacene (form 25% dodecane) after deposition from a drop-on-demand piezoelectric print head [36].
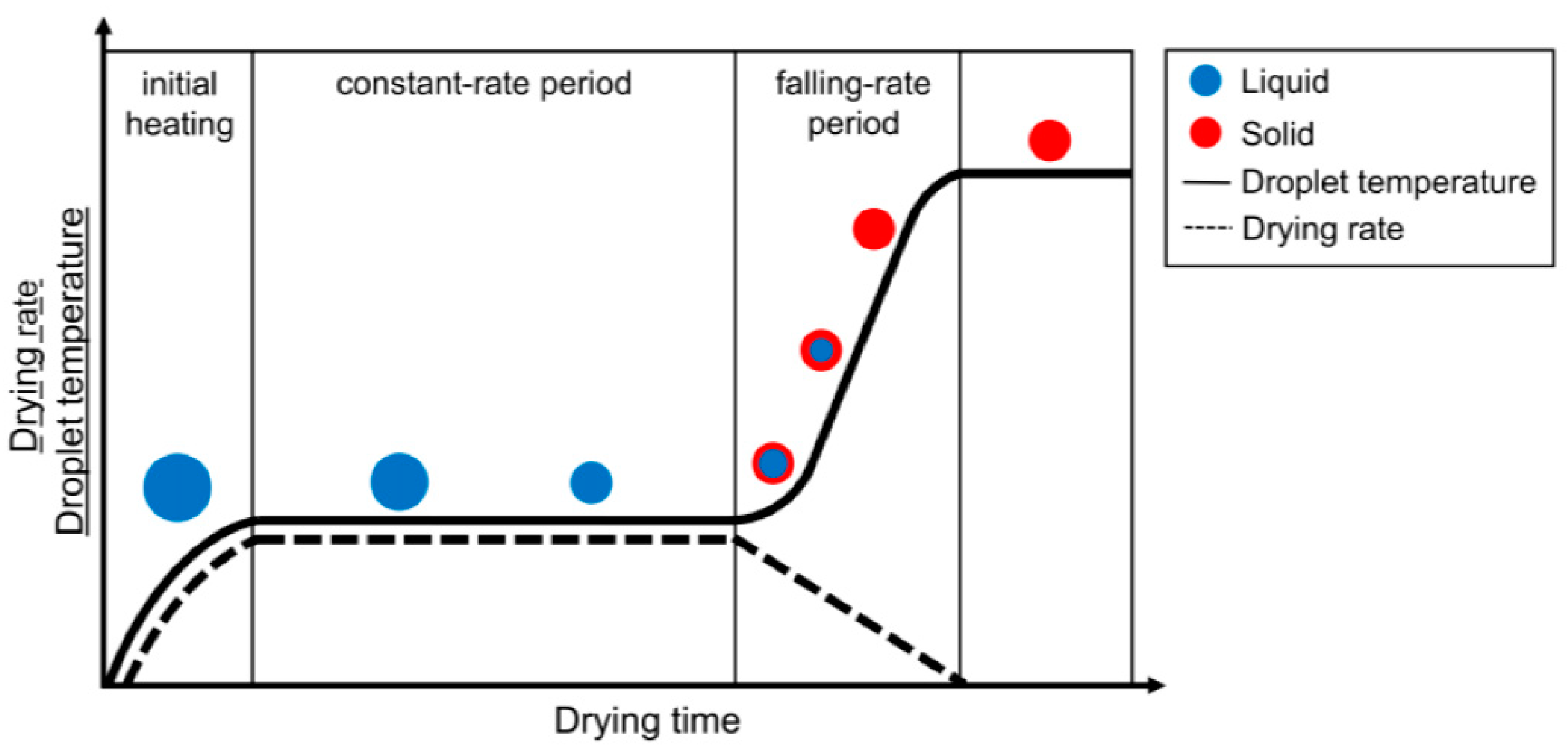
Before the drying process starts, the droplet first reaches an equilibrium temperature due to the continuous heat loss and evaporation of the solvent into a warmer environment of surroundings at a certain pressure and temperature [37][38]. In the first stage of droplet drying, the drying rate (which is commonly expressed in kg m−2 s−1) is limited and determined via the energy essential to evaporate the solvent, which leads the heat transport towards the droplet’s surface [38][39]. After that, the drying process starts from the surface of the droplets, and the molecules of solvent keep drifting towards the surface from the center, which can be mediated via diffusion allied to the solute, convection of liquid within the droplet or capillary fluid flow [37][38][40]. If the temperature of the surroundings is constant, then the drying rate remains unchanged and determined only by the temperature transfer towards the droplet surface [38][41]. For this reason, the first stage of droplet drying is known as the constant-rate drying stage [38].
The second stage of droplet drying is elucidated by the materials present in droplets. Since the evaporation of liquid occurs in the surface of a droplet, the material concentration increases at the surface. This growing concentration gradient results in diffusional material flux far from the surface and towards the center of the droplet, which is a complex phenomenon [38][39]. Consequently, the diffusional motion of the material towards the droplet’s center becomes less than the reduction rate of the droplet diameter because of the constant rate for solvent loss. At this point, crust forms because the higher concentration of materials at the droplet surface leads to a decrease in the drying rate [38][42]. This point is called the locking point or critical point. In the beginning of the second drying stage, a porous solid crust with an internally wet core might be observed in the drying droplet, and drying rate here is now determined by the diffusion or capillary flow rate of the liquid from the wet core via the porous crust. A slowed liquid evaporation still causes the shrinkage of wet core and a considerable increase in the crust towards the droplet’s focal point [42][43]. The condensed crust will influence a growing resistance to mass transfer, and therefore a decrease in the drying rate can be observed. Because of that, this second stage is called the falling-rate stage in the droplet-drying process [37]. It infers the presence of lowest possible amount of residual liquid in a single droplet, which can be either an equilibrium amount or residual solvent that cannot be eliminated by drying [38][44]. Hence, the droplet drying rate in the course of time at the falling-rate period might take on different shapes depending on the mechanism and factors of the drying [43][45]. Figure 3 shows a simplified illustration of the two stages of droplet drying.
In short, the mechanism of inkjet printing comprises three main steps: (1) ejection of ink and droplet formation, (2) liquid–solid interaction after the placement of droplets on the substrate’s surface and (3) drying of ink droplet and subsequent solidifying of the printed features to generate a solid deposit [46][47].
Moreover, inkjet printing does not require sophisticated infrastructure such as clean rooms and large-scale facilities [11]. Merely tiny drops are enough to produce superior quality images with higher resolution [48]. Overall, inkjet printing is a better patterning technique in comparison with some of the other available technologies in the market (Table 1) in terms of cost, efficiency, resolution, compatibility with polymer, process, mode of action, flexibility, requirement of environment and material consumption [49][50].
Table 1. Comparison of some typical patterning technologies [50].
| SN | Properties | Photolithography | Micro-Contact Printing | Shadow Mask | Inkjet Printing |
|---|---|---|---|---|---|
| 1. | Cost | Extremely high | Medium | Low | Low |
| 2. | Efficiency | Low | High | High | High |
| 3. | Resolution | Extremely high | High | Low | High |
| 4. | Compatibility with polymer | Bad | Bad | Good | Excellent |
| 5. | Process | Multi step | Multi step | Multi step | All in one |
| 6. | Mode of action | Noncontact | Contact | Contact | Noncontact |
| 7. | Flexibility | Bad | Bad | Bad | Good, digital lithography |
| 8. | Requirement of environment | Clean rooms, vibration isolation | Medium | Low | Low |
| 9. | Material consumption | High | Low | Medium | Low |
Inkjet printers present some drawbacks. Mainly, they are expensive because of their two basic requirements: (a) printheads must be well suited to different kinds of inks, for example polymeric or metal-based inks and (b) printing cycles must be executed repeatedly. Low-cost inkjet printers that are used generally in household and office might be considered for use as simple devices, but they usually cannot perform due to the incompatibility with the bioinks or inks used for laboratorial research purposes. This is especially the case with metal inks due to their viscosity and nozzle occlusion problems. In addition, the printers’ multilayer patterned structure makes it quite difficult to print with them [51][52][53][54]. An additional issue is the printing of polymers on material surfaces, which leads to adsorbed patterns that are poorly adhesive [55]. Another disadvantage is the coffee ring effect, which causes inkjet-printed insulators to generate a wave-shaped profile where other methods produce perfectly smooth profiles, such as spin-coating [56].
To overcome these issues, researchers have come up with different strategies for both maximizing the benefits that inkjet printing provides and minimizing the disadvantages as well. For example, screen printing provides high-speed printing that is adaptive to commonly available materials and complex multilayer devices. In addition, low-cost inkjet piezoelectric printers provide good spatial resolution (for example, 5760 × 1440 drops per inch), low-cost printing and production and good repeatability (range ~300 µm). Professional inkjet printers provide high spatial resolution and low production cost, are compatible properties with several materials and show repeatability ranges from 5 µm to 25 µm. Finally, mixed-screen printing and low-cost inkjet have demonstrated adaptability to numerous materials, good spatial resolution and repeatability (~300 µm) [57].
There are a few reported studies regarding the development of multilayered structures based on the development of ink properties such as viscosity, surface tension and pH combined with printing parameters (voltage and duration) [58]. Control over the evaporation rate is a key parameter for increasing accuracy and resolution, and the rate can be adjusted by the addition of cosolvents (e.g., alcohol)
1.2. Inks for Inkjet Printing
The ink used for material deposition and its physical properties is considered to be the most crucial part of inkjet printing technology [28]. Inkjet printing involves various processing steps such as droplet generation, motion, interaction with substrate, and drying that are influenced by the quality and properties of the ink for successful printing [11]. The resolution, uniformity and quality of the patterns significantly depend on the viscosity and surface tension of the ink [19][46]. These two parameters can determine the three main steps of inkjet printing process [46]. The speed and accuracy of droplet ejection will decrease if there is an increase in viscosity. At high viscosity and drop rate, the printing process will fail as the ink might not move towards the ink cartridge swiftly to refill it in-between the jetting [19]. Glycols (e.g., glycerol, propylene glycol and polyethylene glycol) are the typically used excipients for viscosity adjustment [59][60][61]. Contrastingly, surface tension influences the propensity of the ink to draw off of the nozzle to produce a droplet, and it is usually adjusted by adding surfactants [19]. Fromm et al. introduced an equation to determine an apt balance among the physical properties in one of his published articles in 1984, which is as follows:
In this equation, the surface tension, density, viscosity and printing mesh aperture diameter are denoted by γ, ρ, η and l, respectively [27][62]. This equation also explains the dimensionless numbers from fluid physics. i.e., Reynolds number, Weber number and Ohnesorge number designated by Re, We and Oh, respectively. It was suggested by Fromm that proper inkjet printing would be possible if Z is greater than 4 (Z > 4). Further investigations led to the introduction of a limiting range for inkjet printing where 1 < Z < 10 [27][63]. This range explains the viscous dissipation of a droplet formation if Z is greater than 1 (Z > 1), and if Z is less than 10 (Z < 10), then the formation of satellite droplets occurs (also termed as secondary droplets) [27]. These satellite droplets have an effect on primary droplets and influence their positioning on the substrate. In fact, the droplet deposition should be accurate, uniform and precise to facilitate successful inkjet printing [1]. Further experimental studies revealed a new range for Z, that is 1 < Z < 14 [62][64][65]. Figure 4 shows the formation of satellite droplets.
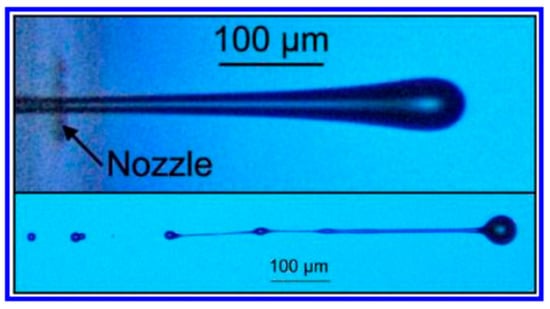
Figure 4. The upper image is a high-speed photograph of a droplet coming out of a nozzle, and the bottom one is a high-speed photograph of a satellite droplet formation [27].
The modulation of droplet size is a major challenge in inkjet printing. To date, a total of eight mechanisms have been recorded that are capable of changing the droplet volume utilizing same ink and printhead.
Usually, optical techniques are used to measure the droplet coming out of the nozzle [66]. To illustrate, a 6 ns short illumination with a laser induced fluorescent stroboscopic recording using iLIF (illumination by laser induced fluorescence) or ultra-high-speed cameras (up to 25 Mfps) are usually used to measure the drop formation with pL sized droplets at approx. 100 kHz repetition rate [67].
One of the inkjet printing limitations is the use of inks of low viscosities. Table 2 lists the compositions of some typically used printing inks including their viscosity and surface tension.
Table 2. Some of the recently used inks in inkjet printing.
| SN | Ink | Ink Viscosity (mPa·s) |
Ink Surface Tension (m Nm−1) | Z Value | Ref. |
|---|---|---|---|---|---|
| 1. | Ethylene glycol | 15.8 | 45.5 | 2.08 | [68] |
| Ethylene Glycol: Water (5/95) | 1.16 | 69.5 | 33.2 | ||
| Ethylene Glycol: Water (10/90) | 1.47 | 68.9 | 26.1 | ||
| Ethylene Glycol: Water (15/85) | 2.32 | 67.7 | 16.5 | ||
| Ethylene Glycol: Water (25/75) | 2.72 | 67.0 | 14.1 | ||
| Ethylene Glycol: Water (50/50) | 5.05 | 46.7 | |||
| Ethylene Glycol: Water (50/50) | 4.39 | 60.3 | 8.40 | ||
| Ethylene Glycol: Water (75/25) | 7.81 | 52.7 | 4.47 | ||
| Ethylene Glycol: Water (85/15) | 10.5 | 50.2 | 3.28 | ||
| 2. | De-ionized water | 1.07 | 72.7 | 36.8 | [68] |
| 3. | Gallium-indium (75/25) | 1.7 | 624 | [46] | |
| 4. | Glycerol-Water | 1–22.5 | 66.4–7.6 | [69] | |
| 5. | CuNO4- Water | ~4.45 | 88 | [70] | |
| 6. | Dowanol | 10.17 | 15.55 | [71] | |
| 7. | Ethyl acetate | 0.452 | 2.367 | [13] | |
| 8. | 5 Fe3O4-95 (nanoparticles + UV Curable matrix resin) | 18.03 | 23.91 | 1.72 | [72] |
| 9. | 10 Fe3O4-90 (nanoparticles + UV Curable matrix resin) | 18.08 | 20.91 | 1.57 | [72] |
| 10. | Hydroxypropyl cellulose:Water (6/94) | 45 | 44.5 | [73] | |
| 11. | Commercial AgNp | 6.8 ± 0.7 | 30 ± 1 | [74] | |
| 12. | Diethylene glycol | 27.1 | 42.7 | 1.17 | [68] |
| 13. | Glycerol | 934.0 | 76.2 | 0.05 | [68] |
| 14. | MnCo2O4 | 10 | 6.17 | [75] | |
| 15. | MnCo1.8Fe0.2O4 | >15 | 4.77 | [75] | |
| 16. | PVDF: BaTiO3 (40/8) | 13.6 | 30.2 | 1.17 | [76] |
| PVDF: BaTiO3 (32/6.4) | 9.7 | 31.7 | 1.72 | [76] | |
| PVDF: BaTiO3 (24/4.8) | 6.0 | 32.4 | 2.79 | [76] | |
| PVDF: BaTiO3 (16/3.2) | 3.7 | 33.5 | 4.59 | [76] | |
| PVDF: BaTiO3 (8/1.6) | 2.1 | 34.8 | 8.23 | [76] | |
| PVDF: BaTiO3 (1/0.2) | 1.3 | 36.0 | 13.56 | [76] | |
| 17. | DNTF: Hexogen (13.86/0) | 1.2 | 23.33 | 36.94 | [77] |
| DNTF: Hexogen (12.47/1.39) | 1.0 | 23.09 | 44.56 | [77] | |
| DNTF: Hexogen (11.09/2.7) | 0.8 | 23.77 | 58.01 | [77] | |
| DNTF: Hexogen (9.70/4.16) | 0.6 | 24.15 | 75.51 | [77] | |
| DNTF: Hexogen (8.32/5.54) | 0.8 | 24.52 | 58.2 | [77] | |
| DNTF: Hexogen (6.93/6.93) | 1.3 | 23.66 | 35.44 | [77] | |
| 18. | 8 mol% Y2O3-stabilized ZrO2 (8YSZ) | 1.5 | 18.8 ± 0.3 | 7.6 | [78] |
Note: concentration of Ethylene Glycol: Water ratios are in v/v; concentration of PVDF: BaTiO3 ratios are in mg mL−1; concentration of DNTF: Hexogen ratios are in wt%; PVDF = Polyvinylidene difluoride; DNTF = 3,4-dinitrofurazanofuroxan.
1.3. Overview of Different Types of Inkjet Printing Technology
Based on the physical process of droplet generation, this automated, high-throughput technology is predominantly classified into two categories: (a) continuous inkjet printing (CIJ) and (b) drop-on-demand printing (DOD) (Figure 5) [16][23][27][29][79]. Continuous inkjet printers generate droplets as a continuous stream of ink discharged on the target, while in drop-on-demand printers, the droplets are ejected in a discontinuous manner only when they are needed [80]. Droplets deposited by continuous inkjet method are usually twice the size of the orifice [27].
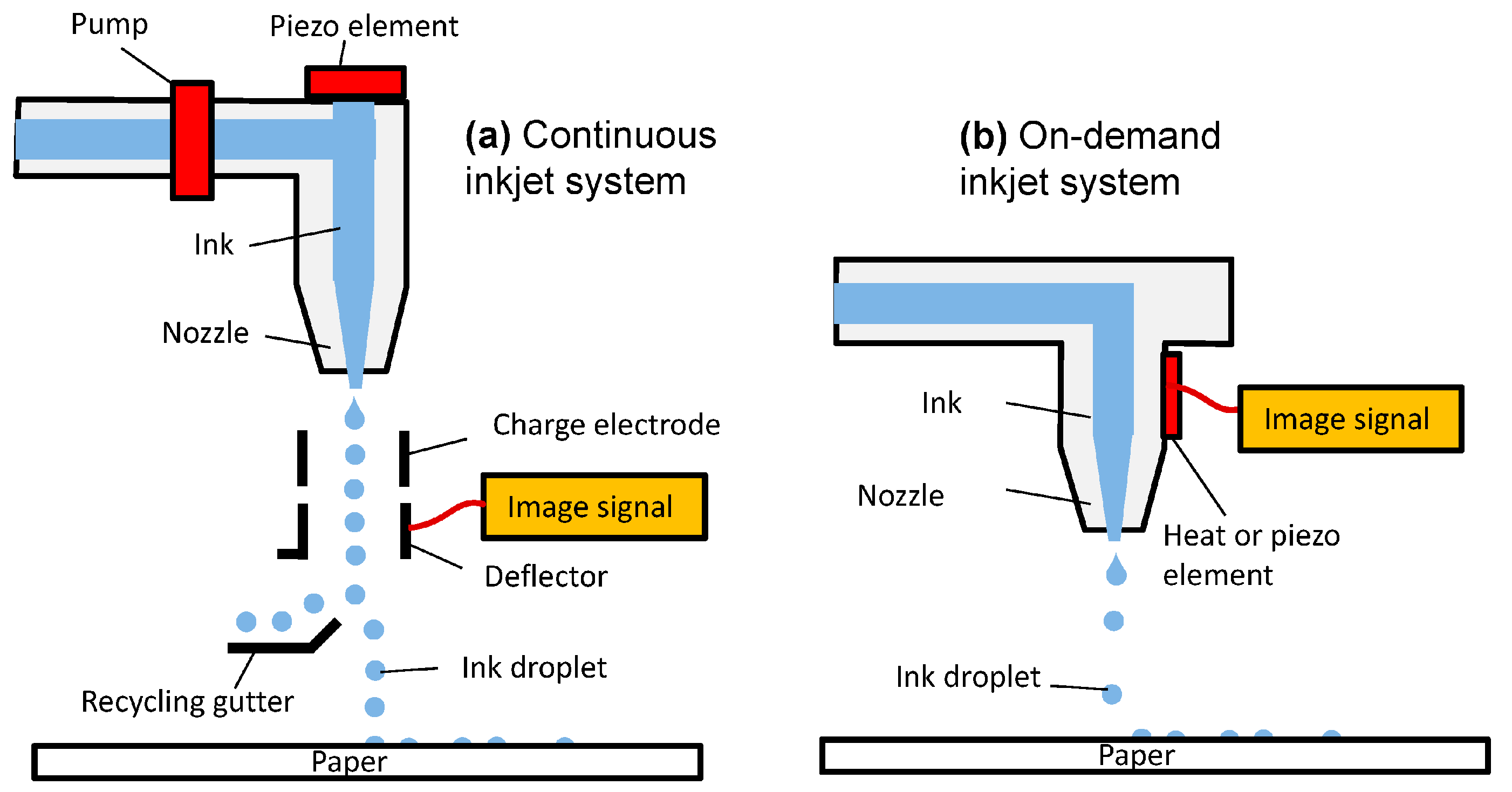
Figure 5. Simplified representation of two different categories of inkjet printing mechanism: (a) continuous inkjet printing (CIJ) and (b) drop-on-demand printing (DOD) [81].
In CIJ printing, a high-pressure pump under an electric field allows the continuous flow of liquid material that is ejected via the orifice, diameter 50–80 µm, which then disintegrate into a stream of droplets under the surface tension forces due to Reayleigh instability [13][15][79]. Continuous inkjet printing is predominantly used in textile printing, labelling and other high-speed graphical works [28]. Depending on actuation technique, IJP can be classified into another two categories which are: (a) piezoelectric and (b) thermal [82][83][84][85]. Both of the techniques reserve the material to be printed in a chamber and emit the droplets through the printhead via a nozzle, but they differ in the process of droplet formation [86][87][88].
In DoD inkjet printing, the liquid droplet is emitted through a nozzle only when it is required. Typically, a DOD printhead consists of multiple nozzles (usually 100–1000, aside from specialized printheads, which might have only one nozzle). The formation of droplets occurs swiftly after the deformation of the piezoelectric wall, which compresses the ink due to the applied wave. The ink material comes out of reservoir as a form of jet through the printhead, gravitates down afterwards and gets ejected via the nozzle under the surface tension forces to generate one or more droplets [15].
In contrast to CIJ, droplets produced by DOD inkjet printing are comparable with the diameter of the orifice, usually ranging from 10 to 50 µm, in accordance with drop volumes, which vary from 1 to 70 pL [15][27]. Due to the capability of smaller droplet formation, it has become a method of choice for several studies [28].
Biological ink materials can be affected by the electrostatic inkjet process due to the shear pressure (sonication with the frequency of 15–25 kHz), and they can clog easily since the diameter of the nozzle is not only fixed but also small [89].
The use of higher amount of solid material in the ink solution can increase the printing efficiency and decrease the cost notably which is one of the advantages of thermal inkjet printing [79].In addition, inks comprising aqueous solvents are usually more feasible for jetting with thermal inkjet printing. In contrast, organic solvents are generally more suitable for piezoelectric inkjet processing. Furthermore, thermal inkjet printers are usually inexpensive compared with the piezoelectric devices [1][90].
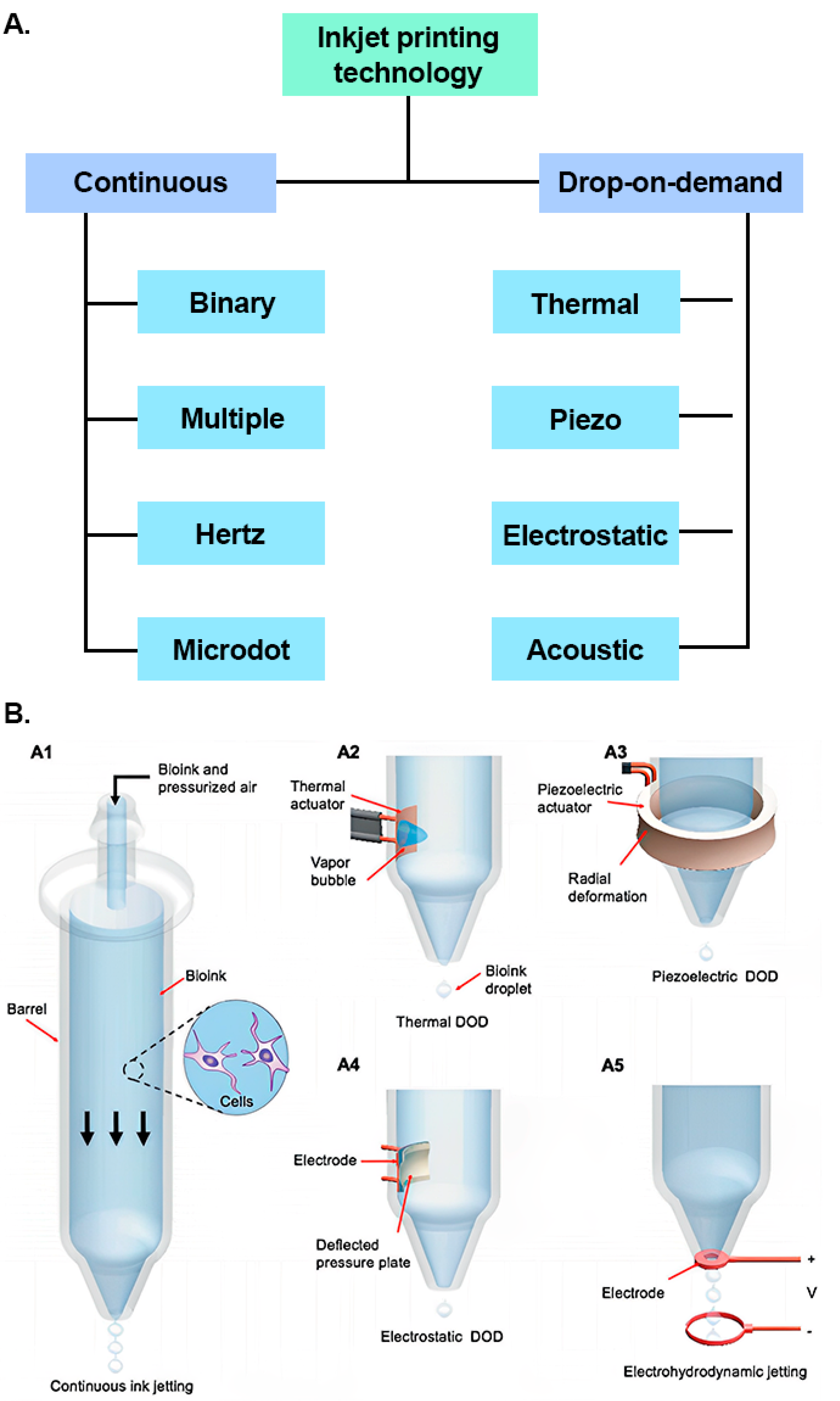
There are several other inkjet printing processes such as electrostatic, electrohydrodynamic and acoustic, but they are not frequently used because of their major drawbacks [82]. Some disadvantages of electrostatic inkjet printing are that it requires high voltage (sometimes over 2 kV) to operate, utilizes conductive metal pipe, requires placing one electrode externally to the device and requires placing a substrate between the nozzle and the electrode [91].
One of the drawbacks of electrohydrodynamic inkjet printing is that it cannot deposit single droplets at a time. The droplet generation occurs using an electric field and not by shrinkage of the ink with thermal energy or chamber deformation [89]. The low throughput (low production speed) of electrohydrodynamic inkjet printing is considered to be the most severe drawback that has retarded its widespread application [92][93]. Figure 6 illustrates an overview of the available inkjet printing methods.
2. Thermal Inkjet Printing
Thermal inkjet printing (TIJP) is a noncontact DOD printing system that was basically developed for printing digital data on media [90][96][97]. It is also known as bubble inkjet printing since the droplet ejection occurs via bubble nucleation [28][87][98]. TIJ printers can eject droplets in a range of 2–180 pL of volume [99][100].
A TIJ printer consists of an ink (desired fluid material to be printed), the cartridge and a printhead. The printhead comprises several nozzles (column like small channels) filled with the fluid material from the ink chamber, and a transducer (which is a thin film resistor for thermal inkjet printing) is attached to each nozzle [79][100].
Due to its reproducibility, low cost and high throughput, this printing technique dominates the market over other printing technologies [47][48][101][102][103][104].
In TIJP, a thin-film resistive heater that creates a frequency ranging from 1 kHz to 5 kHz with an approximate rectangular wave of 3–6 μs pulse width is attached to the printhead, which instantaneously heats the ink in the cartridge. A small vapor bubble forms and puffs up using the heat, which generates a pressure pulse essential for droplet emission through the nozzle. Once a droplet emission is completed, the current is withdrawn, which facilitates a prompt reduction in the vapor pressure and temperature. Consequently, the bubble collapses inside the printhead, which somewhat creates a vacuum (negative pressure) that pulls the liquid ink to refill the chamber [4][47][80][98][105][106][107][108][109][110][111]. Thermal gradient, viscosity of ink material and electric pulse frequency determine the size of the droplets to be generated [22][47][109][111].
In TIJP, the thermal resistor can momentarily (approximately 3 to 10 µs) produce up to 300 °C temperature, and merely around 0.5% of the ink encounters a thermal rise in the nucleation of a vapor bubble [89][105].
Types of Thermal Inkjet Printer
Depending on the droplet emission principle, there are three types of TIJ printer available: (a) side shooter, (b) roof shooter and (c) back shooter [13][48]. For the side shooter printer, the droplet is ejected tangential to the surface of heater. On the other hand, the droplet ejects straight (at 90° angle to the heater surface) in a roof shooter. In the back shooter printer, the droplet ejects straight, but the vapor bubble nucleation occurs in the opposite direction of droplet emission [48]. Simplified versions of the working mechanisms behind the side shooter, roof shooter and back shooter are shown in Figure 7.
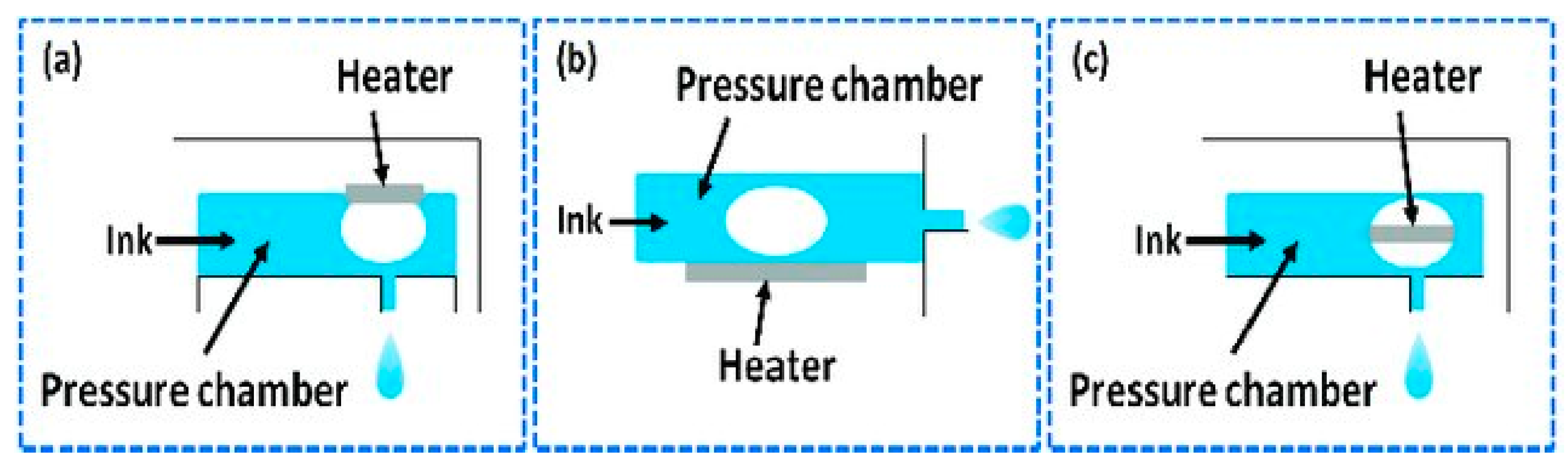
Figure 7. (a) Roof shooter, (b) side shooter, and (c) back shooter system [13].
This entry is adapted from the peer-reviewed paper 10.3390/technologies10050108
References
- Alomari, M.; Mohamed, F.H.; Basit, A.W.; Gaisford, S. Personalised Dosing: Printing a Dose of One’s Own Medicine. Int. J. Pharm. 2015, 494, 568–577.
- Douroumis, D. Hot-Melt Extrusion: Pharmaceutical Applications; Wiley: Hoboken, NJ, USA, 2012.
- Douroumis, D.; Fahr, A. Drug Delivery Strategies for Poorly Water-Soluble Drugs; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013.
- Vaz, V.M.; Kumar, L. 3D Printing as a Promising Tool in Personalized Medicine. AAPS PharmSciTech 2021, 22, 49.
- Scoutaris, N.; Alexander, M.R.; Gellert, P.R.; Roberts, C.J. Inkjet Printing as a Novel Medicine Formulation Technique. J. Control. Release 2011, 156, 179–185.
- Ginsburg, G.S.; McCarthy, J.J. Personalized Medicine: Revolutionizing Drug Discovery and Patient Care. Trends Biotechnol. 2001, 19, 491–496.
- Brittain, H.K.; Scott, R.; Thomas, E. The Rise of the Genome and Personalised Medicine. Clin. Med. 2017, 17, 545.
- Mathur, S.; Sutton, J. Personalized Medicine Could Transform Healthcare. Biomed. Rep. 2017, 7, 3.
- Goetz, L.H.; Schork, N.J. Personalized Medicine: Motivation, Challenges and Progress. Fertil. Steril. 2018, 109, 952.
- Azizi Machekposhti, S.; Mohaved, S.; Narayan, R.J. Inkjet Dispensing Technologies: Recent Advances for Novel Drug Discovery. Expert Opin. Drug Discov. 2019, 14, 101–113.
- Fang, M.; Li, T.; Zhang, S.; Rao, K.V.; Belova, L. Design and Tailoring of Inks for Inkjet Patterning of Metal Oxides. R. Soc. Open Sci. 2020, 7, 200242.
- Gao, Y.; Shi, W.; Wang, W.; Leng, Y.; Zhao, Y. Inkjet Printing Patterns of Highly Conductive Pristine Graphene on Flexible Substrates. Ind. Eng. Chem. Res. 2014, 53, 16777–16784.
- Shah, M.A.; Lee, D.G.; Lee, B.Y.; Hur, S. Classifications and Applications of Inkjet Printing Technology: A Review. IEEE Access 2021, 9, 140079–140102.
- Evans, S.E.; Harrington, T.; Rodriguez Rivero, M.C.; Rognin, E.; Tuladhar, T.; Daly, R. 2D and 3D Inkjet Printing of Biopharmaceuticals—A Review of Trends and Future Perspectives in Research and Manufacturing. Int. J. Pharm. 2021, 599, 120443.
- Daly, R.; Harrington, T.S.; Martin, G.D.; Hutchings, I.M. Inkjet Printing for Pharmaceutics—A Review of Research and Manufacturing. Int. J. Pharm. 2015, 494, 554–567.
- Zhu, X.; Zheng, Q.; Yang, H.; Cai, J.; Huang, L.; Duan, Y.; Xu, Z.; Cen, P. Recent Advances in Inkjet Dispensing Technologies: Applications in Drug Discovery. Expert Opin. Drug Discov. 2012, 7, 761–770.
- Uddin, M.J.; Scoutaris, N.; Klepetsanis, P.; Chowdhry, B.; Prausnitz, M.R.; Douroumis, D. Inkjet Printing of Transdermal Microneedles for the Delivery of Anticancer Agents. Int. J. Pharm. 2015, 494, 593–602.
- Ross, S.; Scoutaris, N.; Lamprou, D.; Mallinson, D.; Douroumis, D. Inkjet Printing of Insulin Microneedles for Transdermal Delivery. Drug Deliv. Transl. Res. 2015, 5, 451–461.
- Ihalainen, P.; Määttänen, A.; Sandler, N. Printing Technologies for Biomolecule and Cell-Based Applications. Int. J. Pharm. 2015, 494, 585–592.
- Zheng, Q.; Lu, J.; Chen, H.; Huang, L.; Cai, J.; Xu, Z. Application of Inkjet Printing Technique for Biological Material Delivery and Antimicrobial Assays. Anal. Biochem. 2011, 410, 171–176.
- Preis, M.; Breitkreutz, J.; Sandler, N. Perspective: Concepts of Printing Technologies for Oral Film Formulations. Int. J. Pharm. 2015, 494, 578–584.
- Scoutaris, N.; Ross, S.; Douroumis, D. Current Trends on Medical and Pharmaceutical Applications of Inkjet Printing Technology. Pharm. Res. 2016, 33, 1799–1816.
- Thabet, Y.; Sibanc, R.; Breitkreutz, J. Printing Pharmaceuticals by Inkjet Technology: Proof of Concept for Stand-Alone and Continuous in-Line Printing on Orodispersible Films. J. Manuf. Process. 2018, 35, 205–215.
- Visser, J.C.; Wibier, L.; Kiefer, O.; Orlu, M.; Breitkreutz, J.; Woerdenbag, H.J.; Taxis, K. A Pediatrics Utilization Study in The Netherlands to Identify Active Pharmaceutical Ingredients Suitable for Inkjet Printing on Orodispersible Films. Pharmaceutics 2020, 12, 164.
- Khan, S.; Ali, S.; Bermak, A. Smart Manufacturing Technologies for Printed Electronics. Hybrid Nanomater. Flex. Electron. Mater. 2019.
- Boland, T.; Xu, T.; Damon, B.; Cui, X. Application of Inkjet Printing to Tissue Engineering. Biotechnol. J. 2006, 1, 910–917.
- Saunders, R.E.; Derby, B. Inkjet Printing Biomaterials for Tissue Engineering: Bioprinting. Int. Mater. Rev. 2014, 59, 430–448.
- De Gans, B.J.; Duineveld, P.C.; Schubert, U.S. Inkjet Printing of Polymers: State of the Art and Future Developments. Adv. Mater. 2004, 16, 203–213.
- Komuro, N.; Takaki, S.; Suzuki, K.; Citterio, D. Inkjet Printed (Bio)Chemical Sensing Devices. Anal. Bioanal. Chem. 2013, 405, 5785–5805.
- Delaney, J.T.; Smith, P.J.; Schubert, U.S. Inkjet Printing of Proteins. Soft Matter 2009, 5, 4866–4877.
- Tekin, E.; Smith, P.J.; Schubert, U.S. Inkjet Printing as a Deposition and Patterning Tool for Polymers and Inorganic Particles. Soft Matter 2008, 4, 703–713.
- Moya, A.; Gabriel, G.; Villa, R.; Javier del Campo, F. Inkjet-Printed Electrochemical Sensors. Curr. Opin. Electrochem. 2017, 3, 29–39.
- Wickström, H.; Nyman, J.O.; Indola, M.; Sundelin, H.; Kronberg, L.; Preis, M.; Rantanen, J.; Sandler, N. Colorimetry as Quality Control Tool for Individual Inkjet-Printed Pediatric Formulations. AAPS PharmSciTech 2017, 18, 293–302.
- Vakili, H.; Wickström, H.; Desai, D.; Preis, M.; Sandler, N. Application of a Handheld NIR Spectrometer in Prediction of Drug Content in Inkjet Printed Orodispersible Formulations Containing Prednisolone and Levothyroxine. Int. J. Pharm. 2017, 524, 414–423.
- Singh, M.; Haverinen, H.M.; Dhagat, P.; Jabbour, G.E. Inkjet Printing—Process and Its Applications. Adv. Mater. 2010, 22, 673–685.
- Lim, J.A.; Lee, W.H.; Lee, H.S.; Lee, J.H.; Park, Y.D.; Cho, K. Self-Organization of Ink-Jet-Printed Triisopropylsilylethynyl Pentacene via Evaporation-Induced Flows in a Drying Droplet. Adv. Funct. Mater. 2008, 18, 229–234.
- Farid, M. A New Approach to Modelling of Single Droplet Drying. Chem. Eng. Sci. 2003, 58, 2985–2993.
- Boel, E.; Koekoekx, R.; Dedroog, S.; Babkin, I.; Vetrano, M.R.; Clasen, C.; Van den Mooter, G. Unraveling Particle Formation: From Single Droplet Drying to Spray Drying and Electrospraying. Pharmaceutics 2020, 12, 625.
- Vehring, R. Pharmaceutical Particle Engineering via Spray Drying. Pharm. Res. 2008, 25, 999–1022.
- Singh, A.; Van den Mooter, G. Spray Drying Formulation of Amorphous Solid Dispersions. Adv. Drug Deliv. Rev. 2016, 100, 27–50.
- Mezhericher, M.; Levy, A.; Borde, I. Modelling the Morphological Evolution of Nanosuspension Droplet in Constant-Rate Drying Stage. Chem. Eng. Sci. 2011, 66, 884–896.
- de Souza Lima, R.; Ré, M.I.; Arlabosse, P. Drying Droplet as a Template for Solid Formation: A Review. Powder Technol. 2020, 359, 161–171.
- Mezhericher, M.; Levy, A.; Borde, I. Modelling of Particle Breakage during Drying. Chem. Eng. Process. Process Intensif. 2008, 47, 1404–1411.
- Perdana, J.; Bereschenko, L.; Fox, M.B.; Kuperus, J.H.; Kleerebezem, M.; Boom, R.M.; Schutyser, M.A.I. Dehydration and Thermal Inactivation of Lactobacillus Plantarum WCFS1: Comparing Single Droplet Drying to Spray and Freeze Drying. Food Res. Int. 2013, 54, 1351–1359.
- Mezhericher, M.; Levy, A.; Borde, I. Spray Drying Modelling Based on Advanced Droplet Drying Kinetics. Chem. Eng. Process. Process Intensif. 2010, 49, 1205–1213.
- Machekposhti, S.A.; Movahed, S.; Narayan, R.J. Physicochemical Parameters That Underlie Inkjet Printing for Medical Applications. Biophys. Rev. 2020, 1, 011301.
- Sohrabi, S.; Liu, Y. Modeling Thermal Inkjet and Cell Printing Process Using Modified Pseudopotential and Thermal Lattice Boltzmann Methods. Phys. Rev. E 2018, 97, 033105.
- Lee, S.W.; Kim, H.C.; Kuk, K.; Oh, Y.S. A Monolithic Inkjet Print Head: DomeJet. Sens. Actuators A Phys. 2002, 95, 114–119.
- Kholghi Eshkalak, S.; Chinnappan, A.; Jayathilaka, W.A.D.M.; Khatibzadeh, M.; Kowsari, E.; Ramakrishna, S. A Review on Inkjet Printing of CNT Composites for Smart Applications. Appl. Mater. Today 2017, 9, 372–386.
- Yin, Z.P.; Huang, Y.A.; Bu, N.B.; Wang, X.M.; Xiong, Y.L. Inkjet Printing for Flexible Electronics: Materials, Processes and Equipments. Chin. Sci. Bull. 2010, 55, 3383–3407.
- Andò, B.; Baglio, S.; Bulsara, A.R.; Emery, T.; Marletta, V.; Pistorio, A. Low-Cost Inkjet Printing Technology for the Rapid Prototyping of Transducers. Sensors 2017, 17, 748.
- Matsuda, Y.; Shibayama, S.; Uete, K.; Yamaguchi, H.; Niimi, T. Electric Conductive Pattern Element Fabricated Using Commercial Inkjet Printer for Paper-Based Analytical Devices. Anal. Chem. 2015, 87, 5762–5765.
- Huang, Q.; Shen, W.; Xu, Q.; Tan, R.; Song, W. Properties of Polyacrylic Acid-Coated Silver Nanoparticle Ink for Inkjet Printing Conductive Tracks on Paper with High Conductivity. Mater. Chem. Phys. 2014, 147, 550–556.
- Huang, Q.; Shen, W.; Xu, Q.; Tan, R.; Song, W. Room-Temperature Sintering of Conductive Ag Films on Paper. Mater. Lett. 2014, 123, 124–127.
- Garcia, A.; Hanifi, N.; Jousselme, B.; Jégou, P.; Palacin, S.; Viel, P.; Berthelot, T. Polymer Grafting by Inkjet Printing: A Direct Chemical Writing Toolset. Adv. Funct. Mater. 2013, 23, 3668–3674.
- Moon, S.J.; Robin, M.; Wenlin, K.; Yann, M.; Bae, B.S.; Mohammed-Brahim, T.; Jacques, E.; Harnois, M. Morphological Impact of Insulator on Inkjet-Printed Transistor. Flex. Print. Electron. 2017, 2, 035008.
- Ando, B.; Baglio, S. All-Inkjet Printed Strain Sensors. IEEE Sens. J. 2013, 13, 4874–4879.
- Salaoru, I.; Zhou, Z.; Morris, P.; Gibbons, G.J. Inkjet-Printed Polyvinyl Alcohol Multilayers. J. Vis. Exp. 2017, 2017, 55093.
- Genina, N.; Fors, D.; Vakili, H.; Ihalainen, P.; Pohjala, L.; Ehlers, H.; Kassamakov, I.; Haeggström, E.; Vuorela, P.; Peltonen, J.; et al. Tailoring Controlled-Release Oral Dosage Forms by Combining Inkjet and Flexographic Printing Techniques. Eur. J. Pharm. Sci. 2012, 47, 615–623.
- Genina, N.; Janßen, E.M.; Breitenbach, A.; Breitkreutz, J.; Sandler, N. Evaluation of Different Substrates for Inkjet Printing of Rasagiline Mesylate. Eur. J. Pharm. Biopharm. 2013, 85, 1075–1083.
- Sandler, N.; Määttänen, A.; Ihalainen, P.; Kronberg, L.; Meierjohann, A.; Viitala, T.; Peltonen, J. Inkjet Printing of Drug Substances and Use of Porous Substrates-towards Individualized Dosing. J. Pharm. Sci. 2011, 100, 3386–3395.
- McManus, D.; Vranic, S.; Withers, F.; Sanchez-Romaguera, V.; Macucci, M.; Yang, H.; Sorrentino, R.; Parvez, K.; Son, S.K.; Iannaccone, G.; et al. Water-Based and Biocompatible 2D Crystal Inks for All-Inkjet-Printed Heterostructures. Nat. Nanotechnol. 2017, 12, 343–350.
- Reis, N.; Ainsley, C.; Derby, B. Ink-Jet Delivery of Particle Suspensions by Piezoelectric Droplet Ejectors. J. Appl. Phys. 2005, 97, 094903.
- Derby, B. Inkjet Printing Ceramics: From Drops to Solid. J. Eur. Ceram. Soc. 2011, 31, 2543–2550.
- Jang, D.; Kim, D.; Moon, J. Influence of Fluid Physical Properties on Ink-Jet Printability. Langmuir 2009, 25, 2629–2635.
- Wijshoff, H. Drop Dynamics in the Inkjet Printing Process. Curr. Opin. Colloid Interface Sci. 2018, 36, 20–27.
- Van Der Bos, A.; Van Der Meulen, M.J.; Driessen, T.; Van Den Berg, M.; Reinten, H.; Wijshoff, H.; Versluis, M.; Lohse, D. Velocity Profile inside Piezoacoustic Inkjet Droplets in Flight: Comparison between Experiment and Numerical Simulation. Phys. Rev. Appl. 2014, 1, 014004.
- Liu, Y.; Derby, B. Experimental Study of the Parameters for Stable Drop-on-Demand Inkjet Performance. Phys. Fluids 2019, 31, 032004.
- Choi, H.W.; Zhou, T.; Singh, M.; Jabbour, G.E. Recent Developments and Directions in Printed Nanomaterials. Nanoscale 2015, 7, 3338–3355.
- Padilla-Martinez, J.P.; Ramirez-San-Juan, J.C.; Berrospe-Rodriguez, C.; Korneev, N.; Aguilar, G.; Zaca-Moran, P.; Ramos-Garcia, R. Controllable Direction of Liquid Jets Generated by Thermocavitation within a Droplet. Appl. Opt. 2017, 56, 7167.
- Oktavianty, O.; Haruyama, S.; Ishii, Y. Enhancing Droplet Quality of Edible Ink in Single and Multi-Drop Methods by Optimization the Waveform Design of DoD Inkjet Printer. Processes 2022, 10, 91.
- Saleh, E.; Woolliams, P.; Clarke, B.; Gregory, A.; Greedy, S.; Smartt, C.; Wildman, R.; Ashcroft, I.; Hague, R.; Dickens, P.; et al. 3D Inkjet-Printed UV-Curable Inks for Multi-Functional Electromagnetic Applications. Addit. Manuf. 2017, 13, 143–148.
- Barlow, N.E.; Kusumaatmaja, H.; Salehi-Reyhani, A.; Brooks, N.; Barter, L.M.C.; Flemming, A.J.; Ces, O. Measuring Bilayer Surface Energy and Curvature in Asymmetric Droplet Interface Bilayers. J. R. Soc. Interface 2018, 15, 20180610.
- Mypati, S.; Dhanushkodi, S.R.; McLaren, M.; Docoslis, A.; Peppley, B.A.; Barz, D.P.J. Optimized Inkjet-Printed Silver Nanoparticle Films: Theoretical and Experimental Investigations. RSC Adv. 2018, 8, 19679–19689.
- Pandiyan, S.; El-Kharouf, A.; Steinberger-Wilckens, R. Formulation of Spinel Based Inkjet Inks for Protective Layer Coatings in SOFC Interconnects. J. Colloid Interface Sci. 2020, 579, 82–95.
- Abdolmaleki, H.; Agarwala, S. PVDF-BaTiO3 Nanocomposite Inkjet Inks with Enhanced β-Phase Crystallinity for Printed Electronics. Polymers 2020, 12, 2430.
- Xu, C.; An, C.; Long, Y.; Li, Q.; Guo, H.; Wang, S.; Wang, J. Inkjet Printing of Energetic Composites with High Density. RSC Adv. 2018, 8, 35863–35869.
- Zhu, Z.; Gong, Z.; Qu, P.; Li, Z.; Rasaki, S.A.; Liu, Z.; Wang, P.; Liu, C.; Lao, C.; Chen, Z. Additive Manufacturing of Thin Electrolyte Layers via Inkjet Printing of Highly-Stable Ceramic Inks. J. Adv. Ceram. 2021, 10, 279–290.
- Li, C.; Shi, H.; Ran, R.; Su, C.; Shao, Z. Thermal Inkjet Printing of Thin-Film Electrolytes and Buffering Layers for Solid Oxide Fuel Cells with Improved Performance. Int. J. Hydrogen Energy 2013, 38, 9310–9319.
- Kolakovic, R.; Viitala, T.; Ihalainen, P.; Genina, N.; Peltonen, J.; Sandler, N. Printing Technologies in Fabrication of Drug Delivery Systems. Expert Opin. Drug Deliv. 2013, 10, 1711–1723.
- Lau, G.K.; Shrestha, M. Ink-Jet Printing of Micro-Electro-Mechanical Systems (MEMS). Micromachines 2017, 8, 194.
- Kumar, P.; Ebbens, S.; Zhao, X. Inkjet Printing of Mammalian Cells—Theory and Applications. Bioprinting 2021, 23, e00157.
- Alamán, J.; Alicante, R.; Peña, J.I.; Sánchez-Somolinos, C. Inkjet Printing of Functional Materials for Optical and Photonic Applications. Materials 2016, 9, 910.
- Murphy, S.V.; Atala, A. 3D Bioprinting of Tissues and Organs. Nat. Biotechnol. 2014, 32, 773–785.
- Calvert, P. Materials Science. Printing Cells. Science 2007, 318, 208–209.
- Gaisford, S. 3D Printed Pharmaceutical Products. 3D Print. Med. 2017, 155–166.
- Özkol, E.; Ebert, J.; Uibel, K.; Wätjen, A.M.; Telle, R. Development of High Solid Content Aqueous 3Y-TZP Suspensions for Direct Inkjet Printing Using a Thermal Inkjet Printer. J. Eur. Ceram. Soc. 2009, 29, 403–409.
- Mannerbro, R.; Ranlöf, M.; Robinson, N.; Forchheimer, R. Inkjet Printed Electrochemical Organic Electronics. Synth. Met. 2008, 158, 556–560.
- Li, X.; Liu, B.; Pei, B.; Chen, J.; Zhou, D.; Peng, J.; Zhang, X.; Jia, W.; Xu, T. Inkjet Bioprinting of Biomaterials. Chem. Rev. 2020, 120, 10793–10833.
- Uzun, S.; Schelling, M.; Hantanasirisakul, K.; Mathis, T.S.; Askeland, R.; Dion, G.; Gogotsi, Y. Additive-Free Aqueous MXene Inks for Thermal Inkjet Printing on Textiles. Small 2021, 17, 2006376.
- Lee, S.; Byun, D.; Jung, D.; Choi, J.; Kim, Y.; Yang, J.H.; Son, S.U.; Tran, S.B.Q.; Ko, H.S. Pole-Type Ground Electrode in Nozzle for Electrostatic Field Induced Drop-on-Demand Inkjet Head. Sensors Actuators A Phys. 2008, 141, 506–514.
- Khan, A.; Rahman, K.; Hyun, M.-T.; Kim, D.-S.; Choi, K.-H.; Khan, A.; Rahman, K.; Hyun, M.; Choi, K.; Kim, D. Multi-Nozzle Electrohydrodynamic Inkjet Printing of Silver Colloidal Solution for the Fabrication of Electrically Functional Microstructures. Appl. Phys. A 2011, 104, 1113–1120.
- Khan, A.; Rahman, K.; Kim, D.S.; Choi, K.H. Direct Printing of Copper Conductive Micro-Tracks by Multi-Nozzle Electrohydrodynamic Inkjet Printing Process. J. Mater. Process. Technol. 2012, 212, 700–706.
- Wijshoff, H. The Dynamics of the Piezo Inkjet Printhead Operation. Phys. Rep. 2010, 491, 77–177.
- Gudapati, H.; Dey, M.; Ozbolat, I. A Comprehensive Review on Droplet-Based Bioprinting: Past, Present and Future. Biomaterials 2016, 102, 20–42.
- Ferris, C.J.; Gilmore, K.G.; Wallace, G.G.; In Het Panhuis, M. Biofabrication: An Overview of the Approaches Used for Printing of Living Cells. Appl. Microbiol. Biotechnol. 2013, 97, 4243–4258.
- Dodoo, C.C.; Alomari, M.; Basit, A.W.; Stapleton, P.; Gaisford, S. A Thermal Ink-Jet Printing Approach for Evaluating Susceptibility of Bacteria to Antibiotics. J. Microbiol. Methods 2019, 164, 105660.
- Prasad, L.K.; Smyth, H. 3D Printing Technologies for Drug Delivery: A Review. Drug Dev. Ind. Pharm. 2016, 42, 1019–1031.
- Vuddanda, P.R.; Alomari, M.; Dodoo, C.C.; Trenfield, S.J.; Velaga, S.; Basit, A.W.; Gaisford, S. Personalisation of Warfarin Therapy Using Thermal Ink-Jet Printing. Eur. J. Pharm. Sci. 2018, 117, 80–87.
- Buanz, A.B.M.; Saunders, M.H.; Basit, A.W.; Gaisford, S. Preparation of Personalized-Dose Salbutamol Sulphate Oral Films with Thermal Ink-Jet Printing. Pharm. Res. 2011, 28, 2386–2392.
- Alper, J. Biology and the Inkjets. Science 2004, 305, 1895.
- Wilson, W.C.; Boland, T. Cell and Organ Printing 1: Protein and Cell Printers. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2003, 272, 491–496.
- Lemmo, A.V.; Rose, D.J.; Tisone, T.C. Inkjet Dispensing Technology: Applications in Drug Discovery. Curr. Opin. Biotechnol. 1998, 9, 615–617.
- Xu, T.; Jin, J.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet Printing of Viable Mammalian Cells. Biomaterials 2005, 26, 93–99.
- Meléndez, P.A.; Kane, K.M.; Ashvar, C.S.; Albrecht, M.; Smith, P.A. Thermal Inkjet Application in the Preparation of Oral Dosage Forms: Dispensing of Prednisolone Solutions and Polymorphic Characterization by Solid-State Spectroscopic Techniques. J. Pharm. Sci. 2008, 97, 2619–2636.
- Patra, S.; Young, V. A Review of 3D Printing Techniques and the Future in Biofabrication of Bioprinted Tissue. Cell Biochem. Biophys. 2016, 74, 93–98.
- Xu, T.; Zhao, W.; Zhu, J.M.; Albanna, M.Z.; Yoo, J.J.; Atala, A. Complex Heterogeneous Tissue Constructs Containing Multiple Cell Types Prepared by Inkjet Printing Technology. Biomaterials 2013, 34, 130–139.
- Gao, G.; Cui, X. Three-Dimensional Bioprinting in Tissue Engineering and Regenerative Medicine. Biotechnol. Lett. 2016, 38, 203–211.
- Zhou, H.; Gué, A.M. Simulation Model and Droplet Ejection Performance of a Thermal-Bubble Microejector. Sens. Actuators B Chem. 2010, 145, 311–319.
- Setti, L.; Piana, C.; Bonazzi, S.; Ballarin, B.; Frascaro, D.; Fraleoni-Morgera, A.; Giuliani, S. Thermal Inkjet Technology for the Microdeposition of Biological Molecules as a Viable Route for the Realization of Biosensors. Anal. Lett. 2007, 37, 1559–1570.
- Cui, X.; Dean, D.; Ruggeri, Z.M.; Boland, T. Cell Damage Evaluation of Thermal Inkjet Printed Chinese Hamster Ovary Cells. Biotechnol. Bioeng. 2010, 106, 963–969.
This entry is offline, you can click here to edit this entry!
