Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The topic of intensification of bioprocesses is widely discussed in agricultural countries and countries with a large amount of organic waste. Therefore, the study of bioprocess intensification methods can help increase qualitative and quantitative indicators of biogas yield by using a combination of intensification methods.
- bioprocesses
- biogas production
- cavitation
- electrolysis
- microbiological methods
1. Introduction
Bioprocesses are usually slower than standard chemical processes. Therefore, a promising area of research is the application of microbiological methods for bioprocess intensification, such as biofilm immobilization in mineral carriers and the formation of stable, permanent conditions for bioprocesses, taking into account the load on the organic matter and the presence of toxicants. Therefore, a combination of physical–chemical methods, such as cavitation, electrolysis, and electromagnetic treatment, is beneficial. Furthermore, the combination of physico-chemical pretreatment methods such as cavitation, electrolysis, and magnetic treatment increase the biodegradability of organics, as with the addition of mineral additives/chemical by-products [1].
2. Cavitation Processes for Intensification of Biogas Yield
Cavitation can occur as a result of a decrease in liquid pressure as its velocity increases or high-intensity sound waves pass through during a half-period. The industrial application of cavitation is for the homogenization, mixing, and precipitation of suspended particles in colloidal liquid compositions. Many industrial mixers are based on this established principle. This is usually achieved by designing a turbine or by passing the mixture through an annular orifice with a narrow inlet and a large outlet: a forced reduction in pressure leads to cavitation as the liquid runs towards the larger volume side. This method can be controlled by hydraulic devices that control the size of the inlet orifice; therefore, the process can be controlled in different mediums [2][3].
Ultrasound is elastic vibrations and waves with frequencies from 15,000 to 20,000 Hz (15 to 20 kHz) and up to 1,000,000,000 Hz (1 GHz), the frequency range of ultrasound between a 1109 and 11,012 Hz is commonly called hypersonic. The ultrasound frequency range can be divided into three subareas: ultrasound at low frequencies (15,000–100,000 Hz)-LFU, ultrasound at medium frequencies (100,000–10,000,000 Hz)-MFU, and ultrasound at high frequencies (10,000,000–1,000,000,000 Hz)-HFU [4]. Thus, the different frequency ranges of ultrasound were shown with the possibility of extending its application to substrate preparation in biogas technology.
As a result of the application of the controlled cavitation process in biomass destructors, they are widely used in biogas production. The percentage of methane in biogas increases to 70–75% [5]. Nykyforov et al. [6] studied anaerobic digestion of blue-green algae that causes blooms in water bodies, it was experimentally established that mechanical cavitation pre-treatment of blue-green algae biomass increases the biogas yield by 21.5%. Furthermore, the biogas produced contains up to 72% methane and hydrogen. Hydrogen is also an important component of biogas, and in the context of the development of hydrogen energy [7] as the most environmentally friendly field, its production and production biologically are relevant.
Table 1. Process characteristics with the additional pretreatment.
| Substrate | Increased Production | Pretreatment Conditions | Limiting Factors | Benefits | Reference | |
|---|---|---|---|---|---|---|
| Wastewater treatment plant | Sewage sludge | Increase the methane yield coefficient up to 95% | USPP: power 150 W; exposure time 15, 30, 45, and 60 min |
|
|
[8] |
| Sewage sludge | Increase the methane content in biogas to 68.3 ± 2.5% | USPP: power 125 W; field intensity 1.9–4.3 W cm−2 |
|
|
[9] | |
| High organic content wastewater | Increase the methane yield up to 60% | USPP: power 400 W; frequency 24 kHz with different amplitude ratios; exposure time 1, 2, 3 h |
|
|
[10] | |
| Waste-activated sludge | Increases biogas production by 25% | USPP: power 225 W, frequency 20 kHz |
|
|
[11] | |
| Food waste | Fruit and vegetable wastes | Increase the methane production by 29–80% | USPP: frequency 20 kHz; the amplitude of 80 μm; exposure time 9 min, 18 min, 27 min |
|
|
[12] |
| Food waste and cardboard | Increase the biogas yield up to 26% | USPP: power 750 kW; frequency of 20 kHz; exposure time 30 min, 45 min, 60 min |
|
|
[13] | |
| Organic fraction of municipal solid waste | Increase the biogas production up to 24% | USPP: density 0.1–0.4 W/mL, exposure time 30 min, 69 min |
|
|
[14] | |
| Increase the biogas production up to 16% | USPP: frequency 20 kHz |
|
|
[15] | ||
| Maximum biogas yield produced after 72 h of digestion increase | USPP: frequency 20 kHz; density 0.2, 0.4, 0.6 W/mL; exposure time 10 min, 20 min, and 30 min. |
|
|
[16] | ||
| Agricultural waste | Maize silage | Increase in biogas and methane production up to 29.5% | USPP: field intensity 40–50 W·cm−2; frequency 20 kHz; production ceases after 300 h |
|
|
[17] |
| Cattle manure mixed with straw wheat (2:1) | Increase in methane production by 1.6–4.1% Increase in biogas yield production by 8.7–64.2% |
USPP: power 400 W; frequency 24 kHz; treatment time 4.41–54.14 s |
|
|
[18] | |
| Increase in methane production by 2.0–5.4% Increase in biogas yield production by 5.7–39.4% |
HCPP: hydrosonic pump 1.2 kW; treatment time 4.41–54.14 s | |||||
| Silage with bovine liquid manure | Increase in biogas yield by 23.5% | USPP: power 400 W; frequency 24 kHz; treatment time 60 s, 120 s, 180 s |
|
|
[19] | |
| Mixture of organic wastes | Increase in methane production by 20% | USPP: intensity 2.3, 7.7, 13.4 W·cm−2 |
|
|
[20] | |
| Algae | residues | Increases the methane yield by 21.5% | HCPP: 4000 rpm for 15 min |
|
|
[6] |
USPP, ultrasound pretreatment parameters; HCPP, hydrothermal cavitation pretreatment parameters; COD, chemical oxygen demand; BOD, biochemical oxygen demand; TS, total solids; VS, volatile solids.
In Polettini et al. [20], the effect of ultrasonic treatment on methane production was evaluated in anaerobic digestion of lignocellulosic waste. It was determined that with a mixture ratio of sonicated and untreated substrate 75/25, methane production improved by 20% and kinetic parameters increased by 64–82%. As can be seen, there is a trend towards an increase in biogas methane production, which has a certain range with a maximum value of 82%, which can be achieved in the study [20], which is consistent with several other studies [5][21].
Joshi et al. [22] showed that the use of ultrasound promotes the solubilization of organic matter present in food waste, and the continued application of ultrasound in the anaerobic digestion process resulted in increased biogas production (almost doubled) of pretreated food waste [13]. The pretreatment of different types of waste is important, as food waste has a significant inclusion of cellulose-containing components, so its treatment with ultrasound can have a positive effect in terms of improving the biodegradability of these compounds and homogenization.
As a result of the high dispersibility and intensification of the anaerobic fermentation processes, the fermentation period of biomass is considerably reduced. The use of ultrasound in secondary sludge at the Molina de Segura wastewater treatment plant [23] significantly reduces the minimum time required for the anaerobic digestion process. By hydrolysis of the existing cell walls in secondary sludge, biodegradable substances increase immediately, accelerating the anaerobic digestion processes and moving to the stages of acidogenesis, acetogenesis, and methanogenesis. The use of an ultrasonic sludge treatment system increased digester capacity by 8%, causing an 18% increase in average biogas production and a 10% decrease in the production of dewatered sludge on average. The cogeneration process at the Molina de Segura wastewater treatment plant reduced the required electricity consumption since cogeneration increased by 20%.
The biological processes are substantially stabilized, leading to the absence of foaming and floating crusts in the upper part of the bioreactor. Thus, the entire productive volume of the reactor is used efficiently. Machnicka et al. [24] showed how the addition of cavitation-disintegrated foam to mesophilic anaerobic digestion can improve the process and the production of biogas. The thermophilic digestion mode is more efficient in terms of biogas yield per unit time, but its use can be limited because of economic indicators of the cost of heating the substrate in the bioreactor, and it depends on external temperatures and thus on the climatic zone. Therefore, in temperate latitudes, including Ukraine, it is relevant to work in the mesophilic mode.
3. Electrolysis Treatment in Processes of Stimulation of the Ecological and Trophic Groups of Microorganisms Required in Biogas Production
The microbial electrolysis cell provides a higher energy efficiency and biogas production, which have been studied and confirmed by several studies. A systematic understanding of microbial interactions and the production of biomethane and hydrogen in the microbial electrolysis cell is limited [25]. Liu et al. [26] showed that biohythane can be produced directly in microbial electrolysis cells by biocathodes using sludge. The predominant population at the alkali-pretreated sludge-fed microbial electrolysis cell anode was represented by exoelectrogenic Geobacter, while fermentative Clostridium dominated at the biocathode. Most of the methanogenic archaea in the cathodes belonged to the hydrogenotrophic Methanobacterium and Methanocorpusculum. The microbial electrolysis cell enhances the production of biomethane and hydrogen from waste sludge through syntrophic interactions between fermentative bacteria, exoelectrogenes, and methanogenic archaea. Furthermore, multiple gas production pathways are observed in the microbial electrolysis cell reactor: fermentation and electrolytic H2 production, as well as hydrogenotrophic methanogenesis and electromethanogenesis. The possibility of additional electrolytic hydrogen production in the electrolysis system also provides perspective on the application in innovative bioprocesses such as photo-fermentation [27] and dark fermentation [28]. Higher efficiency biohydrogen production by two-stage dark fermentation combined with microbial electrolysis was proven on palm oil mill effluent [29] and cassava starch processing wastewater [30]. Furthermore, the integration of microbial electrolysis cells with photo-fermentation positively changed the microbial community with the predominance of electrogenic microorganisms, which increased hydrogen production [31].
In electromethanogenic bioelectrochemical systems, there are three known pathways: CO2 reduction, methylotrophic, and acetoclastic pathways. The CO2 reduction pathway, which stimulates methane production, is considered the main determinant of overall system performance, but other pathways are also important [32]. Therefore, acetoclastic methanogenesis very often prevails in industrial condition [33] biogas production because it is based on the possibility of bioconversion of acetates with the obtaining of methane (CH3COOH → CH4 + CO2).
Some methane production reactions carried out by biocathode communities have been described in the literature, but further research is needed to elucidate the molecular pathways. In a review by Blasco-Gómez et al. [34], some molecular research findings are collected in the field of electromethanogenesis.
Therefore, each of these pathways can be used to produce methane, and it is important to find an integrated opportunity to stimulate their dominant relationships in a consortium of methanogens to improve the quality of biogas.
According to Cerrillo et al. [35], the combination of anaerobic digestion and a microbial electrolysis cell with a methanogenic biocathode also proved to be a promising waste treatment strategy for pig manure. Microbiological analysis showed that the methylotrophic family of Methanossiliicoccaceae (genus Methanomassiliicoccus) was the most abundant among active archaea in anaerobic digestion during the inhibited state. The Methanobacteriaceae family (genera Methanobrevibacter and Methanobacterium), generally considered the most abundant in methanogenic biocathodes, shared dominance with the Methanomassiliicoccaceae families (genus Methanomassiliicoccus) and Methanotrichaceae (genus Methanothrix) in the cathode.
The results of the Liu et al. study [36] showed that stimulation of direct electron transfer in the microbial electrolysis cell improves the processing of biogas. The methane content of biogas increased from 71% to >90% and 8.2% CO2 converted to methane, due to the fact that the acetoclastic methanogen Methanothrix in the cathode used the CO2 reduction pathway, while in the bulk sludge it used the acetate decarboxylation pathway to produce methane. Therefore, methane production was stimulated by hydrogen generation, which was used by autotrophic methanogens as electron donors through the CO2 reduction pathway.
One of the most recent studies by Park et al. [37] focused on microbial communities and the performance of a conventional anaerobic reactor combined with microbial electrolysis cells. According to the study bioelectrochemical reactions can reduce the stabilization period of the reactor and increase the amount of methane produced. Furthermore, an increase in the yield of methane was assumed because e and H+ produced during this process were converted to H2 by electrochemical reactions and then combined with CO2 to produce CH4. Therefore, H2 and CO2 react with the formation of additional methane. However, the hydrogen content of the system with ‘activated water’ (AW) was higher than that of the experiment without electrolysis treatment during the start-up period. On the contrary, the gas compositions in the two reactors were the same during the stationary period. On this basis, it was assumed that the increase in methane yield and production was associated with changes in microbial communities under bioelectrochemical reactions, as shown in Figure 1.
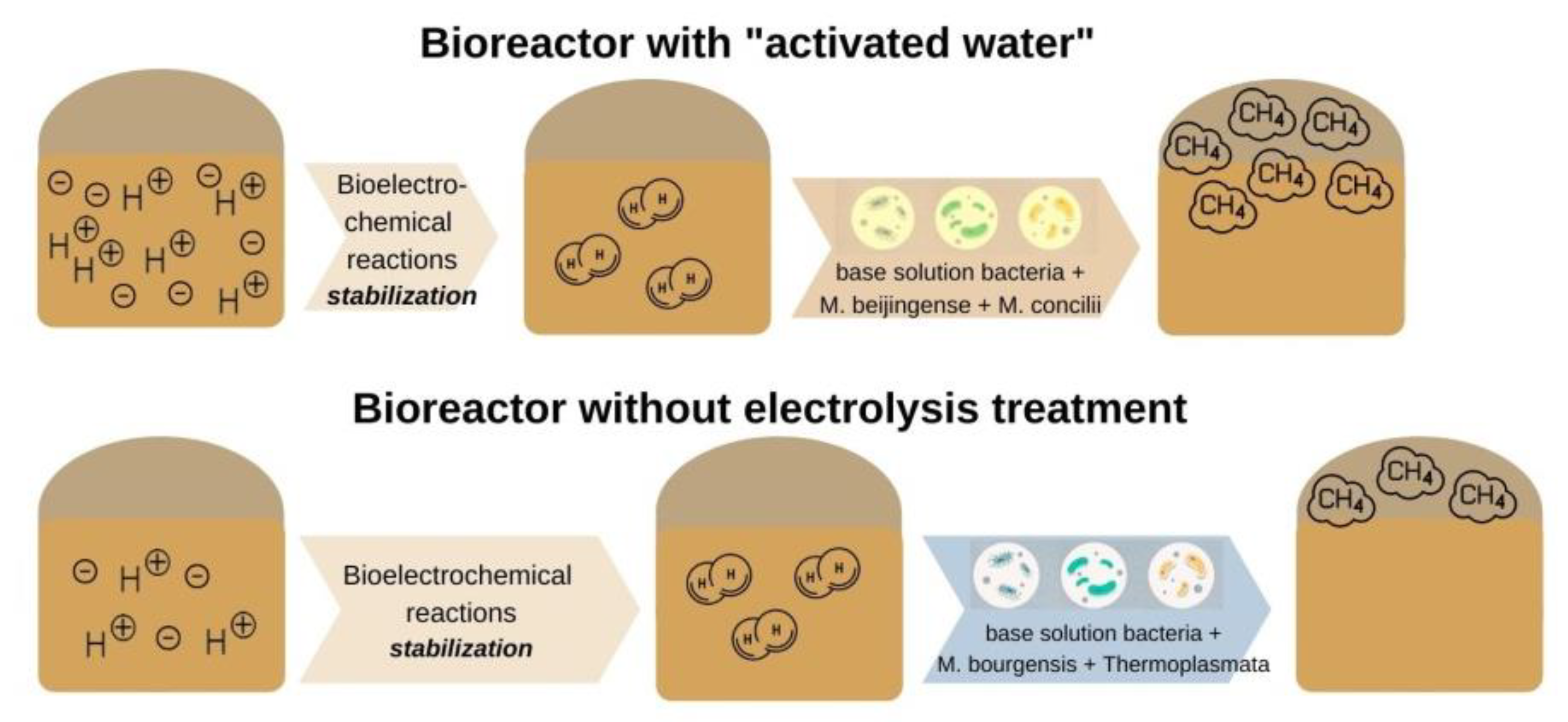
Figure 1. Bioelectrochemical reactions in the system with ‘activated water’ and the experiment without electrolysis treatment during the initial period.
There are definite differences between the microorganisms of the archaea communities in the basic solution, the dominant species in the unclassified solution with AW was Methanobacterium beijingense (63% of the total population), followed by Methanosaeta concilii (20% of the total population). In the unclassified solution without electrolysis treatment, Methanoculleus bourgensis was the dominant species (69% of the total population and unclassified microorganisms, 25%). The Methanoculleus bourgensis and Thermoplasmata classes, which dominated without electrolysis treatment, inhabit the anode surface of bioelectrochemical systems. Methanobacterium beijingense, which represents most of the microbial community with AW, is a hydrogenotrophic methanogen that generates methane from H2, CO2, and formate and does not use substrates from methanol, ethanol, trimethylamine, isobutanol, or isopropanol. Methanosaeta concilii, which is the next largest fraction, is an acetoclastic methanogen that generates methane from acetic acid. Methane formation stops if methanol, trimethylamine, formic acid, propionic acid, butyric acid, and pyruvic acid come into contact with this microorganism. The main microorganisms with Methanobacterium beijingense and Methanosaeta concilii of AW use their respective substrates (acetic acid and formate) to produce methane, and the presence of other compounds negatively affects the formation of methane.
Methanoculleus bourgensis was the dominant species in the reactor without electrolysis treatment. The introduction of additional electron acceptors in the form of hydrogen, as well as the disintegration of complex organic compounds under the influence of redox reactions during electrolysis, was the main contributor to this, which is consistent with [37]. This species uses CO2, H2, formate, 2-propanol, 2-butanol, and other secondary alcohols to generate methane with simultaneous acetoclastic and hydrogenotrophic methanogenesis, with a Gibbs free energy for the production of CO2 methanol lower than for reactions of acetoclastic and hydrogenotrophic methanogenesis. The non-electrolysis approach bioelectrochemically accelerates the methanol pathway and the production rate, and then the methanol produced in the cathode is converted directly to methane by advanced methylotrophic methanogens (Figure 2).
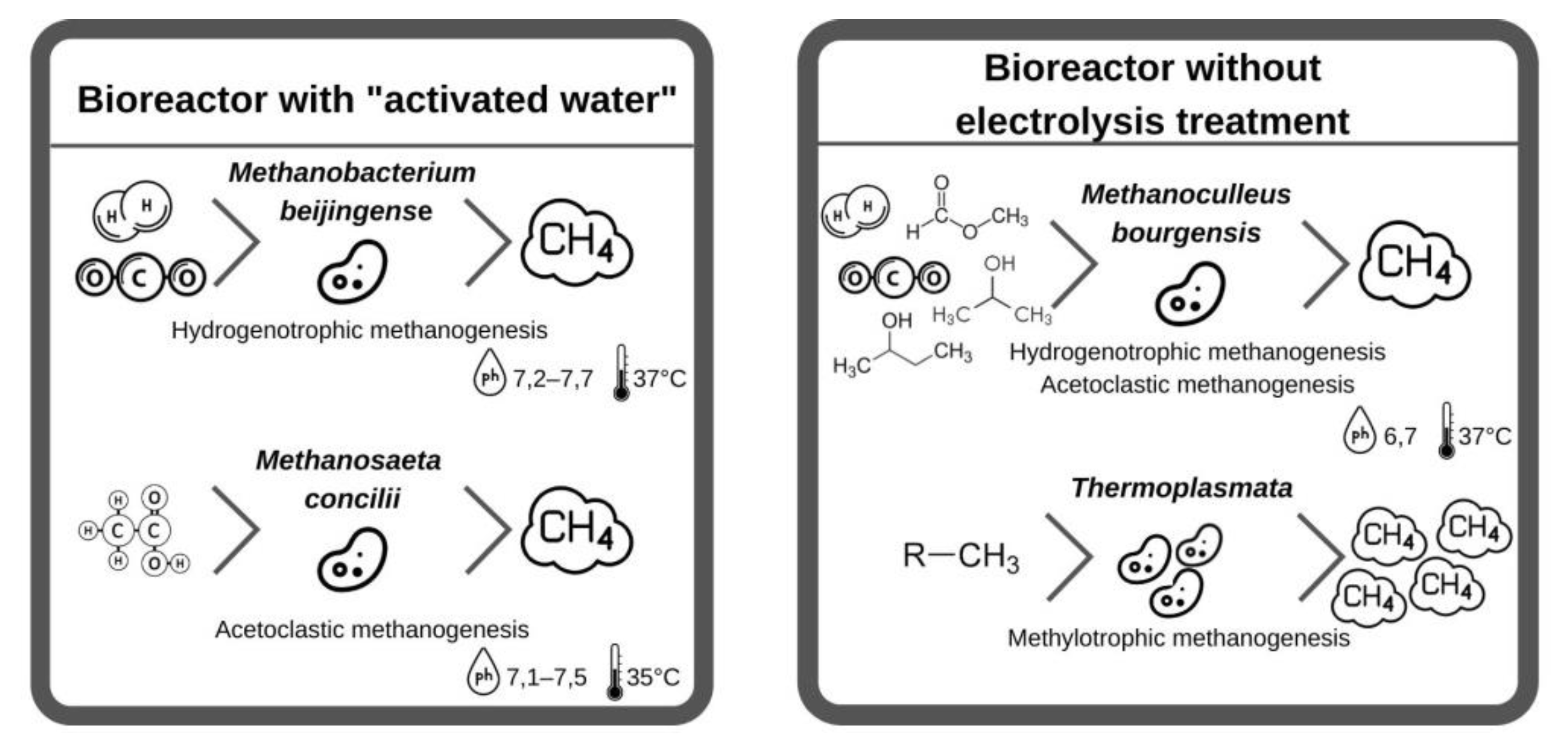
Figure 2. Comparison of bacterial bioprocesses in reactors with ‘activated water’ and without electrolysis treatment.
To summarize the changes in the archaea community in bioreactors with and without electrolysis, microbial communities that generate methane from certain substrates (formate and acetate) were found with AW, while Methanoculleus bourgensis, which generates methane using different substrates (formate, 2-propanol, 2-butanol, etc.), was found with AW, the Thermoplasmata community, which is a methylotrophic methanogen that generates more methane than other methanogens (such as acetoclastic or hydrogenotrophic methanogens), dominated without electrolysis treatment. Combined with the results on species diversity, the diversity and population of archea communities were lower without electrolysis treatment than with AW. This change in the microbial community resulted in a difference in methane production. Furthermore, when analyzing the microbial community without electrolysis treatment using food waste filtrate, the population of Methanosarcina thermophila, which grows with acetate, methanol, methylated amines, etc., and is part of the Methanosarcinaceae family, increased significantly. These results demonstrate that this medium promotes the growth of methylotrophic methanogens in the reactor without electrolysis treatment [37].
Overall, certain aspects ensure higher biogas production with electrolysis treatment for the stimulation bioprocess:
- The microbial electrolysis cell can increase the methane content;
- Electrokinetic decomposition deforms the cell walls of substrates, making their contents easily accessible to bacteria for anaerobic digestion [38];
- Treatment of liquid media with high-voltage electric pulses leads to inactivation of microorganisms at lower temperatures and in a shorter soaking time [39];
- The same increase in the yield and production of methane is associated with changes in the microbial community resulting in a difference in methane production [16].
This entry is adapted from the peer-reviewed paper 10.3390/fermentation8100570
References
- Chernysh, Y.; Balintova, M.; Plyatsuk, L.; Holub, M.; Demcak, S. The Influence of Phosphogypsum Addition on Phosphorus Release in Biochemical Treatment of Sewage Sludge. Int. J. Environ. Res. Public Health 2018, 15, 1269.
- Skripkin, S.; Tsoy, M.; Kuibin, P.; Shtork, S. Swirling Flow in a Hydraulic Turbine Discharge Cone at Different Speeds and Discharge Conditions. Exp. Therm. Fluid Sci. 2019, 100, 349–359.
- Yeh, C.-P.; Tseng, K.-T.; Collicott, S.H. Manipulating Inlet Cavitation to Control Spray Properties. In Proceedings of the 39th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, Huntsville, Alabama, 20–23 July 2003.
- Ranade, N.V.; Nagarajan, S.; Sarvothaman, V.; Ranade, V.V. ANN Based Modelling of Hydrodynamic Cavitation Processes: Biomass Pre-Treatment and Wastewater Treatment. Ultrason. Sonochemistry 2020, 72, 105428.
- Biogas, Z. Available online: https://zorg-biogas.com/ (accessed on 1 August 2022).
- Nykyforov, V.; Malovanyy, M.; Kozlovskaya, T.; Novokhatko, O.; Digtiar, S. The Biotechnological Ways of Blue-Green Algae Complex Processing. East. Eur. J. Enterp. Technol. 2016, 5, 11–18.
- Minutillo, M.; Perna, A.; Sorce, A. Green Hydrogen Production Plants via Biogas Steam and Autothermal Reforming Processes: Energy and Exergy Analyses. Appl. Energy 2020, 277, 115452.
- Martín, M.Á.; González, I.; Serrano, A.; Siles, J.Á. Evaluation of the Improvement of Sonication Pre-Treatment in the Anaerobic Digestion of Sewage Sludge. J. Environ. Manag. 2015, 147, 330–337.
- Zieliński, M.; Dębowski, M.; Krzemieniewski, M.; Rusanowska, P.; Zielińska, M.; Cydzik-Kwiatkowska, A.; Głowacka-Gil, A. Application of an Innovative Ultrasound Disintegrator for Sewage Sludge Conditioning Before Methane Fermentation. J. Ecol. Eng. 2018, 19, 240–247.
- Gunes, B.; Stokes, J.; Davis, P.; Connolly, C.; Lawler, J. Modelling and Optimisation of the Biogas Yield after Hybrid Alkaline-Ultrasonic Pre-Treatment in the Early Stages of Anaerobic Digestion of Pot Ale to Shorten the Processing Time. Process Saf. Environ. Prot. 2021, 146, 43–53.
- Bougrier, C.; Carrère, H.; Delgenès, J.P. Solubilisation of Waste-Activated Sludge by Ultrasonic Treatment. Chem. Eng. J. 2005, 106, 163–169.
- Zeynali, R.; Khojastehpour, M.; Ebrahimi-Nik, M. Effect of Ultrasonic Pre-Treatment on Biogas Yield and Specific Energy in Anaerobic Digestion of Fruit and Vegetable Wholesale Market Wastes. Sustain. Environ. Res. 2017, 27, 259–264.
- Begum, S.; Anupoju, G.R.; Eshtiaghi, N. Anaerobic Co-Digestion of Food Waste and Cardboard in Different Mixing Ratios: Impact of Ultrasound Pre-Treatment on Soluble Organic Matter and Biogas Generation Potential at Varying Food to Inoculum Ratios. Biochem. Eng. J. 2021, 166, 107853.
- Cesaro, A.; Naddeo, V.; Amodio, V.; Belgiorno, V. Enhanced Biogas Production from Anaerobic Codigestion of Solid Waste by Sonolysis. Ultrason. Sonochemistry 2012, 19, 596–600.
- Cesaro, A.; Belgiorno, V. Sonolysis and Ozonation as Pretreatment for Anaerobic Digestion of Solid Organic Waste. Ultrason. Sonochemistry 2013, 20, 931–936.
- Rasapoor, M.; Ajabshirchi, Y.; Adl, M.; Abdi, R.; Gharibi, A. The Effect of Ultrasonic Pretreatment on Biogas Generation Yield from Organic Fraction of Municipal Solid Waste under Medium Solids Concentration Circumstance. Energy Convers. Manag. 2016, 119, 444–452.
- Závacký, M.; Ditl, P.; Prell, A.; Sobotka, M. Increasing Biogas Production from Maize Silage by Ultrasonic Treatment. Chem. Eng. Trans. 2010, 21, 439–444.
- Zieliński, M.; Dębowski, M.; Kisielewska, M.; Nowicka, A.; Rokicka, M.; Szwarc, K. Comparison of Ultrasonic and Hydrothermal Cavitation Pretreatments of Cattle Manure Mixed with Straw Wheat on Fermentative Biogas Production. Waste Biomass Valorization 2019, 10, 747–754.
- Dudek, M.; Rusanowska, P.; Zieliński, M.; Dębowski, M. Influence of Ultrasonic Disintegration on Efficiency of Methane Fermentation of Sida Hermaphrodita Silage. J. Ecol. Eng. 2018, 19, 128–134.
- Boni, M.R.; Polettini, A.; Pomi, R.; Rossi, A. Effect of Ultrasonic Post-Treatment on Anaerobic Digestion of Lignocellulosic Waste. Waste Manag. Res. 2021, 39, 221–232.
- Zablodsky, N.; Klendiy, G.; Klendiy, P. Influence of Low-Frequency Electromagnetic Field on Biophone Explosion Dynamic. Energy Autom. 2019, 2019, 27–36.
- Joshi, S.M.; Gogate, P.R. Intensifying the Biogas Production from Food Waste Using Ultrasound: Understanding into Effect of Operating Parameters. Ultrason. Sonochemistry 2019, 59, 104755.
- Andreu, P.S.; Mifsut, C.L.; Antonio, A.J.; Sánchez, S.B.; García, A. Optimización de La Digestión Anaerobia Mediante La Aplicación de Ultrasonidos En Los Fangos Secundarios de La EDAR Molina de Segura (Murcia). Tecnol. Del Agua 2007, 27, 48–56.
- Machnicka, A.; Grübel, K.; Mirota, K. Considerations of Impact of Venturi Effect on Mesophilic Digestion. Ecol. Chem. Eng. S 2016, 22, 645–658.
- Saravanan, A.; Karishma, S.; Kumar, P.S.; Yaashikaa, P.R.; Jeevanantham, S.; Gayathri, B. Microbial Electrolysis Cells and Microbial Fuel Cells for Biohydrogen Production: Current Advances and Emerging Challenges. Biomass Convers. Biorefinery 2020, 1–21.
- Liu, Q.; Ren, Z.J.; Huang, C.; Liu, B.; Ren, N.; Xing, D. Multiple Syntrophic Interactions Drive Biohythane Production from Waste Sludge in Microbial Electrolysis Cells. Biotechnol. Biofuels 2016, 9, 162.
- Policastro, G.; Cesaro, A.; Fabbricino, M. Photo-Fermentative Hydrogen Production from Cheese Whey: Engineering of a Mixed Culture Process in a Semi-Continuous, Tubular Photo-Bioreactor. Int. J. Hydrogen Energy 2022, 47, 10665–10688.
- Yousuf, A.; Bastidas-Oyanedel, J.-R.; Schmidt, J.E. Effect of Total Solid Content and Pretreatment on the Production of Lactic Acid from Mixed Culture Dark Fermentation of Food Waste. Waste Manag. 2018, 77, 516–521.
- Khongkliang, P.; Jehlee, A.; Kongjan, P.; Reungsang, A.; Sompong, O. High Efficient Biohydrogen Production from Palm Oil Mill Effluent by Two-Stage Dark Fermentation and Microbial Electrolysis under Thermophilic Condition. Int. J. Hydrogen Energy 2019, 44, 31841–31852.
- Khongkliang, P.; Kongjan, P.; Utarapichat, B.; Reungsang, A.; Sompong, O. Continuous Hydrogen Production from Cassava Starch Processing Wastewater by Two-Stage Thermophilic Dark Fermentation and Microbial Electrolysis. Int. J. Hydrogen Energy 2017, 42, 27584–27592.
- Then, M.Y.; Ramesh, S.; Asrul, M.A.M.; Atan, M.F.; Yun, H.A.H.; Lai, J.C.H.; Rahman, M.R.; Abdullah, M.O.; Wahab, N.A. Dynamic Modeling of Hydrogen Production from Photo-Fermentation in Microbial Electrolysis Cell Using Sago Waste. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1101, 012040.
- Amrut Pawar, A.; Karthic, A.; Lee, S.; Pandit, S.; Jung, S.P. Microbial Electrolysis Cells for Electromethanogenesis: Materials, Configurations and Operations. Environ. Eng. Res. 2020, 27, 200484.
- Cai, M.; Wilkins, D.; Chen, J.; Ng, S.-K.; Lu, H.; Jia, Y.; Lee, P.K.H. Metagenomic Reconstruction of Key Anaerobic Digestion Pathways in Municipal Sludge and Industrial Wastewater Biogas-Producing Systems. Front. Microbiol. 2016, 7, 778.
- Blasco-Gómez, R.; Batlle-Vilanova, P.; Villano, M.; Balaguer, M.D.; Colprim, J.; Puig, S. On the Edge of Research and Technological Application: A Critical Review of Electromethanogenesis. Int. J. Mol. Sci. 2017, 18, 874.
- Cerrillo, M.; Viñas, M.; Bonmatí, A. Anaerobic Digestion and Electromethanogenic Microbial Electrolysis Cell Integrated System: Increased Stability and Recovery of Ammonia and Methane. Renew. Energy 2018, 120, 178–189.
- Liu, C.; Sun, D.; Zhao, Z.; Dang, Y.; Holmes, D.E. Methanothrix Enhances Biogas Upgrading in Microbial Electrolysis Cell via Direct Electron Transfer. Bioresour. Technol. 2019, 291, 121877.
- Park, J.-G.; Lee, B.; Lee, U.-J.; Jun, H.-B. An Anaerobic Digester with Microbial Electrolysis Cell Enhances Relative Abundance of Methylotrophic Methanogens in Bulk Solution. Environ. Eng. Res. 2021, 27, 210666.
- Zieliński, M.; Dębowski, M.; Krzemieniewski, M.; Dudek, M.; Grala, A. Effect of Constant Magnetic Field (CMF) with Various Values of Magnetic Induction on Effectiveness of Dairy Wastewater Treatment under Anaerobic Conditions. Pol. J. Environ. Stud. 2014, 23, 255–261.
- Capodaglio, A.G. Pulse Electric Field Technology for Wastewater and Biomass Residues’ Improved Valorization. Processes 2021, 9, 736.
This entry is offline, you can click here to edit this entry!
