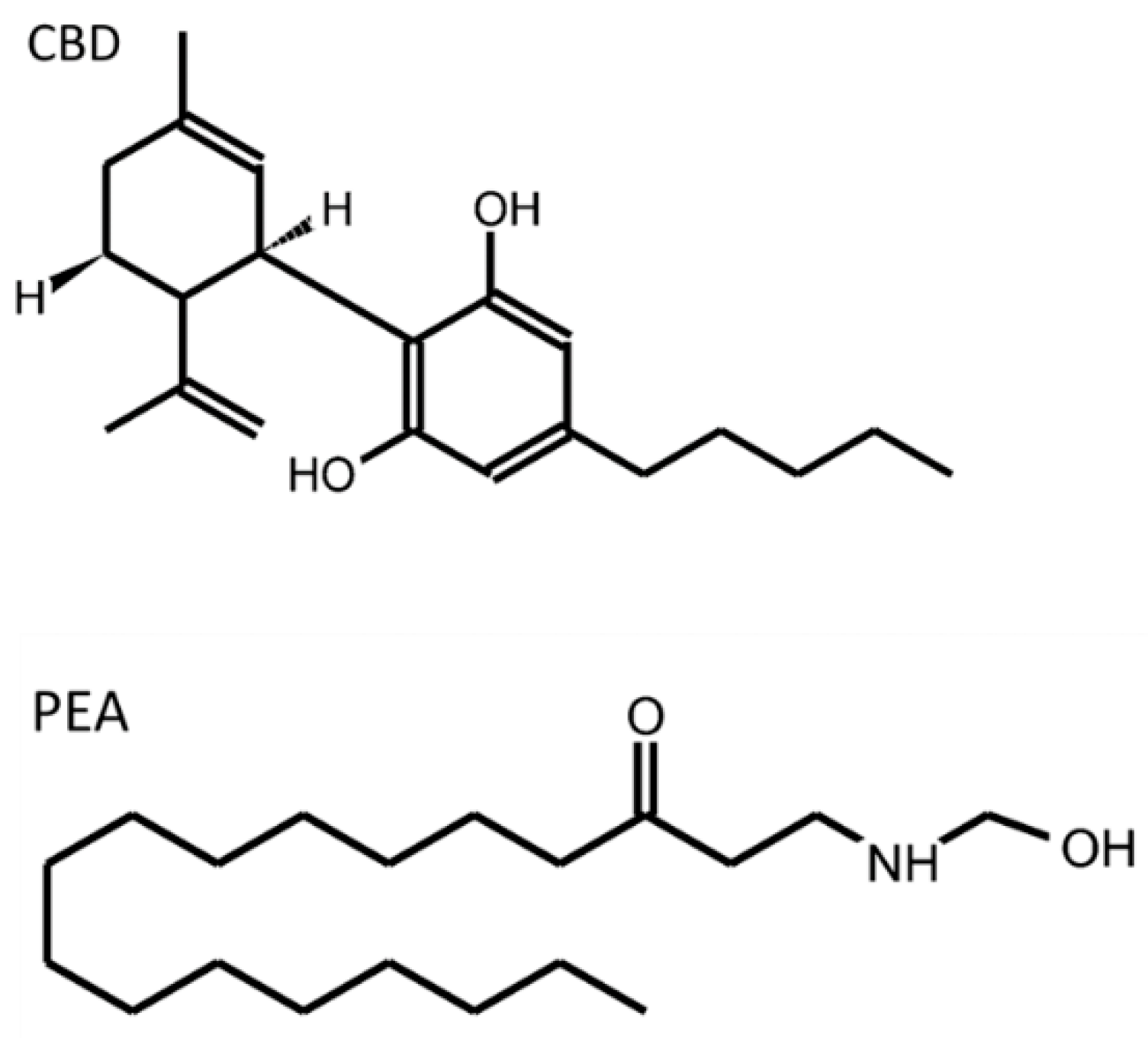
The enteric nervous system (ENS) is a part of the autonomic nervous system that intrinsically innervates the gastrointestinal (GI) tract. Whereas enteric neurons have been deeply studied, the enteric glial cells (EGCs) have received less attention. However, these are immune-competent cells that contribute to the maintenance of the GI tract homeostasis through supporting epithelial integrity, providing neuroprotection, and influencing the GI motor function and sensation. The endogenous cannabinoid system (ECS) includes endogenous classical cannabinoids (anandamide, 2-arachidonoylglycerol), cannabinoid-like ligands (oleoylethanolamide (OEA) and palmitoylethanolamide (PEA)), enzymes involved in their metabolism (FAAH, MAGL, COX-2) and classical (CB1 and CB2) and non-classical (TRPV1, GPR55, PPAR) receptors. The ECS participates in many processes crucial for the proper functioning of the GI tract, in which the EGCs are involved.
- cannabidiol
- endocannabinoid system
- enteric glial cells
- enteric nervous system
- gastrointestinal system
- nutraceuticals
- palmitoylethanolamide
1. Introduction
2. The Enteric Nervous System
2.1. Enteric Neurons
2.2. Enteric Glial Cells
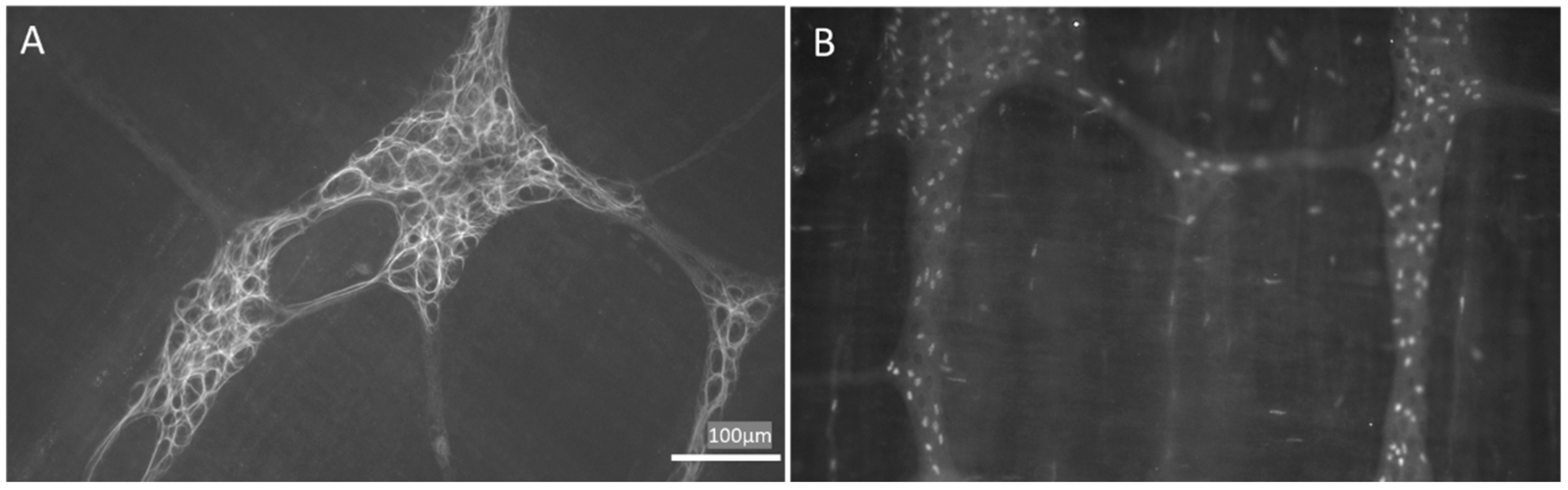
|
Aspect |
Function |
Localization |
Mediators |
References |
|---|---|---|---|---|
|
Epithelial barrier |
Intestinal barrier formation and support Enhancing epithelial healing Neuropods formation |
Mucosa |
proEGF TGF-β S-nitrosoglutathione 15d-PGJ2 NGF-β * Artemin * |
|
|
Intestinal motility |
Control of GI motility # |
Myenteric plexus |
ATP |
|
|
Enteric neurotransmission |
Neuronal communication |
ENS |
ATP NFG GSH |
[49] |
|
Immune response |
Activation of EGCs |
ENS |
MHC II class IL-1β IL-6 TGF-β proEGF GSH PGE2 |
[50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70] |
|
Visceral sensitivity |
Sensitizing/activating nociceptors |
ENS |
ATP GABA IL-1β neurotrophins |
* Mediators released by enteroendocrine cells; # EGC loss results in impaired GI motility. Abbreviations: 15d-PGJ2, 15-deoxy-Δ12,14-prostaglandin J2; ATP, adenosine triphosphate; EGC, enteric glial cell; EGF, Epidermal growth factor; ENS, enteric nervous system; GABA, gamma amino butyric acid; GI, gastrointestinal; GSH, glutathione; IL, interleukin; MHC, major histocompatibility complex; NGF, nerve growth factor; PGE2, prostaglandin E2; proEGF, proepidermal growth factor; TGF, Transforming growth factor.
3. The Endocannabinoid System
This entry is adapted from the peer-reviewed paper 10.3390/molecules27196773
References
- Soty, M.; Gautier-Stein, A.; Rajas, F.; Mithieux, G. Gut-Brain Glucose Signaling in Energy Homeostasis. Cell Metab. 2017, 25, 1231–1242.
- Furness, J.B. The Enteric Nervous System; John Wiley & Sons: Hoboken, NJ, USA, 2008.
- Labanski, A.; Langhorst, J.; Engler, H.; Elsenbruch, S. Stress and the brain-gut axis in functional and chronic-inflammatory gastrointes-tinal diseases: A transdisciplinary challenge. Psychoneuroendocrinology 2020, 111, 104501.
- Fung, C.; Vanden Berghe, P. Functional circuits and signal processing in the enteric nervous system. Cell Mol. Life Sci. 2020, 77, 4505–4522.
- Spencer, N.J.; Hu, H. Enteric nervous system: Sensory transduction, neural circuits and gastrointestinal motility. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 338–351.
- Fleming, M.A.; Ehsan, L.; Moore, S.R.; Levin, D.E. The Enteric Nervous System and Its Emerging Role as a Therapeutic Target. Gastroenterol. Res. Pract. 2020, 2020, 8024171.
- Spear, E.T.; Mawe, G.M. Enteric neuroplasticity and dysmotility in inflammatory disease: Key players and possible therapeutic targets. Am. J. Physiol.-Gastrointest. Liver Physiol. 2019, 317, G853–G861.
- Morales-Soto, W.; Gulbransen, B.D. Enteric Glia: A New Player in Abdominal Pain. CMGH 2019, 7, 433–445.
- Grundmann, D.; Loris, E.; Maas-Omlor, S.; Huang, W.; Scheller, A.; Kirchhoff, F.; Schäfer, K.H. Enteric Glia: S100, GFAP, and Beyond. Anat. Rec. 2019, 302, 1333–1344.
- DeFelice, S.L. The Nutraceutical Revolution: Fueling a Powerful, New International Market; The Foundation for Innovation in Medicine: Mountside, NJ, USA, 1989.
- Sharkey, K.A.; Wiley, J.W. The Role of the Endocannabinoid System in the Brain-Gut Axis. Gastroenterology 2016, 151, 252–266.
- Hasenoehrl, C.; Taschler, U.; Storr, M.; Schicho, R. The gastrointestinal tract-a central organ of cannabinoid signaling in health and disease. Neurogastroenterol. Motil. 2016, 28, 1765–1780.
- Sałaga, M.; Abalo, R.; Fichna, J. Cannabis and Cannabinoids and the Effects on Gastrointestinal Function: An Overview. In Handbook of Cannabis and Related Pathologies; Treat; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 471–480.
- Uranga, J.A.; Vera, G.; Abalo, R. Cannabinoid pharmacology and therapy in gut disorders. Biochem. Pharmacol. 2018, 157, 134–147.
- Meletis, C. The important role of the endocannabinoid system and the endocannabinoidome in gut health. Altern. Ther. Health Med. 2019, 25, 24–27.
- Szczepaniak, A.; Fichna, J. What role do cannabinoids have in modern medicine as gastrointestinal anti-inflammatory drugs? Expert. Opin. Pharmacother. 2020, 21, 1931–1934.
- Cohen, L.B.; Neuman, M.G. Cannabis and the gastrointestinal tract. J. Pharm. Pharm. Sci. 2020, 23, 304–313.
- Martínez, V.; Iriondo De-Hond, A.; Borrelli, F.; Capasso, R.; Del Castillo, M.D.; Abalo, R. Cannabidiol and Other Non-Psychoactive Cannabinoids for Prevention and Treatment of Gastrointestinal Disorders: Useful Nutraceuticals? Int. J. Mol. Sci. 2020, 21, 3067.
- Maselli, D.B.; Camilleri, M. Pharmacology, Clinical Effects, and Therapeutic Potential of Cannabinoids for Gastrointestinal and Liver Diseases. Clin. Gastroenterol. Hepatol. 2020, 19, 1748–1758.
- DeVuono, M.V.; Parker, L.A. Cannabinoid Hyperemesis Syndrome: A Review of Potential Mechanisms. Cannabis Cannabinoid Res. 2020, 5, 132–144.
- Russo, E.B.; Spooner, C.; May, L.; Leslie, R.; Whiteley, V.L. Cannabinoid Hyperemesis Syndrome Survey and Genomic Investigation. Cannabis Cannabinoid Res. 2022, 7, 336–344.
- Kakish, D.; Alaoudi, M.; Welch, B.; Fan, D.; Meghpara, M.; Mandava, N.; Kumthekar, N. Small bowel intussusception in marijuana users. J. Surg. Case Rep. 2020, 2020, rjaa335.
- Furness, J.B. Types of neurons in the enteric nervous system. J. Auton. Nerv. Syst. 2000, 81, 87–96.
- Zeisel, A.; Hochgerner, H.; Lönnerberg, P.; Johnsson, A.; Memic, F.; van der Zwan, J.; Häring, M.; Braun, E.; Borm, L.E.; La Manno, G.; et al. Molecular Architecture of the Mouse Nervous System. Cell 2018, 174, 999–1014.
- Bertrand, P.P.; Kunze, W.A.; Bornstein, J.C.; Furness, J.B. Electrical mapping of the projections of intrinsic primary afferent neurones to the mucosa of the guinea-pig small intestine. Neurogastroenterol. Motil. 1998, 10, 533–542.
- Furness, J.B.; Kunze, W.A.; Bertrand, P.P.; Clerc, N.; Bornstein, J.C. Intrinsic primary afferent neurons of the intestine. Prog. Neurobiol. 1998, 54, 1–18.
- Lomax, A.E.; Furness, J.B. Neurochemical classification of enteric neurons in the guinea-pig distal colon. Cell Tissue Res. 2000, 302, 59–72.
- Neal, K.B.; Bornstein, J.C. Targets of myenteric interneurons in the guinea-pig small intestine. Neurogastroenterol. Motil. 2008, 20, 566–575.
- Grubišić, V.; Gulbransen, B.D. Enteric glia: The most alimentary of all glia. J. Physiol. 2017, 595, 557–570.
- Rosenberg, H.J.; Rao, M. Enteric glia in homeostasis and disease: From fundamental biology to human pathology. iScience 2021, 24, 102863.
- Hanani, M.; Reichenbach, A. Morphology of horseradish peroxidase (HRP)-injected glial cells in the myenteric plexus of the guinea-pig. Cell Tissue Res. 1994, 278, 153–160.
- Ferri, G.L.; Probert, L.; Cocchia, D.; Michetti, F.; Marangos, P.J.; Polak, J.M. Evidence for the presence of S-100 protein in the glial component of the human enteric nervous system. Nature 1982, 297, 409–410.
- Jessen, K.R.; Mirsky, R. Glial cells in the enteric nervous system contain glial fibrillary acidic protein. Nature 1980, 286, 736–737.
- Hoff, S.; Zeller, F.; von Weyhern, C.W.; Wegner, M.; Schemann, M.; Michel, K.; Rühl, A. Quantitative assessment of glial cells in the human and guinea pig enteric nervous system with an anti-Sox8/9/10 antibody. J. Comp. Neurol. 2008, 509, 356–371.
- Hanani, M.; Zamir, O.; Baluk, P. Glial cells in the guinea pig myenteric plexus are dye coupled. Brain Res. 1989, 497, 245–249.
- Christofi, F.L.; Wood, J.D. Effects of PACAP on morphologically identified myenteric neurons in guinea pig small bowel. Am. J. Physiol.-Gastrointest. Liver Physiol. 1993, 264, G414–G421.
- López-Gómez, L.; Szymaszkiewicz, A.; Zielińska, M.; Abalo, R. Nutraceuticals and enteric glial cells. Molecules 2021, 26, 3762.
- Liu, Y.A.; Chung, Y.C.; Pan, S.T.; Shen, M.Y.; Hou, Y.C.; Peng, S.J.; Pasricha, P.J.; Tang, S.C. 3-D imaging, illustration, and quantitation of enteric glial network in transparent human colon mucosa. Neurogastroenterol. Motil. 2013, 25, e324–e338.
- Bohórquez, D.V.; Samsa, L.A.; Roholt, A.; Medicetty, S.; Chandra, R.; Liddle, R.A. Enteroendocrine cell-Enteric glia connection revealed by 3D electron microscopy. PLoS ONE 2014, 9, e89881.
- De Heuvel, E.; Wallace, L.; Sharkey, K.A.; Sigalet, D.L. Glucagon-like peptide 2 induces vasoactive intestinal polypeptide expression in enteric neurons via phophatidylinositol 3-kinase-7 signaling. Am. J. Physiol.-Endocrinol. Metab. 2012, 303, E994–E1005.
- Maudlej, N.; Hanani, M. Modulation of dye coupling among glial cells in the myenteric and submucosal plexuses of the guinea pig. Brain Res. 1992, 578, 94–98.
- Savidge, T.C.; Newman, P.; Pothoulakis, C.; Ruhl, A.; Neunlist, M.; Bourreille, A.; Hurst, R.; Sofroniew, M.V. Enteric Glia Regulate Intestinal Barrier Function and Inflammation Via Release of S-Nitrosoglutathione. Gastroenterology 2007, 132, 1344–1358.
- Bach-Ngohou, K.; Mahé, M.M.; Aubert, P.; Abdo, H.; Boni, S.; Bourreille, A.; Denis, M.G.; Lardeux, B.; Neunlist, M.; Masson, D. Enteric glia modulate epithelial cell proliferation and differentiation through 15-deoxy-? 12,14 -prostaglandin J2. J. Physiol. 2010, 588, 2533–2544.
- Neunlist, M.; Aubert, P.; Bonnaud, S.; Van Landeghem, L.; Coron, E.; Wedel, T.; Naveilhan, P.; Ruhl, A.; Lardeux, B.; Savidge, T.; et al. Enteric glia inhibit intestinal epithelial cell proliferation partly through a TGF-β1-dependent pathway. Am. J. Physiol.-Gastrointest. Liver Physiol. 2007, 292, 231–241.
- Van Landeghem, L.; Chevalier, J.; Mahé, M.M.; Wedel, T.; Urvil, P.; Derkinderen, P.; Savidge, T.; Neunlist, M. Enteric glia promote intestinal mucosal healing via activation of focal adhesion kinase and release of proEGF. Am. J. Physiol.-Gastrointest. Liver Physiol. 2011, 300, G976–G987.
- Aubé, A.C.; Cabarrocas, J.; Bauer, J.; Philippe, D.; Aubert, P.; Doulay, F.; Liblau, R.; Galmiche, J.P.; Neunlist, M. Changes in enteric neurone phenotype and intestinal functions in a transgenic mouse model of enteric glia disruption. Gut 2006, 55, 630–637.
- Nasser, Y.; Fernandez, E.; Keenan, C.M.; Ho, W.; Oland, L.D.; Tibbles, L.A.; Schemann, M.; MacNaughton, W.K.; Rühl, A.; Sharkey, K.A. Role of enteric glia in intestinal physiology: Effects of the gliotoxin fluorocitrate on motor and secretory function. Am. J. Physiol.-Gastrointest. Liver Physiol. 2006, 291, G912–G927.
- Rico, A.L.; Grants, I.; Needleman, B.J.; Williams, K.C.; Soghomonyan, S.; Turco, F.; Christofi, F.L. Gliomodulation of Neuronal and Motor Behavior in the Human GI Tract. Gastroenterology 2015, 148, S-18.
- Fields, R.D.; Ni, Y. Nonsynaptic communication through ATP release from volume-activated anion channels in axons. Sci. Signal. 2010, 3, ra-73.
- De Filippis, D.; Esposito, G.; Cirillo, C.; Cipriano, M.; De Winter, B.Y.; Scuderi, C.; Sarnelli, G.; Cuomo, R.; Steardo, L.; De Man, J.G.; et al. Cannabidiol Reduces Intestinal Inflammation through the Control of Neuroimmune Axis. PLoS ONE 2011, 6, e28159.
- Bush, T.G.; Savidge, T.C.; Freeman, T.C.; Cox, H.J.; Campbell, E.A.; Mucke, L.; Johnson, M.H.; Sofroniew, M.V. Fulminant jejuno-ileitis following ablation of enteric gila in adult transgenic mice. Cell 1998, 93, 189–201.
- Jessen, K.R.; Mirsky, R. Astrocyte-like glia in the peripheral nervous system: An immunohistochemical study of enteric glia. J. Neurosci. 1983, 3, 2206–2218.
- Cirillo, C.; Sarnelli, G.; Esposito, G.; Turco, F.; Steardo, L.; Cuomo, R. S100B protein in the gut: The evidence for enteroglial sustained intestinal inflammation. World J. Gastroenterol. 2011, 17, 1261–1266.
- Bradley, J.S.; Parr, E.J.; Sharkey, K.A. Effects of inflammation on cell proliferation in the myenteric plexus of the guinea-pig ileum. Cell Tissue Res. 1997, 289, 455–461.
- Pochard, C.; Coquenlorge, S.; Freyssinet, M.; Naveilhan, P.; Bourreille, A.; Neunlist, M.; Rolli-Derkinderen, M. The multiple faces of inflammatory enteric glial cells: Is crohn’s disease a gliopathy? Am. J. Physiol.-Gastrointest. Liver Physiol. 2018, 315, G1–G11.
- Von Boyen, G.B.; Steinkamp, M.; Reinshagen, M.; Schäfer, K.H.; Adler, G.; Kirsch, J. Nerve Growth Factor Secretion in Cultured Enteric Glia Cells is Modulated by Proinflammatory Cytokines. J. Neuroendocrinol. 2006, 18, 820–825.
- Von Boyen, G.B.; Degenkolb, N.; Hartmann, C.; Adler, G.; Steinkamp, M. The endothelin axis influences enteric glia cell functions. Med. Sci. Monit. 2010, 16, 161–167.
- Esposito, G.; Capoccia, E.; Turco, F.; Palumbo, I.; Lu, J.; Steardo, A.; Cuomo, R.; Sarnelli, G.; Steardo, L. Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-α activation. Gut 2014, 63, 1300–1312.
- Murakami, M.; Ohta, T.; Ito, S. Interleukin-1beta enhances the action of bradykinin in rat myenteric neurons through up-regulation of glial B1 receptor expression. Neuroscience 2008, 151, 222–231.
- Turco, F.; Sarnelli, G.; Cirillo, C.; Palumbo, I.; De Giorgi, F.; D’Alessandro, A.; Cammarota, M.; Giuliano, M.; Cuomo, R. Enteroglial-derived S100B protein integrates bacteria-induced Toll-like receptor signalling in human enteric glial cells. Gut 2014, 63, 105–115.
- Geboes, K.; Rutgeerts, P.; Ectors, N.; Mebis, J.; Penninckx, F.; Vantrappen, G.; Desmet, V.J. Major histocompatibility class II expression on the small intestinal nervous system in Crohn’s disease. Gastroenterology 1992, 103, 439–447.
- Koretz, K.; Momburg, F.; Otto, H.F.; Möller, P. Sequential induction of MHC antigens on autochthonous cells of ileum affected by Crohn’s disease. Am. J. Pathol. 1987, 129, 493–502.
- Cirillo, C.; Sarnelli, G.; Esposito, G.; Grosso, M.; Petruzzelli, R.; Izzo, P.; Calì, G.; D’Armiento, F.P.; Rocco, A.; Nardone, G.; et al. Increased mucosal nitric oxide production in ulcerative colitis is mediated in part by the enteroglial-derived S100B protein. Neurogastroenterol. Motil. 2009, 21, 1209-e112.
- Von Boyen, G.B.; Steinkamp, M.; Reinshagen, M.; Schäfer, K.H.; Adler, G.; Kirsch, J. Proinflammatory cytokines increase glial fibrillary acidic protein expression in enteric glia. Gut 2004, 53, 222–228.
- Rosenbaum, C.; Schick, M.A.; Wollborn, J.; Heider, A.; Scholz, C.J.; Cecil, A.; Niesler, B.; Hirrlinger, J.; Walles, H.; Metzger, M. Activation of myenteric glia during acute inflammation in vitro and in vivo. PLoS ONE 2016, 11, e0151335.
- Cirillo, C.; Sarnelli, G.; Turco, F.; Mango, A.; Grosso, M.; Aprea, G.; Masone, S.; Cuomo, R. Proinflammatory stimuli activates human-derived enteroglial cells and induces autocrine nitric oxide production. Neurogastroenterol. Motil. 2011, 23, e372–e382.
- Esposito, G.; Cirillo, C.; Sarnelli, G.; De Filippis, D.; D’Armiento, F.P.; Rocco, A.; Nardone, G.; Petruzzelli, R.; Grosso, M.; Izzo, P.; et al. Enteric Glial-Derived S100B Protein Stimulates Nitric Oxide Production in Celiac Disease. Gastroenterology 2007, 133, 918–925.
- Burns, A.; Pachnis, V. Development of the enteric nervous system: Bringing together cells, signals and genes. Neurogastroenterol. Motil. 2009, 21, 100–102.
- Von Boyen, G.B.; Steinkamp, M.; Geerling, I.; Reinshagen, M.; Schäfer, K.H.; Adler, G.; Kirsch, J. Proinflammatory cytokines induce neurotrophic factor expression in enteric glia: A key to the regulation of epithelial apoptosis in crohn’s disease. Inflamm. Bowel Dis. 2006, 12, 346–354.
- Sharkey, K.A. Emerging roles for enteric glia in gastrointestinal disorders. J. Clin. Investig. 2015, 125, 918–925.
- Grubišić, V.; McClain, J.L.; Fried, D.E.; Grants, I.; Rajasekhar, P.; Csizmadia, E.; Ajijola, O.A.; Watson, R.E.; Poole, D.P.; Robson, S.C.; et al. Enteric Glia Modulate Macrophage Phenotype and Visceral Sensitivity following Inflammation. Cell Rep. 2020, 32, 108100.
- Lucarini, E.; Seguella, L.; Vincenzi, M.; Parisio, C.; Micheli, L.; Toti, A.; Corpetti, C.; Del Re, A.; Squillace, S.; Maftei, D.; et al. Role of Enteric Glia as Bridging Element between Gut Inflammation and Visceral Pain Consolidation during Acute Colitis in Rats. Biomedicines 2021, 9, 1671.
- Sheikh, N.K.; Dua, A. Cannabinoids. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2022.
- Duggan, P.J. The chemistry of cannabis and cannabinoids. Aust. J. Chem. 2021, 74, 369–387.
- Zou, S.; Kumar, U. Cannabinoid receptors and the endocannabinoid system: Signaling and function in the central nervous system. Int. J. Mol. Sci. 2018, 19, 833.
- Hanus, L.; Abu-Lafi, S.; Fride, E.; Breuer, A.; Vogel, Z.; Shalev, D.E.; Kustanovich, I.; Mechoulam, R. 2-Arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc. Natl. Acad. Sci. USA 2001, 98, 3662–3665.
- Bisogno, T.; Melck, D.; Bobrov, M.Y.; Gretskaya, N.M.; Bezuglov, V.V.; De Petrocellis, L.; Di Marzo, V. N-acyl-dopamines: Novel synthetic CB1 cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo. Biochem. J. 2000, 351, 817–824.
- Porter, A.C.; Sauer, J.M.; Knierman, M.D.; Becker, G.W.; Berna, M.J.; Bao, J.; Nomikos, G.G.; Carter, P.; Bymaster, F.P.; Leese, A.B.; et al. Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J. Pharmacol. Exp. Ther. 2002, 301, 1020–1024.
- Starowicz, K.; Nigam, S.; Di Marzo, V. Biochemistry and pharmacology of endovanilloids. Pharmacol. Ther. 2007, 114, 3–33.
- Di Marzo, V.; De Petrocellis, L. Why do cannabinoid receptors have more than one endogenous ligand? Philos. Trans. R Soc. B Biol. Sci. 2012, 367, 3216–3228.
- Ahluwalia, J.; Urban, L.; Capogna, M.; Bevan, S.; Nagy, I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience 2000, 100, 685–688.
- Ahluwalia, J.; Urban, L.; Bevan, S.; Capogna, M.; Nagy, I. Cannabinoid 1 receptors are expressed by nerve growth factor- and glial cell-derived neurotrophic factor-responsive primary sensory neurones. Neuroscience 2002, 110, 747–753.
- Binzen, U.; Greffrath, W.; Hennessy, S.; Bausen, M.; Saaler-Reinhardt, S.; Treede, R. Co-expression of the voltage-gated potassium channel Kv1.4 with transient receptor potential channels (TRPV1 and TRPV2) and the cannabinoid receptor CB1 in rat dorsal root ganglion neurons. Neuroscience 2006, 14, 527–539.
- Ralevic, V.; Kendall, D. Cannabinoid Modulation of Perivascular Sympathetic and Sensory Neurotransmission. Curr. Vasc. Pharmacol. 2009, 7, 15–25.
- Weller, K.; Reeh, P.W.; Sauer, S.K. TRPV1, TRPA1, and CB1 in the isolated vagus nerve-Axonal chemosensitivity and control of neuropeptide release. Neuropeptides 2011, 45, 391–400.
- Cristino, L.; de Petrocellis, L.; Pryce, G.; Baker, D.; Guglielmotti, V.; Di Marzo, V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience 2006, 139, 1405–1415.
- Maione, S.; De Petrocellis, L.; de Novellis, V.; Moriello, A.S.; Petrosino, S.; Palazzo, E.; Rossi, F.S.; Woodward, D.F.; Di Marzo, V. Analgesic actions of N-arachidonoyl-serotonin, a fatty acid amide hydrolase inhibitor with antagonistic activity at vanilloid TRPV1 receptors. Br. J. Pharmacol. 2007, 150, 766–781.
- Micale, V.; Cristino, L.; Tamburella, A.; Petrosino, S.; Leggio, G.M.; Drago, F.; Di Marzo, V. Anxiolytic effects in mice of a dual blocker of fatty acid amide hydrolase and transient receptor potential vanilloid type-1 channels. Neuropsychopharmacology 2009, 34, 593–606.
- Golech, S.A.; McCarron, R.M.; Chen, Y.; Bembry, J.; Lenz, F.; Mechoulam, R.; Shohami, E.; Spatz, M. Human brain endothelium: Coexpression and function of vanilloid and endo-cannabinoid receptors. Mol. Brain Res. 2004, 132, 87–92.
- Domenicali, M.; Ros, J.; Fernández-Varo, G.; Cejudo-Martín, P.; Crespo, M.; Morales-Ruiz, M.; Briones, A.M.; Campistol, J.M.; Arroyo, V.; Vila, E.; et al. Increased anandamide induced relaxation in mesenteric arteries of cirrhotic rats: Role of cannabinoid and vanilloid receptors. Gut 2005, 54, 522–527.
- Lu, T.; Newton, C.; Perkins, I.; Friedman, H.; Klein, T.W. Role of cannabinoid receptors in Delta-9-tetrahydrocannabinol suppression of IL-12p40 in mouse bone marrow-derived dendritic cells infected with Legionella pneumophila. Eur. J. Pharmacol. 2006, 532, 170–177.
- Cavuoto, P.; McAinch, A.J.; Hatzinikolas, G.; Janovská, A.; Game, P.; Wittert, G.A. The expression of receptors for endocannabinoids in human and rodent skeletal muscle. Biochem. Biophys. Res. Commun. 2007, 364, 105–110.
- Rossi, F.; Siniscalco, D.; Luongo, L.; De Petrocellis, L.; Bellini, G.; Petrosino, S.; Torella, M.; Santoro, C.; Nobili, B.; Perrotta, S.; et al. The endovanilloid/endocannabinoid system in human osteoclasts: Possible involvement in bone formation and resorption. Bone 2009, 44, 476–484.
- Tóth, B.I.; Dobrosi, N.; Dajnoki, A.; Czifra, G.; Oláh, A.; Szöllosi, A.G.; Juhász, I.; Sugawara, K.; Paus, R.; Bíró, T. Endocannabinoids modulate human epidermal keratinocyte proliferation and survival via the sequential engagement of cannabinoid receptor-1 and transient receptor potential vanilloid-1. J. Investig. Dermatol. 2011, 131, 1095–1104.
- Pucci, M.; Pasquariello, N.; Battista, N.; Di Tommaso, M.; Rapino, C.; Fezza, F.; Zuccolo, M.; Jourdain, R.; Finazzi Agrò, A.; Breton, L.; et al. Endocannabinoids stimulate human melanogenesis via type-1 cannabinoid receptor. J. Biol. Chem. 2012, 287, 15466–15478.
- Oka, S.; Nakajima, K.; Yamashita, A.; Kishimoto, S.; Sugiura, T. Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem. Biophys. Res. Commun. 2007, 362, 928–934.
- Ryberg, E.; Larsson, N.; Sjögren, S.; Hjorth, S.; Hermansson, N.O.; Leonova, J.; Elebring, T.; Nilsson, K.; Drmota, T.; Greasley, P.J. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2007, 152, 1092–1101.
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.; Di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB1 and CB2. Pharmacol. Rev. 2010, 62, 588–631.
- Kano, M.; Ohno-Shosaku, T.; Hashimotodani, Y.; Uchigashima, M.; Watanabe, M. Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev. 2009, 89, 309–380.
- Feledziak, M.; Lambert, D.M.; Marchand-Brynaert, J.; Muccioli, G.G. Inhibitors of the endocannabinoid-degrading enzymes, or how to increase endocannabinoid’s activity by preventing their hydrolysis. Recent Pat. CNS Drug Discov. 2012, 7, 49–70.
- Rouzer, C.A.; Marnett, L.J. Endocannabinoid oxygenation by cyclooxygenases, lipoxygenases, and cytochromes P450: Cross-talk between the eicosanoid and endocannabinoid signaling pathways. Chem. Rev. 2011, 111, 5899–5921.
