Macrophages are the most abundant immune cells of the tumor microenvironment (TME) and have multiple important functions in cancer. Macrophages comprise a heterogeneous and functionally versatile population of innate immune cells. Compelling evidence indicate that the high degree of plasticity of macrophages and their ability to self-renew majorly impact tumor progression and resistance to therapy. In addition, the microenvironmental factors largely affect the metabolism of macrophages and may have a major influence on TAMs proliferation and subsets functions. Thus, understanding the signaling pathways regulating TAMs self-renewal capacity may help to identify promising targets for the development of novel anticancer agents.
1. Introduction
Macrophages comprise a heterogeneous and functionally versatile population of innate immune cells [
1,
2,
3]. Every adult tissue contains an abundant population of macrophages either as resident cells or monocyte-derived cells that play tissue-specific functions and are essential for organ homeostasis [
4,
5,
6]. Tissue-resident macrophages (TRMs) are long-lived cells and can be phenotypically distinct from monocyte-derived macrophages (MDMs). Initially, it was believed that TRMs in an adult are maintained by a constant replenishment of bone marrow-derived circulating monocytes in the steady state. This model was based on the assumption that terminally differentiated macrophages were unable to enter the cell cycle [
7]. This was refuted by strong evidence that differentiated macrophages in several tissues were derived from precursors in yolk sac or fetal liver, independently of bone marrow, and self-maintained throughout life by local proliferation [
8,
9,
10,
11]. Accordingly, mature macrophages can proliferate in response to specific stimuli indefinitely and can also be expanded and maintained in long-term culture without loss of differentiation [
12]. These findings demonstrated that macrophages exhibit a self-renewal potential similar to that of stem cells [
12,
13]. Distinct lineage-specific enhancer platforms regulate a shared network of genes that control self-renewal capacity in both stem and mature cells. In particular, single cell analyses identified key transcription factors, e.g., c-myelocytomatosis (c-Myc), Krüppel-like factor 2 (KLF2) and Krüppel-like factor 4 (KLF4), involved in macrophage self-renewal [
14]. Conversely, MafB and c-Maf repress the self-renewal of resident macrophages [
14,
15]. Moreover, several in vivo studies have demonstrated that bone marrow-derived monocytes can also differentiate into self-renewing tissue-resident macrophages [
16,
17,
18,
19]. Similar to embryo-derived macrophages, KLF2 was strongly upregulated in the self-renewing bone marrow-derived macrophages. This was accompanied by the downregulation of MafB, unravelling a molecular mechanism of proliferation amongst self-renewing macrophages of different origins [
13].
The discovery that TRMs were capable of self-renewal led to the new concept that macrophage proliferation played a key role in the expansion of macrophage populations in the TME, which is composed of the stroma and the tumor cells. During tumor growth, both TRMs and newly recruited monocyte-derived macrophages can give rise to TAMs which have been correlated with poor clinical outcome in most cancers [
20,
21,
22,
23,
24,
25,
26]. Emerging evidence showed that TAMs retained the self-renewal capacity [
23,
25,
27,
28,
29,
30]. This unique feature was shown to markedly contribute to increasing the pool of protumoral TAMs, whether they derive from the yolk sac or the bone marrow, in mammary [
23] and pancreatic [
25] tumors.
High plasticity is a characteristic feature of macrophages that enables them to rapidly change phenotypes in response to environmental cues [
31]. One of the best examples is the macrophage adaptation to the tumor environment, which has commonly been discussed in terms of a spectrum of polarization states from anti-tumor M1 to protumor M2 phenotypes [
32]. Macrophage plasticity also concerns their metabolism and its modulation has emerged as a key factor in controlling macrophage activation and functions [
33,
34,
35]. Although metabolic plasticity is associated with and participates to macrophage polarization state [
36], it is becoming clear that specific metabolic signatures have an impact on self-renewing macrophages.
2. Macrophages Proliferation in Health and Disease
Macrophages are considered key players in innate and adaptive immune response, and in tissue repair. They protect the organism from infection both directly by pathogens phagocytosis and indirectly by acting as antigens presenting cells. Moreover, in light of recent discoveries, it has become obvious that resident macrophages derived from temporally and spatially distinct hematopoietic waves are critically important for tissue homeostasis [
1,
37]. Macrophages display different microanatomical localization in tissues and exhibits distinct rate of replacement via monocyte-derived and/or self-renewal capabilities [
38]. The first wave of hematopoietic stem cells (HSCs) in human is detected at around day 18–19 of estimated gestational age (EGA) in the blood islands of the yolk sac (YS) where stem cells restricted to myelo-erythroid development are produced. These primitive macrophages are the first wave of colonization of the brain and other fetal organs, and microglial cells are the only macrophages that predominantly arise from yolk sac progenitors [
39,
40]. From 5 to 7 weeks of EGA, a second wave of hematopoietic cell development occurs in the aorta–gonads–mesonephros (AGM) when hematopoiesis temporally moved to the fetal liver. With the exception of microglia in the brain, most TRMs originate from monocytes produced in the fetal liver from around 4–5 weeks of EGA until 22 weeks of EGA [
40]. Finally, at 10.5 weeks of EGA, hematopoiesis is definitively established in the bone marrow to give rise to monocyte-derived macrophages in adulthood [
39,
40,
41]. To summarize, most adult tissue-resident macrophages are settled before birth and are maintained locally throughout adulthood by self-renewal. Adult bone marrow-derived circulating monocytes can be recruited to inflammatory sites to support TRMs populations [
25].
Interestingly, the number of macrophages can increase as a consequence of proliferation, leading to chronic low-grade inflammation. This phenomenon can cause a variety of alterations associated with certain pathological conditions [
42,
43]. For example, under normal conditions, adipose tissue macrophages (ATMs) whose function is to remove cellular pathogens, dispose of dying adipocytes and process lipids, constitute approximately 10% of all cells in fat tissue. In obesity, the proportion of ATMs increases to 50%, thereby leading to a persistent inflammatory state that results in adipose tissue fibrosis, insulin resistance and type-2 diabetes mellitus (T2DM) caused by β-cell dysfunction in pancreatic islets [
43]. Initially, increased ATM numbers were believed to be a result of blood monocyte recruitment in the affected tissues. This view has changed in light of recent evidence that the accumulation of ATMs in crown-like structures around dead adipocytes is mainly a consequence of in situ proliferation at the early stage of obesity and is further promoted by the recruitment of monocytes at the late stage [
43]. Pancreatic inflammation associated with obesity is characterized by the accumulation of immune cells and elevated production of inflammatory cytokines and chemokines [
44,
45,
46]. A high number of islet-resident macrophages is a typical hallmark of T2DM associated with obesity [
42,
47]. Interestingly, intra-islet macrophages proliferate and expand locally, independent of recruitment from circulating monocytes, and may impair β cell function by restricting insulin secretion, suggesting a complex crosstalk network between proliferating macrophages and β cells.
Macrophages with self-renewal capabilities have also been involved in non-resolving inflammation associated with cancer. TAMs represent the most abundant inflammatory cells in the TME [
48,
49,
50]. Crosstalks between macrophages and the other cells of the TME, via cell-to-cell contact or the production of soluble factors, are essential for promoting cancer cell proliferation, angiogenesis, and metastasis [
3,
51,
52]. The accumulation of TAMs in tumors results from the constant recruitment of circulating monocytic precursors from the blood [
23,
53]. However, recent studies revealed that pools of TAMs can originate from proliferating TRMs [
23,
28,
29,
30,
54]. This is significant given that proliferative macrophages were detected in sarcomas [
28], fibrosarcoma [
55], gastric cancer [
56], colorectal carcinoma CRC [
57], prostate cancer [
58], non-small cell lung cancer (NSCLC) [
59,
60], ovarian cancer [
61], CNS cancer [
62], breast carcinomas [
29,
30], and pancreatic cancer [
25]. In a murine model of human pancreatic ductal adenocarcinomas (PDAC), circulating monocytes were found to be dispensable for tumor growth, while the expansion of TRMs through in situ proliferation played a critical role to regulate TAMs population supporting cancer progression [
25]. In addition, whereas monocyte-derived TAMs played a more potent roles in antigen presentation, proliferating embryonically derived macrophages exhibited a profibrotic transcriptional profile [
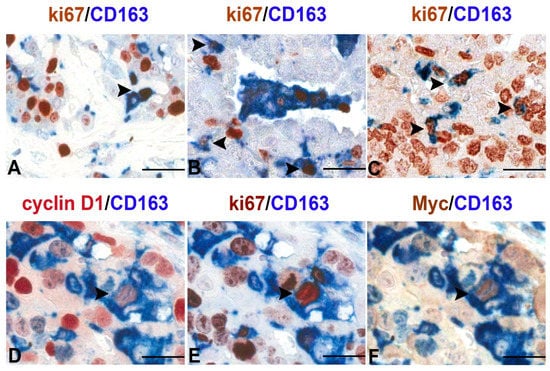
25], indicative of their role in producing and remodeling molecules in the extracellular matrix. More recently, using tissue microarrays in a large set of human primary malignant lesions, we demonstrated a noticeable increase in the number of proliferating macrophages (Ki67
+CD163
+ cells) compared with normal tissues [
63] (
Figure 1). Moreover, TAMs accumulation through proliferation was a significant feature of malignancy, clearly indicating that local macrophage proliferation was a common hallmark of human tumors and a potentially important prognostic marker of malignancy [
63].
Figure 1. Proliferating TAMs in human cancers. Sections are from human pleural mesothelioma (A), lung adenosquamous carcinoma (B), serous ovarian carcinoma (C) and transitional bladder carcinoma (D–F) and stained as labelled. A variable fraction of CD163+ TAMs co-express the proliferation marker ki-67 as demonstrated by double immunostaining (A–C,E); a minor fraction of TAMs also co-express nuclear Myc and Cyclin D1 as revealed by sequential double immunostains (D–F). Images are obtained from 40× digitalized slides and resized using Adobe Photoshop. Scale bar (A–C), 44 μM; (D–F), 33 μM.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10112709