Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
β-cyclocitral (βCC), a main apocarotenoid of β-carotene, increases plants’ resistance against stresses. It has recently appeared as a novel bioactive composite in a variety of organisms from plants to animals. In plants, βCC marked as stress signals that accrue under adverse ecological conditions. βCC regulates nuclear gene expression through several signaling pathways, leading to stress tolerance.
- β-carotene
- plant
- apocarotenoid
1. Biosynthesis of β-Cyclocitral
The formation of β-cyclocitral (βCC) occurs either by direct oxidation of β-carotene through ROS (1O2) or by an enzymatic pathway. A family of non-heme iron-dependent enzymes in plants catalyzes the carotenoids by an enzymatic cleavage via 9-cis-epoxycarotenoid cleavage dioxygenases (NCEDs) and carotenoid cleavage dioxygenases (CCDs), resulting in apocarotenoids, an oxidation product [1][2]. The first step in abscisic acid (ABA) production is catalyzed by NCED enzymes cleaving the 11, 12 (11′, 12′) double bond of 9-cis-violaxanthin or 9-cisneoxanthin [3]. Furthermore, CCD enzymes and NCED enzymes do not share cleavage specificities. In Arabidopsis, there are four CCDs (CCD1, CCD4, CCD7, and CCD8). It is unknown whether one of these CCDs creates βCC from carotene in plant leaves. In each of the four CCDs in Arabidopsis deficient mutants, the accumulation of βCC was not affected, which suggests that β-carotene oxidation mediated by CCD in this species is not a major source of this apocarotenoid [4], despite the fact that between 4 CCDs functional redundancy cannot be ruled out. This is similar in cyanobacteria, where βCC formation aided by CCD was not found [5]. Unlike CCDs that are plastidial, cytosolic CCD1 cleaves the double bonds of 9, 10 (9′, 10′) to produce varying volatiles and apocarotenoids of extensive acyclic or monocyclic apocarotenoids and carotenoids.
The strigolactones biosynthesis is dependent on CCD8 and CCD7 [6]. Since CCD4 has a specific cleavage activity at 9, 10 (9′, 10′) and 5, 6 (5′, 6′) double bond, it does not generate βCC [1][2]. Furthermore, in high light conditions, CCD4 is highly downregulated, which activates the accumulation of βCC [4]. However, the cleavage of β-carotene in citrus from the location 7, 8 (7′, 8′), CCD4b is reported under CCD4 enzyme, which results in the production of βCC [7]. Similarly, another CCD4c in the Crocus stigma from CCD4 can cleave carotenoids at 9-10 (9′, 10′), resulting into β-ionone and produces βCC with low efficiency at 7 and 8 (7′, 8′) [8]. For the production of βCC, CCD4b gene in Vitis vinifera in the carotenoid-accumulating yeast strain is also reported [9]. Another way for the oxidation of carotenoids can be provided by lipoxygenase [10]. Similarly, in leaves of Solanum lycopersicum and Arabidopsis, knockout mutants for 13-lipoxygenase LOX2 were reported to have low levels of βCC [11]. On the other hand, in the βCC accumulation under high light and 1O2 stresses, it is unknown if this enzyme is involved despite the fact that LOX2 is induced under these circumstances [4]. Eventually, from the fungus Lepista irina, extracellular fluid purified a peroxidase which produces βCC and other unstable apocarotenoids from the cleavage of β-carotene [12].
When compared to photosystem II, it is thought that photosystem I does not produce considerable amounts of 1O2. Auto-oxidation of β-carotene can also produce βCC, especially when attacked by the reactive specie 1O2 [13]. Carotenoids quench 1O2 through a physical mechanism that involves energy transfer from 1O2 to the carotenoid, followed by the excited quencher’s thermal decay [14]. However, carotene can be oxidised by 1O2, allowing 1O2 to be chemically quenched. 1O2 is an electrophilic molecule that has a strong affinity for double bonds in carotenoid molecules, oxidizing them and creating a range of apocarotenoids, including βCC [15]. In microalgae, the principal oxidation products of β-carotene are β-ionone and βCC, which release large amounts of these chemicals during summer blooms [16].
2. Signalling of β-Cyclocitral in Plants
The βCC has emerged as a new dimension for acting as a stress tolerant molecule in adverse conditions. The signaling pathway has been disclosed along with the transportation function within plants. The βCC performs in a hormone-induced marker line and the corresponding mutant responds to phytohormone pathway signals like auxin and brassinosteroids (BRs) and eventually results in cell enlargement [17]. Enzymatic action produces CCD4b from the genetic variance of Crocus sativus through β-carotene cleavage in the model plant Arabidopsis, which hastens to reduce the dehydration, salinity and oxidation rate [8][18]. The endo-metabolic substances in vascular plants took part in the xenobiotic response with diverse detoxifying agents, such as SCL14, ANAC102, ANAC001 and ANAC031 for oxidation resistance [19]. The βCC induced plants build interdependence with PAP signaling and down-regulate carotenoid substances while ST2A acts as a sulfate donor, and SAL1 has a deleterious effect during the plethora of light and drought stress [20][21]. Along with PAP, Methylerythritol cyclodiphosphate (MEcPP) substrates are also redox regularized and trigger the augmentation rate of the ROS level [22][23][24]. Sustaining photosensitivity during oxidative stress environment in mbs1 mutant crops for signaling pathway, procurement of protein and partial replacement in the nuclei occurs [25].
3. Functions of β-Cyclocitral in Plants
The βCC is a volatile organic compound that has been reported to have multiple functions in non-vascular plants (Figure 1). Microalgae discharge βCC, which is responsible for transferring stress signals to homogeneous algae and inducing defence. The former compound plays an allelopathic function on heterogeneous algae and aquatic macrophyte for opposite nutrients, as well as providing protection in opposition to predators [26]. The βCC has been reported to make cell rupture in Nitzschia palea, a diatom [27]. Ikawa et al. (2001) [28] reported that in cyanobacterium Microcystis, βCC is one of the main emitted volatile organic compounds. Sun et al. (2020) [29] suggested that the toxicity of βCC to cells might be associated with nuclear variation, DNA laddering, caspase 9- and caspase 3-like performance, signifying the initiation of a programmed cell death mechanism. In the case of cyanobacterial bloom, β-cyclocitric acid is produced by the oxidation of the βCC compound by Microcystis, indirectly causing toxicity. The production of this acidic compound leads to water acidification, causing chlorophyll loss, cell lysis, and phycocyanin pigment release, resulting in a characteristic blue colour [30]. These studies suggest that βCC and other volatile apocarotenoids are the principal allelopathic agents in cyanobacterial volatile organic compounds, but that at high concentrations, these compounds may be harmful to the emitters. However, no evidence has been found that low levels of βCC can elicit defence mechanisms in photosynthetic bacteria, such as those found in vascular plants. Mosses have also been observed to release volatile chemical compounds that could be used in interspecies communication [31]. Experimental evidence exhibited that photosynthetic activators and enzymatic variance treated with βCC in plants increased the photosynthetic rate, root-shoot expansion and carbon assimilation [32].
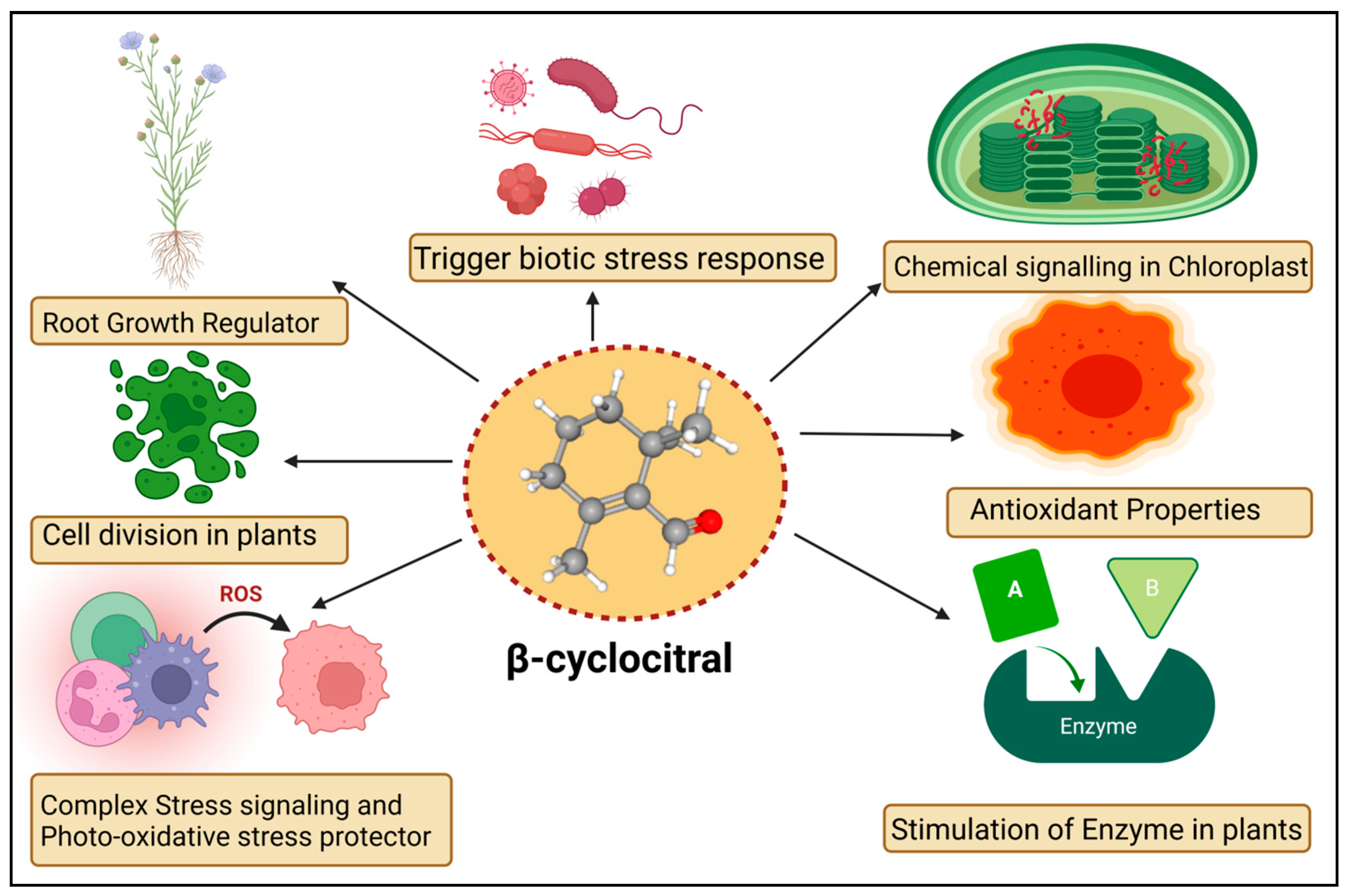
Figure 1. Diagrammatic sketch describing different functions of β-cyclocitral in plants.
The mosses Hamatocaulis vernicosus and Sphagnum fexuosum compete for the release of volatile organic compounds via increasing emission of a molecule called methyl 2,6, 6-trimethyl-1-cyclohexene-1-carboxylate, which is chemically linked to βCC. As a result, an alarmed mechanism could be set off, signalling the competitive strength of their neighboring moss species. The former is an enzyme that can convert carotene to βCC. The βCC is an intermediary in the 1O2 signalling pathway, which controls gene expression reprogramming. It eventually causes plant cells to shift from active growth to cellular defence, resulting in stress and adaptation. The bulk of the downregulated gene encoded proteins involved in the development, growth, and biogenesis of cellular components [15]. Upregulated genes, on the other hand, were linked to environmental interactions, stress responses, and cellular mobility. Under normal or light stress conditions, βCC produces a tiny zinc finger protein (MBS1; methylene blue sensitivity 1) that is needed for the proper expression of 1O2-responsive genes [33]. The βCC is said to have increased the former protein levels while also causing the protein to relocalize to the nucleus [25]. Further research revealed that the Arabidopsis mbs1 mutant (deficient in MBS1) was insensitive to βCC and therefore lacked an increase in photo-tolerance after treatment with βCC [25]. MBS1 is thought to be downstream of βCC in the 1O2 signalling pathway, although it’s precise function remains uncertain. Exogenous reactive substances are inactivated by typical detoxifying enzymes in vascular plants, which remove these molecules in three phases. In the first stage, side groups are introduced or modified in harmful substances such as herbicides, pollutants, and so on. The modified molecule is conjugated to sugar moieties or glutathione in the second stage. Finally, inactivated chemical compounds are compartmentalized [34]. The transcriptome of βCC-treated Arabidopsis plants showed activation of detoxification pathways [35]. Several glutathione-S-transferases (GST) and UDP-glycosyltransferases (UDP-glycosyltransferases) were involved in the xenobiotic detoxification process. The GRAS protein (SCL14; SCARECROW LIKE 14) and the glutaredoxin (GRX480/ROXY19) fight for interaction with TGAII transcription factors and mediate the activation/inhibition of a large number of detoxifying enzymes during the modification phase [36]. SCL14-controlled xenobiotic detoxification was induced by βCC and photooxidative stress conditions. Similarly, the scl14 knockout mutant did not respond to βCC and remained susceptible to high light stress even after treatment with βCC [37]. A few signalling cascade components downstream of βCC have been identified. The SCL14/TGA II complex, in particular, modulates the transcriptional levels of a transcription factor that regulates other downstream transcription regulators and, eventually, the redox enzymes of the first phase of the detoxification response [37], thus improving cellular detoxification capabilities. Surprisingly, the loss of MBS1 had no effect on βCC-induced cellular detoxification, indicating that there are two pathways in βCC signalling, one regulated by SCL14 and the other dependent on MBS1 [37]. Lipid peroxidation produces the chemicals that are characteristic of photooxidation and contributes unwaveringly to its toxicity. They decompose into reactive aldehydes (acrolein or 4-hydroxynonenal), which obstruct macromolecule function and cause cell death [38]. Diversified functions of βCC in plants are given in Table 1.
In plants including Solanum lycopersicum, Piper nigrum, and Arabidopsis thaliana, chemical βCC can cause changes in gene expression and promote drought tolerance [39]. The available literature on βCC shows that it may activate a signalling cascade that has yet to be fully described. Dickinson et al. (2019) [40] found that Arabidopsis seedlings grown in Petri plates treated with βCC stimulated the growth of primary roots. Increased root lengths may be beneficial under salt and water stress conditions, allowing for better soil exploration and water uptake. βCC has the ability to influence root development in Solanum lycopersicum and Oryza sativa without relying on auxin or brassinosteroid signalling. However, it is unclear whether βCC causes root growth directly through cell division and elongation or indirectly through the activation of cellular detoxification and resistance to oxidative stress. The molecular processes underlying βCC control of root development will need to be clarified in future research [13].
Table 1. Various functions of βCC in Plants.
| Plant Species | Functions | References |
|---|---|---|
 Nitzschia palea |
Cell rupture at 0.1–0.5 mg mL−1 dose, cyanobacterial cell degradation, change in water color | [27] |
 Cyanidium caldarium |
Unpalatable water odor | [41] |
 Chlorella pyrenoidosa |
Inhibition of cell growth and development | [28] |
 Chlamydomonas reinhardtii |
Induce programmed cell death, cause poison to other algae | [29] |
 Microcystis aeruginosa |
Increase βCC emission, expose high ion concentration | [42] |
 Solanum lycopersicum |
Retro nasal olfactory (smell) add to flavor to the fruit, volatile compound induces taste | [43] |
 Oryza sativa |
Scented rice varieties have aroma, more leaves present in vegetative stage | [44] |
 Petroselinum crispum |
Helps to produce essential oil and contribute in anti-fungal activity | [45] |
 Camellia sinensis |
Improve odorant properties and structural functions | [46] |
 Grapevines |
Inhibit infestation of spider mite and reduce symptoms | [47] |
This entry is adapted from the peer-reviewed paper 10.3390/molecules27206845
References
- Giuliano, G.; Al-Babili, S.; Von Lintig, J. Carotenoid oxygenases: Cleave it or leave it. Trends Plant Sci. 2003, 8, 145–149.
- Hou, X.; Rivers, J.; León, P.; McQuinn, R.P.; Pogson, B.J. Synthesis and function of apocarotenoid signals in plants. Trends Plant Sci. 2016, 21, 792–803.
- Nambara, E.; Marion-Poll, A. Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 2005, 56, 165.
- Ramel, F.; Mialoundama, A.S.; Havaux, M. Nonenzymic carotenoid oxidation and photooxidative stress signalling in plants. J. Exp. Bot. 2013, 64, 799–805.
- Cui, H.; Wang, Y.; Qin, S. Genomewide analysis of carotenoid cleavage dioxygenases in unicellular and filamentous cyanobacteria. Comp. Funct. Genom. 2012, 2012, 164690.
- Al-Babili, S.; Bouwmeester, H.J. Strigolactones, a novel carotenoid-derived plant hormone. Annu. Rev. Plant Biol. 2015, 66, 161–186.
- Rodrigo, M.J.; Alquézar, B.; Alós, E.; Medina, V.; Carmona, L.; Bruno, M.; Al-Babili, S.; Zacarías, L. A novel carotenoid cleavage activity involved in the biosynthesis of Citrus fruit-specific apocarotenoid pigments. J. Exp. Bot. 2013, 64, 4461–4478.
- Rubio-Moraga, A.; Rambla, J.L.; Fernández-de-Carmen, A.; Trapero-Mozos, A.; Ahrazem, O.; Orzáez, D.; Granell, A.; Gómez-Gómez, L. New target carotenoids for CCD4 enzymes are revealed with the characterization of a novel stress-induced carotenoid cleavage dioxygenase gene from Crocus sativus. Plant Mol. Biol. 2014, 86, 555–569.
- Meng, N.; Yan, G.-L.; Zhang, D.; Li, X.-Y.; Duan, C.-Q.; Pan, Q.-H. Characterization of two Vitis vinifera carotenoid cleavage dioxygenases by heterologous expression in Saccharomyces cerevisiae. Mol. Biol. Rep. 2019, 46, 6311–6323.
- Hayward, S.; Cilliers, T.; Swart, P. Lipoxygenases: From isolation to application. Compr. Rev. Food Sci. Food Saf. 2017, 16, 199–211.
- Miras-Moreno, B.; Pedreño, M.A.; Fraser, P.D.; Sabater-Jara, A.B.; Almagro, L. Effect of diflufenican on total carotenoid and phytoene production in carrot suspension-cultured cells. Planta 2019, 249, 113–122.
- Zorn, H.; Langhoff, S.; Scheibner, M.; Nimtz, M.; Berger, R.G. A peroxidase from Lepista irina cleaves β, β-carotene to flavor compounds. Biol. Chem. 2003, 384, 1049–1056.
- D’Alessandro, S.; Havaux, M. Sensing β-carotene oxidation in photosystem II to master plant stress tolerance. New Phytol. 2019, 223, 1776–1783.
- Edge, R.; McGarvey, D.J.; Truscott, T.G. The carotenoids as anti-oxidants—A review. J. Photochem. Photobiol. B Biol. 1997, 41, 189–200.
- Ramel, F.; Birtic, S.; Ginies, C.; Soubigou-Taconnat, L.; Triantaphylidès, C.; Havaux, M. Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl. Acad. Sci. USA 2012, 109, 5535–5540.
- Ma, Z.; Niu, Y.; Xie, P.; Chen, J.; Tao, M.; Deng, X. Off-flavor compounds from decaying cyanobacterial blooms of Lake Taihu. J. Environ. Sci. 2013, 25, 495–501.
- Felemban, A.; Braguy, J.; Zurbriggen, M.D.; Al-Babili, S. Apocarotenoids involved in plant development and stress response. Front. Plant Sci. 2019, 10, 1168.
- Baba, S.A.; Jain, D.; Abbas, N.; Ashraf, N. Overexpression of Crocus carotenoid cleavage dioxygenase, CsCCD4b, in Arabidopsis imparts tolerance to dehydration, salt and oxidative stresses by modulating ROS machinery. J. Plant Physiol. 2015, 189, 114–125.
- Moretto, J.A.S.; de Freitas, P.N.N.; de Almeida, E.C.; Altarugio, L.M.; da Silva, S.V.; Fiore, M.F.; Pinto, E. Effects of different cultivation conditions on the production of β-cyclocitral and β-ionone in Microcystis aeruginosa. BMC Microbiol. 2022, 22, 78.
- Estavillo, G.M.; Crisp, P.A.; Pornsiriwong, W.; Wirtz, M.; Collinge, D.; Carrie, C.; Giraud, E.; Whelan, J.; David, P.; Javot, H. Evidence for a SAL1-PAP chloroplast retrograde pathway that functions in drought and high light signaling in Arabidopsis. Plant Cell 2011, 23, 3992–4012.
- Wilson, P.B.; Estavillo, G.M.; Field, K.J.; Pornsiriwong, W.; Carroll, A.J.; Howell, K.A.; Woo, N.S.; Lake, J.A.; Smith, S.M.; Harvey Millar, A. The nucleotidase/phosphatase SAL1 is a negative regulator of drought tolerance in Arabidopsis. Plant J. 2009, 58, 299–317.
- Rossel, J.B.; Walter, P.B.; Hendrickson, L.; Chow, W.S.; Poole, A.; Mullineaux, P.M.; Pogson, B.J. A mutation affecting ASCORBATE PEROXIDASE 2 gene expression reveals a link between responses to high light and drought tolerance. Plant Cell Environ. 2006, 29, 269–281.
- Kim, B.; Von Arnim, A.G. FIERY1 regulates light-mediated repression of cell elongation and flowering time via its 3′(2′), 5′-bisphosphate nucleotidase activity. Plant J. 2009, 58, 208–219.
- Li, Z.; Sharkey, T.D. Metabolic profiling of the methylerythritol phosphate pathway reveals the source of post-illumination isoprene burst from leaves. Plant Cell Environ. 2013, 36, 429–437.
- Shumbe, L.; d’Alessandro, S.; Shao, N.; Chevalier, A.; Ksas, B.; Bock, R.; Havaux, M. Methylene Blue Sensitivity 1 (MBS1) is required for acclimation of Arabidopsis to singlet oxygen and acts downstream of β-cyclocitral. Plant Cell Environ. 2017, 40, 216–226.
- Zuo, Z. Why algae release volatile organic compounds—The emission and roles. Front. Microbiol. 2019, 10, 491.
- Chang, D.W.; Hsieh, M.-L.; Chen, Y.-M.; Lin, T.-F.; Chang, J.-S. Kinetics of cell lysis for Microcystis aeruginosa and Nitzschia palea in the exposure to β-cyclocitral. J. Hazard. Mater. 2011, 185, 1214–1220.
- Ikawa, M.; Sasner, J.J.; Haney, J.F. Activity of cyanobacterial and algal odor compounds found in lake waters on green alga Chlorella pyrenoidosa growth. Hydrobiologia 2001, 443, 19–22.
- Sun, Q.; Zhou, M.; Zuo, Z. Toxic mechanism of eucalyptol and β-cyclocitral on Chlamydomonas reinhardtii by inducing programmed cell death. J. Hazard. Mater. 2020, 389, 121910.
- Arii, S.; Tsuji, K.; Tomita, K.; Hasegawa, M.; Bober, B.; Harada, K.-I. Cyanobacterial blue color formation during lysis under natural conditions. Appl. Environ. Microbiol. 2015, 81, 2667–2675.
- Saritas, Y.; Sonwa, M.M.; Iznaguen, H.; König, W.A.; Muhle, H.; Mues, R. Volatile constituents in mosses (Musci). Phytochemistry 2001, 57, 443–457.
- Schwachtje, J.; Minchin, P.E.H.; Jahnke, S.; van Dongen, J.T.; Schittko, U.; Baldwin, I.T. SNF1-related kinases allow plants to tolerate herbivory by allocating carbon to roots. Proc. Natl. Acad. Sci. USA 2006, 103, 12935–12940.
- Shao, N.; Duan, G.Y.; Bock, R. A mediator of singlet oxygen responses in Chlamydomonas reinhardtii and Arabidopsis identified by a luciferase-based genetic screen in algal cells. Plant Cell 2013, 25, 4209–4226.
- Riechers, D.E.; Kreuz, K.; Zhang, Q. Detoxification without intoxication: Herbicide safeners activate plant defense gene expression. Plant Physiol. 2010, 153, 3–13.
- Sandermann, H., Jr. Plant metabolism of xenobiotics. Trends Biochem. Sci. 1992, 17, 82–84.
- Huang, L.J.; Li, N.; Thurow, C.; Wirtz, M.; Hell, R.; Gatz, C. Ectopically expressed glutaredoxin ROXY19 negatively regulates the detoxification pathway in Arabidopsis thaliana. BMC Plant Biol. 2016, 16, 200.
- D’alessandro, S.; Ksas, B.; Havaux, M. Decoding β-cyclocitral-mediated retrograde signaling reveals the role of a detoxification response in plant tolerance to photooxidative stress. Plant Cell 2018, 30, 2495–2511.
- Mano, J.; Biswas, M.S.; Sugimoto, K. Reactive carbonyl species: A missing link in ROS signaling. Plants 2019, 8, 391.
- D’Alessandro, S.; Mizokami, Y.; Légeret, B.; Havaux, M. The apocarotenoid β-cyclocitric acid elicits drought tolerance in plants. iScience 2019, 19, 461–473.
- Dickinson, A.J.; Lehner, K.; Mi, J.; Jia, K.-P.; Mijar, M.; Dinneny, J.; Al-Babili, S.; Benfey, P.N. β-Cyclocitral is a conserved root growth regulator. Proc. Natl. Acad. Sci. USA 2019, 116, 10563–10567.
- Xu, Q.; Yang, L.; Yang, W.; Bai, Y.; Hou, P.; Zhao, J.; Zhou, L.; Zuo, Z. Volatile organic compounds released from Microcystis flos-aquae under nitrogen sources and their toxic effects on Chlorella vulgaris. Ecotoxicol. Environ. Saf. 2017, 135, 191–200.
- Hasegawa, M.; Nishizawa, A.; Tsuji, K.; Kimura, S.; Harada, K. Volatile organic compounds derived from 2-keto-acid decarboxylase in Microcystis aeruginosa. Microbes Environ. 2012, 27, 525–528.
- Tieman, D.; Bliss, P.; McIntyre, L.M.; Blandon-Ubeda, A.; Bies, D.; Odabasi, A.Z.; Rodríguez, G.R.; van der Knaap, E.; Taylor, M.G.; Goulet, C. The chemical interactions underlying tomato flavor preferences. Curr. Biol. 2012, 22, 1035–1039.
- Hinge, V.; Patil, H.; Nadaf, A. Comparative characterization of aroma volatiles and related gene expression analysis at vegetative and mature stages in basmati and non-basmati rice (Oryza sativa L.) cultivars. Appl. Biochem. Biotechnol. 2016, 178, 619–639.
- Linde, G.A.; Gazim, Z.C.; Cardoso, B.K.; Jorge, L.F.; Tešević, V.; Glamočlija, J.; Soković, M.; Colauto, N.B. Antifungal and antibacterial activities of Petroselinum crispum essential oil. Genet. Mol. Res. 2016, 15, gmr.15038538.
- Ojha, P.K.; Roy, K. PLS regression-based chemometric modeling of odorant properties of diverse chemical constituents of black tea and coffee. RSC Adv. 2018, 8, 2293–2304.
- Lazazzara, V.; Bueschl, C.; Parich, A.; Pertot, I.; Schuhmacher, R.; Perazzolli, M. Downy mildew symptoms on grapevines can be reduced by volatile organic compounds of resistant genotypes. Sci. Rep. 2018, 8, 1618.
This entry is offline, you can click here to edit this entry!
