Platinum nanoparticles (PtNPs) have superior physicochemical properties and great potential in biomedical applications. Eco-friendly and economic approaches for the synthesis of PtNPs have been developed to overcome the shortcomings of the traditional physical and chemical methods. Various biogenic entities have been utilized in the green synthesis of PtNPs, including mainly plant extracts, algae, fungi bacteria, and their biomedical effects were assessed. Other biological derivatives have been used in the synthesis of PtNPs such as egg yolk, sheep milk, honey, and bovine serum albumin protein. The green approaches for the synthesis of PtNPs have reduced the reaction time, the energy required, and offered ambient conditions of fabrication. Plant extracts contain diverse primary and secondary metabolites, which could serve as natural reducing and capping agents. The plant-mediated biosynthesis of MNPs is a simple and rapid process involving mixing the plant extract with the metal ions solution at an optimized temperature and pH. The nanoparticle generation is indicated by the change in color of the reaction medium.
- green synthesis
- biosynthesis
- platinum nanoparticles
- anticancer
1. Green Synthesis of PtNPs Using Plant Extracts
| Plant | Part Used | Reaction Conditions | Average Size (nm) | Shape | Biomedical Application | Ref. |
|---|---|---|---|---|---|---|
| Azadirachta indica | Leaf extract | 100 °C; 60 min | 5–50 | Spherical | - | [1] |
| Nigella sativa (Black cumin) | Seeds | 75 °C; 2 days | 3.47 | Spherical |
|
[2] |
| Terminalia chebula | Fruit extract | 100 °C; 10 min | <4 | Nearly spherical | - | [3] |
| Tea polyphenol | - | Room temperature 1 h |
30–60 | Flower-shaped |
|
[4] |
| Ocimum sanctum (Tulsi) | Leaf extract | 100 °C; 1 h | 23 | Irregular structure | - | [5] |
| Ocimum sanctum (Tulsi) | Leaf extract | Room temperature. Plant extract: Pt ions (1:9) >20 min | 2 | Irregular structure | - | [6] |
| Punica granatum (Pomegranate) | Crusts | Room temperature Ultrasonication 24 h | 20.12 | Spherical and cubes |
|
[7] |
| Doipyros kaki (Persimmon) | Leaf extract | 95 °C; 2–3 h; Leaf broth concentration: >10% | 2−12 | Spheres and plates | - | [8] |
| Anacardium occidentale (Cashew) | Leaf extract | 95 °C; pH 6–9 | - | Irregular rod shaped |
- | [9] |
| Bacopa monnieri (Water hyssop) | Leaf extract | Room temperature | 5–20 | Spherical |
|
[10] |
| Cochlospermum gossypium | Gum | 120 °C; pH 8 | 2.4 | Spherical | - | [11] |
| Dioscorea bulbifera | Tuber extract | 100 °C; 5 h | 2–5 | Spherical |
|
[12] |
| Eichhornia crassipes (Water hyacinth) | Leaf extract | 90 °C; 1 h | TEM: 3.74 DLS: 73.3 |
Spherical | - | [13] |
| Green tea | Powder extract | 50 °C; 4 h | 2 | Spherical |
|
[13] |
| Ononis spinose | Radix extract | 80 °C; 10 h | 4 | Spherical and hexagonal |
|
[14] |
| Maytenus royleanus | Leaf extract | 90 °C; 3 h | 5 | Spherical |
|
[15] |
| Mentha piperita (Peppermint) |
Leaf extract | 60 °C; 2 h | 54.3 | Spherical |
|
[16] |
| Taraxacum laevigatum | Plant extract | 90 °C; 10 min | 2–7 | Spherical |
|
[17] |
| Prunus x yedoensis | Gum extract | pH 8; Gum extract concentrations of 7% and 8% 30 min |
10–50 | Spherical |
|
[18] |
2. Characterization and Biological Activities of PtNPs Prepared Using Plant Extracts
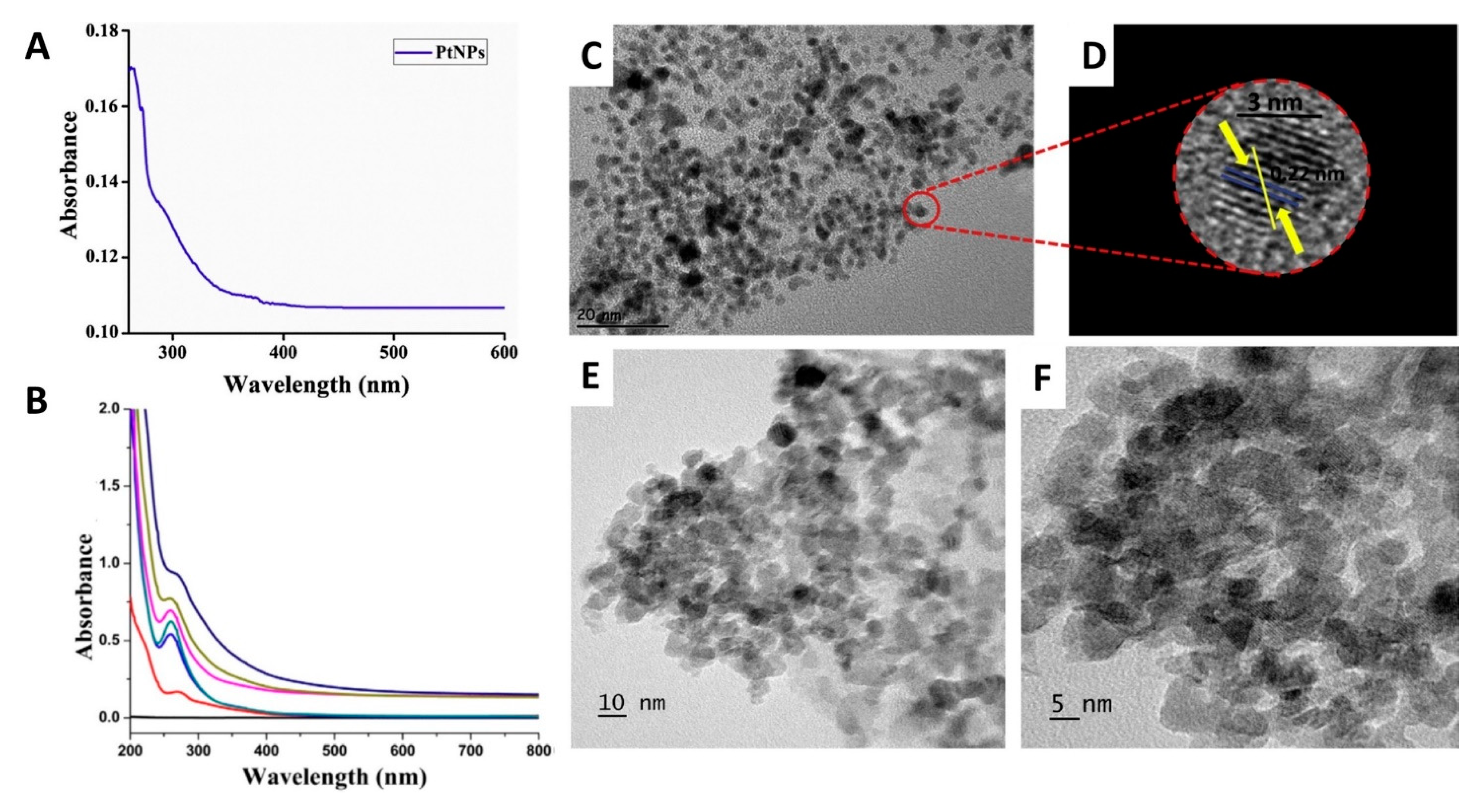
This entry is adapted from the peer-reviewed paper 10.3390/molecules25214981
References
- Thirumurugan, A.; Aswitha, P.; Kiruthika, C.; Nagarajan, S.; Christy, A.N. Green synthesis of platinum nanoparticles using Azadirachta indica—An eco-friendly approach. Mater. Lett. 2016, 170, 175–178.
- Ayguna, A.; Gülbagcaa, F.; Ozerb, L.Y.; Ustaogluc, B.; Altunogluc, Y.C.; Balogluc, M.C.; Atalard, M.N.; Almae, M.H.; Sena, F. Biogenic platinum nanoparticles using black cumin seed and their potential usage as antimicrobial and anticancer agent. J. Pharm. Biomed. Anal. 2020, 179, 112961.
- Kumar, K.M.; Mandal, B.K.; Tammina, S.K. Green synthesis of nano platinum using naturally occurring polyphenols. RSC Adv. 2013, 3, 4033.
- Alshatwi, A.A.; Periasamy, J.A.; Subbarayan, V. Green synthesis of platinum nanoparticles that induce cell death and G2/M-phase cell cycle arrest in human cervical cancer cells. J. Mater. Sci. Mater. Med. 2015, 26, 7.
- Soundarrajan, C.; Sankari, A.; Dhandapani, P.; Maruthamuthu, S.; Ravichandran, S.; Sozhan, G.; Palaniswamy, N. Rapid biological synthesis of platinum nanoparticles using Ocimum sanctum for water electrolysis applications. Bioprocess Biosyst. Eng. 2012, 35, 827–833.
- Prabhu, N.; Gajendran, T. Green Synthesis of Noble Metal of Platinum Nanoparticles from Ocimum sanctum (Tulsi) Plant- Extracts. J. Biotechnol. Biochem. 2017, 3, 107–112.
- Sahin, B.; Aygün, A.; Gündüz, H.; Sahin, K.; Demir, E.; Akocak, S.; Sen, F. Cytotoxic effects of platinum nanoparticles obtained from pomegranate extract by the green synthesis method on the MCF-7 cell line. Colloids Surf. B Biointerfaces 2018, 163, 119–124.
- Song, J.Y.; Kwon, E.Y.; Kim, B.S. Biological synthesis of platinum nanoparticles using Diopyros kaki leaf extract. Bioprocess Biosyst. Eng. 2010, 33, 159–164.
- Sheny, D.S.; Philip, D.; Mathew, J. Synthesis of platinum nanoparticles using dried Anacardium occidentale leaf and its catalytic and thermal applications. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 114, 267–271.
- Nellore, J.; Pauline, C.; Amarnath, K. Bacopa monnieri Phytochemicals Mediated Synthesis of Platinum Nanoparticles and Its Neurorescue Effect on 1-Methyl 4-Phenyl 1,2,3,6 Tetrahydropyridine-Induced Experimental Parkinsonism in Zebrafish. J. Neurodegener. Dis. 2013, 2013, 1–8.
- Vinod, V.; Saravanan, P.; Sreedhar, B.; Devi, D.K.; Sashidhar, R. A facile synthesis and characterization of Ag, Au and Pt nanoparticles using a natural hydrocolloid gum kondagogu (Cochlospermum gossypium). Colloids Surf. B Biointerfaces 2011, 83, 291–298.
- Ghosh, S.; Nitnavare, R.; Dewle, A.; Tomar, G.B.; Chippalkatti, R.; More, P.; Chopade, B.A. Novel platinum–palladium bimetallic nanoparticles synthesized by Dioscorea bulbifera: Anticancer and antioxidant activitiesInt. J. Nanomed. 2015, 10, 7477–7490.
- Depciuch, J.; Stec, M.; Maximenko, A.; Drzymała, E.; Pawlyta, M.; Baran, J.; Parlinska-Wojtan, M. Synthesis method-dependent photothermal effects of colloidal solutions of platinum nanoparticles used in photothermal anticancer therapy. Appl. Organometal. Chem. 2020, 34, e5401.
- Dobrucka, R.; Romaniuk-Drapała, A.; Kaczmarek, M. Evaluation of biological synthesized platinum nanoparticles using Ononidis radix extract on the cell lung carcinoma A549. Biomed. Microdevices 2019, 21, 75.
- Ullah, S.; Ahmad, A.; Wang, A.; Raza, M.; Jan, A.U.; Tahir, K.; Rahman, A.U.; Qipeng, Y. Bio-fabrication of catalytic platinum nanoparticles and their in vitro efficacy against lungs cancer cells line (A549). J. Photochem. Photobiol. B Biol. 2017, 173, 368–375.
- Yang, C.; Wang, M.; Zhou, J.; Chi, Q. Bio-synthesis of peppermint leaf extract polyphenols capped nano-platinum and their in-vitro cytotoxicity towards colon cancer cell lines (HCT 116). Mater. Sci. Eng. C 2017, 77, 1012–1016.
- Tahir, K.; Nazir, S.; Ahmad, A.; Li, B.; Khan, A.U.; Khan, Z.U.H.; Khan, F.U.; Khan, Q.U.; Khan, A.; Rahman, A.U. Facile and green synthesis of phytochemicals capped platinum nanoparticles and in vitro their superior antibacterial activity. J. Photochem. Photobiol. B Biol. 2017, 166, 246–251.
- Velmurugan, P.; Shim, J.; Kim, K.; Oh, B.T. Prunus × yedoensis tree gum mediated synthesis of platinum nanoparticles with antifungal activity against phytopathogens. Mater. Lett. 2016, 174, 61–65.
- Anyik, J.L.; Oluwafemi, O.S. Plant-mediated synthesis of platinum nanoparticles using water hyacinth as an efficient biomatrix source—An eco-friendly development. Mater. Lett. 2017, 196, 141–144.
- Nadaroglu, H.; Gungor, A.A.; Ince, S.; Babagil, A. Green synthesis and characterisation of platinum nanoparticles using quail egg yolk. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 172, 43–47.
- Baskaran, B.; Muthukumarasamy, A.; Chidambaram, S.; Sugumaran, A.; Ramachandran, K.; Rasu Manimuthu, T. Cytotoxic potentials of biologically fabricated platinum nanoparticles from Streptomyces sp. on MCF-7 breast cancer cells. IET Nanobiotechnol. 2017, 11, 241–246.
- Gaidhani, S.V.; Yeshvekar, R.K.; Shedbalkar, U.U.; Bellare, J.H.; Chopade, B.A. Bio-reduction of hexachloroplatinic acid to platinum nanoparticles employing Acinetobacter calcoaceticus. Process. Biochem. 2014, 49, 2313–2319.
- Gholami-Shabani, M.; Gholami-Shabani, Z.; Shams-Ghahfarokhi, M.; Akbarzadeh, A.; Riazi, G.; Razzaghi-Abyaneh, M. Biogenic Approach using Sheep Milk for the Synthesis of Platinum Nanoparticles: The Role of Milk Protein in Platinum Reduction and Stabilization. Int. J. Nanosci. Nanotechnol. 2016, 12, 199–206.
- Venu, R.; Ramulu, T.S.; Anandakumar, S.; Rani, V.S.; Kim, C.G. Bio-directed synthesis of platinum nanoparticles using aqueous honey solutions and their catalytic applications. Colloid Surf. A 2011, 384, 733–738.
